Abstract
The objective of the present study was to examine the feasibility of the production of autologous porcine somatic cell nuclear transfer (SCNT) blastocysts using oocytes and donor cells from slaughtered ovaries. Therefore, we attempted to optimize autologous SCNT by examining the effects of electrical fusion conditions and donor cell type on cell fusion and the development of SCNT embryos. Four types of donor cells were used: 1) denuded cumulus cells (DCCs) collected from in vitro-matured (IVM) oocytes; 2) cumulus cells collected from oocytes after 22 h of IVM and cultured for 18 h (CCCs); 3) follicular cells obtained from follicular contents and cultured for 40 h (CFCs); and 4) adult skin fibroblasts. The DCCs showed a significantly (p < 0.01) lower rate of fusion than the CCCs when two pulses of 170 V/mm DC were applied for 50 µsec (19 ± 2% vs. 77 ± 3%). The rate of DCC fusion with oocytes was increased by the application of two DC pulses of 190 V/mm for 30 µsec, although this was still lower than the rate of fusion in the CCCs (33 ± 1% vs. 80 ± 2%). The rates of cleavage (57 ± 5%) and blastocyst formation (1 ± 1%) in the DCC-derived embryos did not differ from those (55 ± 6% and 3 ± 1%, respectively) in the CCC-derived SCNT embryos. Autologous SCNT embryos derived from CFCs (5 ± 2%) showed higher levels of blastocyst formation (p < 0.01) than CCC-derived autologous SCNT embryos (1 ± 0%). In conclusion, the results of the present study show that culturing cumulus and follicular cells before SCNT enhances cell fusion with oocytes and that CFCs are superior to CCCs in the production of higher numbers of autologous SCNT blastocysts.
Keywords: autologous, cumulus cells, follicular cells, nuclear transfer, pigs
Introduction
Nuclear transfer (NT) techniques that use a variety of somatic cells have been used to produce genetically superior animals or transgenic animals that can secrete valuable proteins or pharmaceuticals used in human medicine in their urine or milk [3,14,17], as well as to establish embryonic stem cell lines in several mammalian species [9,35]. It is currently possible to produce somatic cell-cloned animals from several mammalian species, including cattle, pigs, horses, and sheep [10,14,25,38]. The pig has been widely used as a target animal for the generation of a bio-organ donor for xenotransplantation by deleting or inserting specific genes that are associated with the human immune system [16,27,32].
The mass production of somatic cell nuclear transfer (SCNT) embryos is usually carried out using immature pig oocytes that are obtained from slaughtered ovaries and used as cytoplasts after in vitro maturation (IVM). However, these slaughterhouse-derived oocytes originate from various maternal lineages and therefore have different types of mitochondrial DNA (mtDNA) depending on their origin. SCNT embryos prepared from recipient oocytes and donor cells with different maternal lineages are not true clones, but are rather genetic copies with mtDNAs from two different sources. Together with the reprogramming of donor cell nuclei in recipient oocytes, previous studies have demonstrated that mtDNA influences the in vitro developmental capacity of SCNT embryos and the normality of newborn animals derived from SCNT embryos [7,22]. It has also been reported that the fusion rate and the number of transferable blastocysts are affected by the source of recipient oocytes in bovine SCNT [4] and that heterologous mtDNA introduced by NT of donor cells influences the in vitro development of SCNT mouse embryos [22]. Mitochondria mediate cell metabolism, growth, and differentiation by means of ATP production. In addition to these physiological roles, mitochondria are known to influence the economic value of domestic animals due to their influence on meat quality, milk yield traits, and fertility [20,21,28,31]. It is necessary to establish an efficient system for the generation of autologous SCNT embryos in order to apply SCNT to the production of a superior animal with maternally inherited traits [11,33,34]. In cattle, autologous SCNT was used to examine the effect of nuclear-cytoplasmic interactions on embryo viability in vitro and in vivo [4,12,39], but there are few reports on autologous SCNT in pigs.
