Abstract
Aims
Our aim was to determine the contribution of the three angiotensin (Ang) II receptor subtypes (AT1a, AT1b, AT2) to coronary responsiveness, cardiac histopathology, and tissue Ang II levels using mice deficient for one, two, or all three Ang II receptors.
Methods and results
Hearts of knockout mice and their wild-type controls were collected for histochemistry or perfused according to Langendorff, and kidneys were removed to measure tissue Ang II. Ang II dose-dependently decreased coronary flow (CF) and left ventricular systolic pressure (LVSP), and these effects were absent in all genotypes deficient for AT1a, independently of AT1b and AT2. The deletion of Ang II receptors had an effect neither on the morphology of medium-sized vessels in the heart nor on the development of fibrosis. However, the lack of both AT1 subtypes was associated with atrophic changes in the myocardium, a reduced CF and a reduced LVSP. AT1a deletion alone, independently of the presence or absence of AT1b and/or AT2, reduced renal Ang II by 50% despite a five-fold rise of plasma Ang II. AT1b deletion, on top of AT1a deletion (but not alone), further decreased tissue Ang II, while increasing plasma Ang II. In mice deficient for all three Ang II receptors, renal Ang II was located only extracellularly.
Conclusion
The lack of both AT1 subtypes led to a baseline reduction of CF and LVSP, and the effects of Ang II on CF and LVSP were found to be exclusively mediated via AT1a. The lack of AT1a or AT1b does not influence the development or maintenance of normal cardiac morphology, whereas deficiency for both receptors led to atrophic changes in the heart. Renal Ang II levels largely depend on AT1 binding of extracellularly generated Ang II, and in the absence of all three Ang II receptors, renal Ang II is only located extracellularly.
Keywords: Angiotensin II, G protein-coupled receptors, Genetically modified animals
1. Introduction
The biological actions of angiotensin (Ang) II are mediated via Ang II type 1 (AT1) and Ang II type 2 (AT2) receptors. In rodents, two subtypes of AT1 have been identified: AT1a and AT1b1 which share 94% sequence homology2 and have similar ligand binding affinities and signal transduction properties. To date, there are no pharmacological antagonists that discriminate between AT1a and AT1b, and the function of AT2 is still only partly understood.
To get more insight in the function of the three Ang II receptors, we generated mice which are deficient for either one, two, or all three Ang II receptors.3 AT2 deletion increased baseline mean arterial pressure (MAP), whereas mice lacking AT1a were hypotensive and displayed a reduced heart weight/body weight (HW/BW) ratio. Blood pressure and HW/BW ratio dropped further in mice lacking both AT1 subtypes. AT1a deletion impaired the in vivo pressor response to Ang II bolus injections, whereas deficiency for AT1b and/or AT2 had no impact. However, the additional lack of AT1b in AT1a-deficient mice further impaired the vasoconstrictive capacity of Ang II. Ang II failed to alter MAP in mice lacking all three Ang II receptors (triple knockouts), indicating that no other receptors than the AT1a, AT1b, and/or AT2 mediate the pressor effects of Ang II.
In the present study, we set out to quantify, in the above-described knockout mice, the contribution of the three Ang II receptors to cardiac haemodynamics in vitro, using the Langendorff heart preparation. Given the reduced heart size in AT1a knockout mice, we also quantified brain natriuretic peptide (BNP) expression and studied morphological changes such as fibrosis and remodelling. Finally, we measured tissue Ang II levels in these mice to verify earlier findings by us and others showing that tissue Ang II is AT1-bound.4–8
2. Methods
2.1. Animals
Males (aged ∼5 months) of all eight possible genotypes (AT1a/AT1b/AT2: +/+//+/+//+/+, −/−//+/+//+/+, +/+//−/−//+/+, +/+//+/+//−/−, +/+//−/−//−/−, −/−//+/+//−/−, −/−//−/−//+/+, −/−//−/−//−/−) of the recently described Ang II receptor deficient mice3 were used in this study. In addition, age-matched animals lacking angiotensinogen (Agt)9 were used for histological comparison. Experiments on adult mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), the regulations of the Animal Care Committee of the Erasmus MC, and the Federal Law on the Use of Experimental Animals in Germany and were approved by the local authorities (Landesamt für Gesundheit und Soziales des Landes Berlin).