The objective of the present study was to examine the feasibility of producing autologous porcine SCNT blastocysts using autologous oocytes and donor cells from slaughtered ovaries. Therefore, we attempted to optimize autologous pig SCNT by examining the effects of electrical fusion and donor cell type on cell fusion and the subsequent in vitro development of porcine SCNT embryos.
Materials and Methods
Culture media
All chemicals were purchased from Sigma-Aldrich (USA), unless otherwise stated. IVM was carried out in TCM-199 media (Invitrogen, USA) supplemented with 10% (v/v) porcine follicular fluid, 0.6 mM cysteine, 0.91 mM pyruvate, 10 ng/ml epidermal growth factor, 75 µg/ml kanamycin, and 1 µg/ml insulin. Porcine follicular fluid (pFF) was collected from follicles of 3-8 mm in diameter, centrifuged at 1900 × g for 15 min, filtered through a 0.2-µm filter, and stored at -30℃ until use. The same batch of pFF was used in all experiments. The in vitro culture (IVC) medium for embryo development was North Carolina State University -23 medium containing 0.4% (w/v) bovine serum albumin (BSA) [24], which was modified by replacing glucose with 0.5 mM pyruvate and 5.0 mM lactate [23].
Oocyte collection and IVM
Porcine ovaries were collected from prepubertal gilts at a local slaughterhouse and transported to the laboratory in saline at approximately 37℃. Follicles of 3-8 mm in diameter were aspirated using an 18 G needle fixed to a 10-ml disposable syringe, and the follicular contents were pooled into 15-ml conical tubes and allowed to settle as sediment. The sediment was suspended in HEPES-buffered Tyrode's medium (TLH) containing 0.05% (w/v) polyvinyl alcohol (PVA) (TLH-PVA) [1] and observed under the microscope. Only cumulus-oocyte complexes (COCs) with more than three layers of compact cumulus cells were selected for IVM. For the autologous SCNT in Experiment 3, immature oocytes from pairs of ovaries from individual pigs were collected and matured separately. All oocytes, with the exception of morphologically degenerated oocytes, were cultured for IVM to obtain as many oocytes as possible during autologous SCNT. After washing twice in TLH-PVA and once in IVM medium, groups of 20-80 COCs were placed into individual wells of a 4-well multi-dish (Nunc, Denmark) that contained 500 µl of IVM medium with 5 IU/ml eCG (Intervet, Holland) and 5 IU/ml hCG (Intervet, Holland). COCs were cultured at 39℃ in a humidified atmosphere of 5% CO2 in air. After 22 h of maturation culture, the COCs were washed three times in fresh, hormone-free IVM medium and then cultured for an additional 18 h.
Preparation of donor cells
Four types of somatic cells were used as donor cells: 1) denuded cumulus cells (DCCs); 2) cultured cumulus cells (CCCs); 3) cultured follicular cells (CFCs); and 4) adult skin fibroblasts. DCCs were collected from IVM oocytes by repeated pipetting in IVM medium that contained 0.1% (w/v) hyaluronidase, washed twice in TLH-PVA by centrifugation, and resuspended in TLH containing 0.4% (w/v) BSA (TLH-BSA) prior to nuclear transfer. CCCs collected from oocytes at 22 h of IVM were cultured for 18 h. Follicular cells collected from the follicular contents at the time of oocyte aspiration were washed twice in TLH-PVA by centrifugation and cultured for 44 h before use. Pig ear skin fibroblasts were cultured until contact inhibited, as described previously [15,37]. The cells were cultured in Dulbecco's modified Eagle medium with nutrient mixture F-12 (Invitrogen, USA) supplemented with 10% fetal bovine serum, with the exception of DCCs. The cells were then trypsinized, centrifuged, and resuspended in TLH-BSA prior to use.
Nuclear transfer
The base medium for oocyte manipulation was calcium-free TLH-BSA containing 5 µg/ml cytochalasin B. After 40 h of maturation culture, denuded oocytes were incubated for 15 min in a manipulation medium that contained 5 µg/ml Hoechst 33342, washed twice in fresh medium, and then placed into a manipulation medium droplet that was overlaid with mineral oil. Metaphase II (MII) oocytes were enucleated by aspirating the first polar body and MII chromosomes using a 17-µm beveled glass pipette (Humagen, USA), and enucleation was confirmed under an epifluorescent microscope (TE300; Nikon, Japan).
After enucleation, a single cell was inserted into the perivitelline space of each oocyte. Cell-oocyte couplets were placed on a 1-mm fusion chamber that was overlaid with 1 ml of 280 mM mannitol that contained 0.001 mM CaCl2 and 0.05 mM MgCl2. Membrane fusion was induced by applying an alternating current field of 2 V, 1 MHz for 2 sec, followed by two direct current (DC) pulses of 170 V for 50 µsec or 190 V for 30 µsec using a cell fusion generator (LF101; NepaGene, Japan). The oocytes were incubated for 1 h in TLH-BSA and examined for fusion under a stereomicroscope prior to activation.
Activation and embryo culture
Reconstructed oocytes were activated by two pulses of 120 V/mm DC for 60 µsec in 280 mM mannitol that contained 0.01 mM CaCl2 and 0.05 mM MgCl2. The oocytes were thoroughly washed in IVC medium, transferred into 30-µl droplets of medium under mineral oil, and cultured for 6 days at 39℃ in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2. Cleavage and blastocyst formation were evaluated on Days 2 and 6, respectively (the day of SCNT was designated as Day 0). The total cell numbers in the blastocysts were assessed using Hoechst 33342 staining under an epifluorescent microscope.
Experimental design and statistical analysis
All of the oocytes used in the respective experiments were randomly allocated to each treatment group, and at least three replications were performed in each experiment. In Experiment 1, SCNT embryos were produced using two types of donor cells (DCC and CCC). The cell fusion rate was examined after applying two electrical fusion stimuli to CCCs and DCCs (2 × 2 factorial design). The in vitro developmental capacities of the SCNT embryos derived from DCCs or CCCs were examined in Experiment 2. Based on the results of Experiment 1, two DC pulses of 190 V/mm for 30 µsec and 170 V/mm for 50 µsec were applied to the DCCs and CCCs, respectively, in order to fuse the cell with a cytoplast. In Experiments 1 and 2, SCNT embryos were produced by donor cells of heterologous origin. In Experiment 3, autologous SCNT embryos were produced using CCCs or CFCs as donor cells, and their in vitro developmental potentials were examined. In addition, the developmental capacities of the autologous SCNT embryos were compared with those of heterologous SCNT embryos derived from adult skin fibroblasts.
The data were analyzed using the general linear model procedure in the Statistical Analysis System version 9.1 software (SAS Institute, USA), followed by the least significant difference mean separation procedure when treatments differed at p < 0.05. Percentage data were subjected to arcsine transformation before analysis in order to maintain the homogeneity of variance. The results are expressed as mean ± SE of the mean.
Results
Effects of electrical field strength and donor cell type on fusion (Experiment 1)
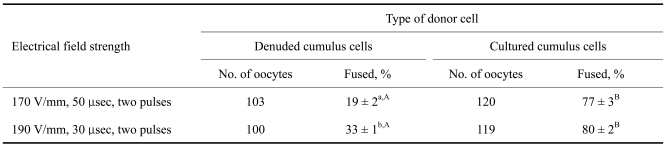
Two electrical field strengths were applied to reconstructed oocytes derived from DCCs or CCCs. As shown in Table 1, the application of two DC pulses of 190 V/mm for 30 µsec resulted in a significantly (p < 0.01) higher fusion rate (33 ± 1%) than the application of two DC pulses of 170 V/mm for 50 µsec (19 ± 2%) when DCC were used as donor cells. However, fusion efficiency was not altered by two different electrical field strengths when CCCs were used as donor cells (77 ± 3% vs. 80 ± 2%). The CCCs showed significantly higher fusion rates (77-80%) than the DCC (19-33%), regardless of the strength of the electrical field applied. There was no interaction between electrical field strength and type of donor cell with respect to fusion rate.