2.2. Langendorff heart preparation
Mice were killed by cervical dislocation. The heart was rapidly excised and placed in ice-cold modified Krebs-Henseleit (KH) buffer (composition in mmol/L: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2,CaCl2 1.2, d-glucose 11, NaHCO3 25, pyruvic acid 2), gassed with 95% O2 and 5% CO2.10–12 The aorta was immediately cannulated with a 19G needle (with a small circumferential grove close to the blunt tip) and perfused with gassed KH buffer according to Langendorff at a constant perfusion pressure of 80 mmHg.13 Two needle electrodes were placed at the right atrium and the hearts were paced at ∼600 b.p.m. (10 Hz, 4 ms duration, 4 V) using a Grass stimulator (Grass Instruments Co., Quincy, MA, USA). Left ventricular systolic pressure (LVSP) was measured with a water-filled balloon (made of domestic food wrap) connected to a disposable pressure transducer (Braun, Melsungen, Germany). The left atrium was removed, and the balloon was inserted into the left ventricle.13,14 Left ventricular end-diastolic pressure was set at 3–5 mmHg by adjusting the balloon volume. Coronary flow (CF) was measured with a flow probe (Transonic systems, Ithaca, NY, USA). After a stabilization period of 10–15 min, baseline values of CF and LVSP were obtained. Next, bolus injections (100 µL) of modified KH buffer were applied three times to determine injection-induced changes in CF and LVSP. Subsequently, bolus injections (100 µL) of Ang II (concentration range 0.1 nmol/L to 0.1 mmol/L) were applied.
2.3. RNA isolation and RNase protection assay
Total RNA was isolated from heart using the TRIzol reagent (Invitrogen GmbH, Karlsruhe, Germany) as described earlier.15 Mouse BNP mRNA expression was identified by RPA using the Ambion RPA II kit (Ambion Europe Ltd, Huntingdon, UK) as described elsewhere.15 In brief, SP6-RNA polymerase transcribed a radioactive antisense probe complementary to BNP mRNA (290 bp).16 RNA complementary to 127 nucleotides of rL32 mRNA was used as a positive control.17,18 Thirty microgram of each RNA sample was hybridized with of the radiolabelled antisense probes in the same reaction. The hybridized fragments protected from RNase A + T1 digestion were separated by electrophoresis and analyzed using a FUJIX BAS 2000 Phospho-Imager system (Raytest GmbH, Straubenhardt, Germany). Quantitative analyses were performed by measuring the intensity of the target bands normalized by the intensity of rL32.
2.4. Histology
Hearts were isolated and fixed in 4% buffered formalin and processed according to standard protocols. In brief, the hearts were embedded in paraffin and sectioned at 2 µm. Slides were deparaffinized and hydrated. Sections of hearts were stained for histology with haematoxylin-eosin (HE) solution, periodic acid-Schiff (PAS) solution, or van Gieson solution as described previously.18,19 Tissue sections (n ≥ 4) were evaluated in four planes for morphological changes and connective tissue production.
2.5. Ang II levels in kidney
Kidneys were removed from the abdomen, rapidly frozen in liquid nitrogen, and stored at −80°C until processing. Ang II was determined by radioimmunoassay following SepPak extraction and high-performance liquid chromatography separation as described before.20
2.6. Data analysis
CF and LVSP data were recorded and digitalized using WinDaq waveform recording software (Dataq Instruments, Akron, OH, USA). After a manual selection of the desired signals pre- and post-injection, data were analysed using Matlab (Mathworks, Inc., Natick, MA, USA). Six consecutive beats were selected for the determination of CF and LVSP. Results are represented as mean ± SEM or geometric mean and range. Concentration–response curves were analysed as described,21 using Graph Pad Prism 3.01 (Graph Pad Software, Inc., San Diego, CA, USA), to obtain pEC50 (−10log EC50) values. The pEC50 values refer to the agonist concentration in the injection fluid and do not reflect the actual concentrations seen by the receptor. Statistical analysis was performed using the SPSS 11.0 statistical package (SPSS, Inc., Chicago, IL, USA). Multiple regression analysis was conducted to determine the contribution of the three receptors as independent variables and, in case one of the receptors did not exert an independent effect, their interaction. P < 0.05 was considered significant.
3. Results
3.1. Langendorff heart studies
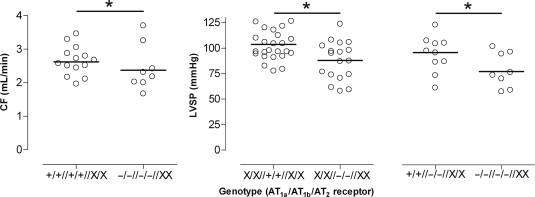
Table 1 shows the baseline CF and LVSP values in the eight genotypes. Regression analysis revealed that the deletion of individual Ang II receptor subtypes did not affect baseline CF. However, combined deletion of AT1a and AT1b, independently of the presence or absence of AT2, lowered baseline CF (P = 0.020; Figure 1, left panel). Although baseline LVSP was unaffected by individual AT1a or AT2 deletion, it was lower in hearts lacking AT1b, independently of the presence of AT1a or AT2 (P = 0.005; Figure 1, middle panel). In addition, AT1a deletion on top of AT1b deletion further decreased baseline LVSP (P = 0.003; Figure 1, right panel).