Table 1.
Fusion rates of reconstructed oocytes in relation to electrical field strength and donor cell type
abWithin a column, values with different superscripts are significantly different (p<0.01). ABWithin a row, values with different superscripts are significantly different (p<0.01).
in vitro development of SCNT embryos derived from DCCs or CCCs (Experiment 2)
SCNT embryos derived from DCCs and CCCs were cultured and examined for their developmental capacities in vitro (Table 2). The fusion rate was significantly (p < 0.01) higher when CCCs (75 ± 2%) were used as donor cells than when DCCs (31 ± 1%) were used. The rates of cleavage and blastocyst formation in SCNT embryos derived from DCCs were 57 ± 5% and 1 ± 1%, respectively, which were not significantly different from those of the CCC-derived SCNT embryos (55 ± 6% and 3 ± 1%, respectively). The number of blastocyst cells was not altered by the type of donor cell (28 ± 0 and 33 ± 3 cells/blastocyst for DCCs and CCCs, respectively).
Table 2.
in vitro development of somatic cell nuclear transfer embryos derived from denuded (DCC) or cultured cumulus cells (CCC)
abWithin a column, values with different superscripts are significantly different (p< 0.01).
in vitro development of SCNT embryos derived from autologous or heterologous donor cells (Experiment 3)
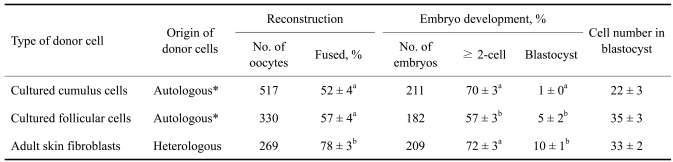
For autologous somatic cell cloning, 1,243 immature oocytes were obtained from 40 pairs of ovaries (31.1 oocytes/pig) and cultured for IVM. After IVM, 74.1% (921/1,243) of the oocytes reached the MII stage. The fusion rate (78 ± 3%) after SCNT was significantly (p < 0.01) higher when heterologous skin fibroblasts were used as donor cells than when cumulus or follicular cells were used as donor cells (52 ± 4% and 57 ± 4%, respectively). SCNT embryos derived from autologous follicular cells showed a significantly (p < 0.01) lower cleavage rate (57 ± 3%) than embryos derived from cumulus cells or skin fibroblasts (70 ± 3% and 72 ± 3%, respectively). A significantly lower rate of blastocyst formation (1 ± 0%) was obtained from autologous SCNT using oocytes and cumulus cells from the same pig than from SCNT using autologous follicular cells and heterologous skin fibroblasts (5 ± 2% and 10 ± 1%). There was no significant difference in blastocyst cell number (22-35 cells) among the three treatment groups (Table 3).
Table 3.
in vitro development of somatic cell nuclear transfer embryos derived from autologous or heterologous donor cells
*Somatic cell nuclear transfer embryos were produced from donor cells and recipient oocytes that originated from the same pig. abWithin a column, values with different superscripts are significantly different (p< 0.01).
Discussion
A series of experiments was performed to produce cloned blastocysts by autologous SCNT using recipient oocytes and donor cells from the same pig. Our results demonstrated that higher rates of fusion with recipient oocytes could be obtained by culturing cumulus or follicular cells before SCNT. In addition, autologous SCNT blastocysts could be produced via the reconstruction of oocytes with cumulus cells and follicular cells from slaughtered ovaries from the same pig, but their developmental capacities tended to be lower than those of heterologous SCNT embryos derived from adult skin fibroblasts.
Donor cell nuclei are usually introduced into enucleated oocytes by cell fusion [19,37] or direct injection into the cytoplast [5,36]. The successful fusion of donor cells with oocytes is a prerequisite for normal embryonic development in SCNT using the cell fusion method. The DCCs from IVM oocytes showed very low fusion rates after NT in comparison to the CCCs, which is inconsistent with the previous finding in goats [18], where cumulus cells from IVM oocytes showed comparable fusion rates to cultured fetal fibroblasts after NT. It is not clear whether this discrepancy between the results of the present study and those of the previous study is due to species difference. The fusion rate of uncultured cumulus cells (DCCs) was greatly increased when the cells were cultured (CCCs) before electrical fusion. This result indicates that the cell culture process may have induced changes in the properties of the membrane, such that the sensitivities of the cultured cumulus cells to electrical pulsing were altered. Cell size is known to be one of the factors that influence cell fusion in reconstructed oocytes [26]. In this study, the CCCs tended to be larger in diameter than the DCCs collected from oocytes immediately after the end of IVM, which may have contributed to the disparity in cell fusion rates between the two types of cumulus cells.
Even though there was a difference in cell fusion rates, the SCNT embryos derived from DCCs and CCCs did not show any differences in the rates of embryo cleavage or blastocyst formation, which means that the cell culture process itself does not influence embryonic development after SCNT. The donor cell cycle is an important factor in the development of SCNT embryos [6,38]. Cells in the G0/G1 phases are superior to cells in other phases of the cycle in terms of their ability to support NT embryo development when MII oocytes are used as recipient cytoplasts [8,36]. Schultz et al. [29] reported that more than 90% of the cumulus cells that surround recently ovulated mouse oocytes are in the G0/G1 phases of the cell cycle. Another study found that the rate of cell growth in the G0/G1 phases decreased in actively growing cells, as compared to that of cells that were serum-starved or confluent [2]. Although we did not analyze the cell cycles of the DCCs and CCCs, we suspect that the cycles of those two types of cumulus cell were different since the CCCs were cultured for only 18 h and were probably in the actively growing phase. However, this probable difference in the cell cycle was not reflected in the development of the SCNT embryos.
The mean nuclear maturation rate in oocytes collected from individual pigs was 74.1%, which was lower than that (88-98%) observed for heterogeneous oocytes matured in our laboratory [23,30]. We used almost all of the oocytes derived from each pair of ovaries in order to produce as many autologous SCNT embryos as possible. Therefore, it seems likely that the inclusion of low-quality oocytes in the oocyte selection process contributed to the decrease in the rate of nuclear maturation. The rate of MII oocytes derivation from individual pigs ranged from 34.2% to 100%, while embryo cleavage and blastocyst formation after SCNT were not correlated with the maturation rate (data not shown).
The fusion rate (52%) of autologous cell-oocyte couplets reconstructed from CCCs (Experiment 3) was low in comparison to that observed in Experiments 1 and 2 (75-77%). It is unclear whether the decreased fusion rate in Experiment 3 can be attributed to the heterologous or autologous origin of the donor cells or to the difference in the batch of oocytes used. Comparative study using heterologous and autologous donor cells with the same batch of oocytes should be performed to clarify the effect of donor cell origin on cell fusion and subsequent SCNT embryo development.