Table 1.
Baseline coronary flow (CF) and left ventricular pressure (LVSP) according to genotype
| Genotype | +/+//+/+//+/+ | +/+//−/−//+/+ | +/+//+/+//−/− | +/+//−/−//−/− | −/−//+/+//+/+ | −/−//+/+//−/− | −/−//−/−//+/+ | −/−//−/−//−/− |
| n | 8 | 4 | 6 | 7 | 5 | 7 | 4 | 4 |
| CF (mL/min) | 2.5 ± 0.2 | 3.0 ± 0.3 | 2.8 ± 0.1 | 3.1 ± 0.1 | 2.7 ± 0.1 | 3.0 ± 0.2 | 2.4 ± 0.3 | 2.5 ± 0.3 |
| LVSP (mmHg) | 98.5 ± 3.7 | 98.2 ± 6.3 | 96.5 ± 3.5 | 98.9 ± 8.3 | 114.3 ± 6.8 | 112.6 ± 7.1 | 86.0 ± 6.2 | 72.3 ± 6.7 |
Data are mean ± SEM.
Figure 1.
Baseline coronary flow (CF) following deletion of AT1a and AT1b, independently of the presence or absence of AT2 (left panel), baseline left ventricular systolic pressure (LVSP) following deletion of AT1b, independently of the presence of AT1a or AT2 (middle panel), and baseline LVSP following deletion of AT1a on top of AT1b, independently of the presence of AT2 (right panel). *P < 0.05; data (n = 8–26) are represented as scatter dot plot. The horizontal bar represents the mean. The use of ‘X’ implies that for that specific receptor, both +/+ and −/− animals were included, i.e. that the comparison occurred independently of the presence or absence of that receptor.
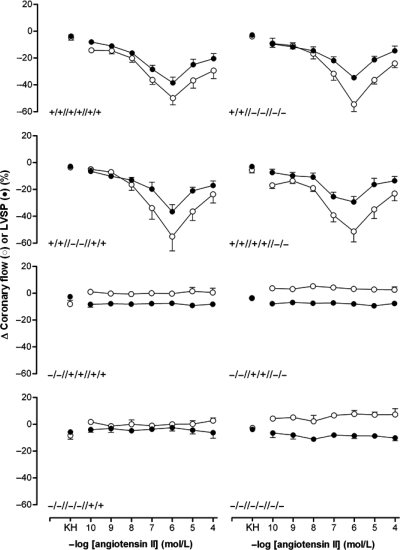
In hearts of wild-type mice, bolus injections of Ang II dose-dependently decreased CF and LVSP (Figure 2) by maximally 56 ± 5% and 39 ± 4%, respectively (pEC50 7.51 ± 0.17 and 7.35 ± 0.13). Ang II concentrations >1 µmol/L did not result in effects that were larger than those observed at 1 µmol/L, in agreement with the concept of receptor desensitization.22–24
Figure 2.
Changes in coronary flow (CF; open symbols) and left ventricular systolic pressure (LVSP; closed symbols) after a bolus injection of Ang II in the Langendorff heart according to genotype. Data (mean + SEM of 4–8 experiments) represent percentage change from baseline. KH, bolus injection of Krebs-Henseleit buffer. The x-axis displays the Ang II concentration in the injection fluid.
Bolus injections of Ang II exerted no effects in hearts of mice deficient for AT1a receptors. Interestingly, no differences were found between hearts of mice lacking AT1a alone and hearts of mice deficient for AT1b and/or AT2 on top of AT1a deficiency. The effects of Ang II bolus injections in hearts of mice lacking AT1b and/or AT2 were identical to those in wild-type mice. This suggests that the cardiac effects of Ang II are exclusively AT1a mediated.
3.2. Cardiac histopathological examination and expression of BNP
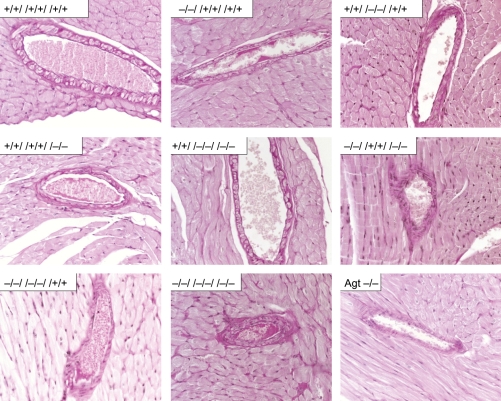
We investigated the impact of the Ang II receptors on morphological changes in medium-sized vessels in the heart using PAS-stained sections. Deficiency for one, two, or all three Ang II receptor subtypes did not influence the morphology of medium-sized arteries in the heart (Figure 3). To investigate the impact of lack in Ang II receptor expression on fibroblast quantities, we determined the basal production of connective tissue by van Gieson staining. In none of the eight possible receptor combinations, a change in basal connective tissue production was detected (Figure 4).