CCC-derived autologous SCNT embryos showed lower developmental capacities to the blastocyst stage than those reconstructed from autologous CFCs and heterologous skin fibroblasts. This result was not consistent with the previous finding in pigs [19], which showed no significant difference in the rate of blastocyst formation between adult fibroblast-derived and cumulus cell-derived SCNT embryos. Although CFC-derived SCNT embryos showed lower embryo cleavage rates than CCC-derived embryos, they showed higher rates of blastocyst formation than CCC-derived embryos. It is not clear whether the differences in the developmental competence of CCC-, CFC-, and skin fibroblast-derived embryos are attributable to the autologous or heterologous origin of the donor cells or to the difference in the type of donor cell used. In cattle, it has been reported that cumulus cells in cumulus-enclosed oocytes spontaneously undergo apoptosis during IVM, and it was suggested that the degree of apoptosis might be correlated with the developmental competence of the oocytes [13]. The cumulus cells used in this study were obtained from IVM oocytes. In addition, cumulus cells and follicular cells were cultured for 18 and 40 h, respectively, before they were used as donor cells, whereas skin fibroblasts were cultured for more than 5 days until confluent. It is possible that differences in the cell cycle and the degree of apoptosis among the CCCs, CFCs, and skin fibroblasts due to the use of different methods for donor cell preparation influenced the developmental capacities of the SCNT embryos. Cell cycle and apoptosis analysis would be helpful in optimizing autologous SCNT using CCCs or CFCs.
In conclusion, the results of the present study show that the culturing of cumulus or follicular cells before nuclear transfer enhances the rate of fusion and that CFCs are superior to CCCs in the production of greater numbers of autologous SCNT blastocysts. The SCNT method established in the present study can be applied to the analysis of the role of mitochondria in the development of autologous or heterologous SCNT embryos. Notwithstanding the successful production of autologous SCNT blastocysts in this study, the low developmental capacity of autologous SCNT embryos remains to be improved. Further studies are needed to establish an effective method for the production of autologous SCNT piglets and to examine the effects of autologous SCNT on economic traits, such as meat quality, milk yield, and fertility.
Acknowledgments
The authors thank Mr. Bohyun Kwon and Ms. Inyoung Lee for their assistance in the collection and transportation of the ovaries, and Veterinary Services of Gyonggi and Gangwon provinces for their generous donation of porcine ovaries. This work was supported by a Korea Research Foundation Grant (KRF-2004-041-E00342).
References
- 1.Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod. 1983;28:235–247. doi: 10.1095/biolreprod28.1.235. [DOI] [PubMed] [Google Scholar]
- 2.Boquest AC, Day BN, Prather RS. Flow cytometric cell cycle analysis of cultured porcine fetal fibroblast cells. Biol Reprod. 1999;60:1013–1019. doi: 10.1095/biolreprod60.4.1013. [DOI] [PubMed] [Google Scholar]
- 3.Brophy B, Smolenski G, Wheeler T, Wells D, L'Huillier P, Laible G. Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat Biotechnol. 2003;21:157–162. doi: 10.1038/nbt783. [DOI] [PubMed] [Google Scholar]
- 4.Bruggerhoff K, Zakhartchenko V, Wenigerkind H, Reichenbach HD, Prelle K, Schernthaner W, Alberio R, Kuchenhoff H, Stojkovic M, Brem G, Hiendleder S, Wolf E. Bovine somatic cell nuclear transfer using recipient oocytes recovered by ovum pick-up: effect of maternal lineage of oocyte donors. Biol Reprod. 2002;66:367–373. doi: 10.1095/biolreprod66.2.367. [DOI] [PubMed] [Google Scholar]
- 5.Bui HT, Van Thuan N, Wakayama T, Miyano T. Chromatin remodeling in somatic cells injected into mature pig oocytes. Reproduction. 2006;131:1037–1049. doi: 10.1530/rep.1.00897. [DOI] [PubMed] [Google Scholar]
- 6.Campbell KHS, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 7.Cibelli JB, Campbell KH, Seidel GE, West MD, Lanza RP. The health profile of cloned animals. Nat Biotechnol. 2002;20:13–14. doi: 10.1038/nbt0102-13. [DOI] [PubMed] [Google Scholar]
- 8.Collas P, Balise JJ, Robl JM. Influence of cell cycle stage of the donor nucleus on development of nuclear transplant rabbit embryos. Biol Reprod. 1992;46:492–500. doi: 10.1095/biolreprod46.3.492. [DOI] [PubMed] [Google Scholar]
- 9.Fang ZF, Gai H, Huang YZ, Li SG, Chen XJ, Shi JJ, Wu L, Liu A, Xu P, Sheng HZ. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp Cell Res. 2006;312:3669–3682. doi: 10.1016/j.yexcr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, Ponderato N, Duchi R, Lazzari G. Pregnancy: a cloned horse born to its dam twin. Nature. 2003;424:635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- 11.Hiendleder S, Zakhartchenko V, Wenigerkind H, Reichenbach HD, Bruggerhoff K, Prelle K, Brem G, Stojkovic M, Wolf E. Heteroplasmy in bovine fetuses produced by intra- and inter-subspecific somatic cell nuclear transfer: neutral segregation of nuclear donor mitochondrial DNA in various tissues and evidence for recipient cow mitochondria in fetal blood. Biol Reprod. 2003;68:159–166. doi: 10.1095/biolreprod.102.008201. [DOI] [PubMed] [Google Scholar]
- 12.Hiendleder S, Prelle K, Bruggerhoff K, Reichenbach HD, Wenigerkind H, Bebbere D, Stojkovic M, Muller S, Brem G, Zakhartchenko V, Wolf E. Nuclear-cytoplasmic interactions affect in utero developmental capacity, phenotype, and cellular metabolism of bovine nuclear transfer fetuses. Biol Reprod. 2004;70:1196–1205. doi: 10.1095/biolreprod.103.023028. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Imai H, Yamada M. Apoptosis in cumulus cells during in vitro maturation of bovine cumulus-enclosed oocytes. Reproduction. 2003;125:369–376. [PubMed] [Google Scholar]
- 14.Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Lee SL, Ock SA, Balasubramanian S, Choe SY, Rho GJ. Development of cloned pig embryos by nuclear transfer following different activation treatments. Mol Reprod Dev. 2005;70:308–313. doi: 10.1002/mrd.20211. [DOI] [PubMed] [Google Scholar]
- 16.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 18.Lan GC, Chang ZL, Luo MJ, Jiang YL, Han D, Wu YG, Han ZB, Ma SF, Tan JH. Production of cloned goats by nuclear transfer of cumulus cells and long-term cultured fetal fibroblast cells into abattoir-derived oocytes. Mol Reprod Dev. 2006;73:834–840. doi: 10.1002/mrd.20443. [DOI] [PubMed] [Google Scholar]
- 19.Lee GS, Hyun SH, Kim HS, Kim DY, Lee SH, Lim JM, Lee ES, Kang SK, Lee BC, Hwang WS. Improvement of a porcine somatic cell nuclear transfer technique by optimizing donor cell and recipient oocyte preparations. Theriogenology. 2003;59:1949–1957. doi: 10.1016/s0093-691x(02)01294-3. [DOI] [PubMed] [Google Scholar]
- 20.Mannen H, Kojima T, Oyama K, Mukai F, Ishida T, Tsuji S. Effect of mitochondrial DNA variation on carcass traits of Japanese Black cattle. J Anim Sci. 1998;76:36–41. doi: 10.2527/1998.76136x. [DOI] [PubMed] [Google Scholar]
- 21.Mannen H, Morimoto M, Oyama K, Mukai F, Tsuji S. Identification of mitochondrial DNA substitutions related to meat quality in Japanese Black cattle. J Anim Sci. 2003;81:68–73. doi: 10.2527/2003.81168x. [DOI] [PubMed] [Google Scholar]
- 22.Nagao Y, Totsuka Y, Atomi Y, Kaneda H, Yonekawa H, Takahashi S, Imai H. Heterogeneous mitochondria DNA introduced by nuclear transfer influences the developmental ability of mouse embryos in vitro. Theriogenology. 1997;47:233. [Google Scholar]
- 23.Park Y, Hong J, Yong H, Lim J, Lee E. Effect of exogenous carbohydrates in a serum-free culture medium on the development of in vitro matured and fertilized porcine embryos. Zygote. 2005;13:269–275. doi: 10.1017/s0967199405003369. [DOI] [PubMed] [Google Scholar]
- 24.Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl. 1993;48:61–73. [PubMed] [Google Scholar]
- 25.Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KHS. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 26.Prather RS, Barnes FL, Sims MM, Robl JM, Eyestone WH, First NL. Nuclear transplantation in the bovine embryo: assessment of donor nuclei and recipient oocyte. Biol Reprod. 1987;37:859–866. doi: 10.1095/biolreprod37.4.859. [DOI] [PubMed] [Google Scholar]
- 27.Ramsoondar JJ, Machaty Z, Costa C, Williams BL, Fodor WL, Bondioli KR. Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod. 2003;69:437–445. doi: 10.1095/biolreprod.102.014647. [DOI] [PubMed] [Google Scholar]
- 28.Schutz MM, Freeman AE, Beitz DC, Mayfield JE. The importance of maternal lineage on milk yield traits of dairy cattle. J Dairy Sci. 1992;75:1331–1341. doi: 10.3168/jds.S0022-0302(92)77884-9. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz AW, Whittingham DG, Snowden R. Alterations in the cell cycle of mouse cumulus granulosa cells during expansion and mucification in vivo and in vitro. Reprod Fertil Dev. 1996;8:935–943. doi: 10.1071/rd9960935. [DOI] [PubMed] [Google Scholar]
- 30.Song K, Lee E. Modification of maturation condition improves oocyte maturation and in vitro development of somatic cell nuclear transfer pig embryos. J Vet Sci. 2007;8:81–87. doi: 10.4142/jvs.2007.8.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutarno, Cummins JM, Greeff J, Lymbery AJ. Mitochondrial DNA polymorphisms and fertility in beef cattle. Theriogenology. 2002;57:1603–1610. doi: 10.1016/s0093-691x(02)00664-7. [DOI] [PubMed] [Google Scholar]
- 32.Takahagi Y, Fujimura T, Miyagawa S, Nagashima H, Shigehisa T, Shirakura R, Murakami H. Production of alpha 1,3-galactosyltransferase gene knockout pigs expressing both human decay-accelerating factor and N-acetylglucosaminyltransferase III. Mol Reprod Dev. 2005;71:331–338. doi: 10.1002/mrd.20305. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Takahashi S, Onishi A, Goto Y, Miyazawa A, Imai H. Dominant distribution of mitochondrial DNA from recipient oocytes in bovine embryos and offspring after nuclear transfer. J Reprod Fertil. 1999;116:253–259. doi: 10.1530/jrf.0.1160253. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Akagi S, Kaneyama K, Kojima T, Takahashi S, Imai H, Yamanaka M, Onishi A, Hanada H. Proliferation of donor mitochondrial DNA in nuclear transfer calves (Bos taurus) derived from cumulus cells. Mol Reprod Dev. 2003;64:429–437. doi: 10.1002/mrd.10279. [DOI] [PubMed] [Google Scholar]
- 35.Wakayama S, Ohta H, Kishigami S, Thuan NV, Hikichi T, Mizutani E, Miyake M, Wakayama T. Establishment of male and female nuclear transfer embryonic stem cell lines from different mouse strains and tissues. Biol Reprod. 2005;72:932–936. doi: 10.1095/biolreprod.104.035105. [DOI] [PubMed] [Google Scholar]
- 36.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 37.Walker SC, Shin T, Zaunbrecher GM, Romano JE, Johnson GA, Bazer FW, Piedrahita JA. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002;4:105–112. doi: 10.1089/153623002320253283. [DOI] [PubMed] [Google Scholar]
- 38.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 39.Yang XY, Li H, Ma QW, Yan JB, Zhao JG, Li HW, Shen HQ, Liu HF, Huang Y, Huang SZ, Zeng YT, Zeng F. Improved efficiency of bovine cloning by autologous somatic cell nuclear transfer. Reproduction. 2006;132:733–739. doi: 10.1530/rep.1.01118. [DOI] [PubMed] [Google Scholar]