Figure 3.
Medium-sized arteries in hearts of wild-type (+/+//+/+//+/+), AT1a- (−/−//+/+//+/+), AT1b- (+/+//−/−//+/+), and AT2- (+/+//+/+//−/−) single-knockout mice, mice exclusively expressing AT1a (+/+//−/−//−/−), AT1b (−/−//+/+//−/−), or AT2 (−/−//−/−//+/+), triple-knockout mice (−/−//−/−//−/−), and angiotensinogen-deficient (Agt −/−) mice do not show genotype-related morphological alterations (PAS-stained; magnification: 400-fold; representative sections for all groups from n ≥ 4 sections).
Figure 4.
Sections of hearts from wild-type (+/+//+/+//+/+), AT1a- (−/−//+/+//+/+), AT1b- (+/+//−/−//+/+), and AT2- (+/+//+/+//−/−) receptor single-knockout mice, mice exclusively expressing AT1a (+/+//−/−//−/−), AT1b (−/−//+/+//−/−), or AT2 (−/−//−/−//+/+) receptors, triple-knockout mice (−/−//−/−//−/−), and angiotensinogen-deficient (Agt −/−) mice do not show changes in basal connective tissue production (van Gieson-stained; magnification: 400-fold; representative sections for all groups from n ≥ 4 sections).
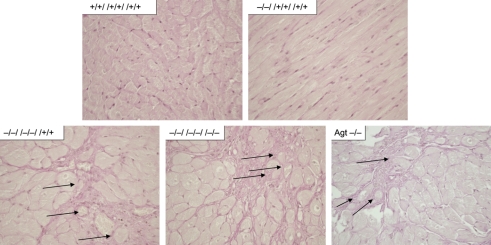
As we have reported earlier, all genotypes lacking the AT1a receptor showed a significantly reduced HW/BW ratio.3 Interestingly, there was oligo- to multi-focal atrophy of cardiac muscle evident in animals lacking both AT1 subtypes (−/−//−/−//+/+ and −/−//−/−//−/−) and Agt-deficient mice, whereas animals deficient for none or only one of the AT1 subtypes showed no structural changes within the heart (Figure 5). Atrophic muscle fibres revealed a more pronounced, dense, reticular network, with partial collapse of the reticulin fibres. Atrophic muscle fibres had an angular outline, and widened partially enlarged interstitial spaces.
Figure 5.
Oligo- to multi-focal atrophy of cardiac muscle is evident in animals lacking both AT1 subtypes (−/−//−/−//+/+ and −/−//−/−//−/−) and angiotensinogen-deficient (Agt −/−) mice, whereas animals lacking none or only one AT1-subtype (exemplarily shown +/+//+/+//+/+ and −/−//+/+//+/+). Arrows indicate dense reticular network, partially collapsed, with individualized muscle fibres, and occasional angular outline, as well as loss of muscle fibres (HE-stained; magnification: 400-fold representative sections for all groups from n ≥ 4 sections).
BNP is a marker for heart failure. Thus, we tested whether the deficiency of one of the receptors and/or the structural changes have an impact on BNP expression in all eight groups. None of the eight genotypes showed increased BNP expression (data not shown).
3.3. Ang II levels in plasma and kidney
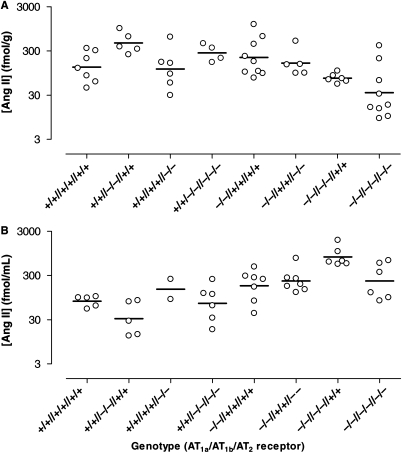
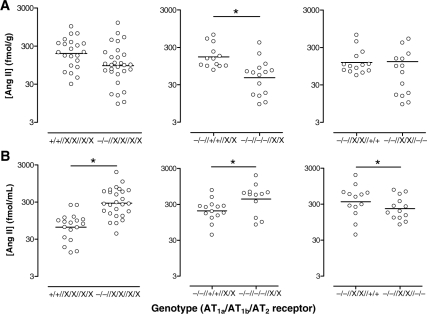
As the hearts had already been used for ex vivo haemodynamic studies, these measurements were performed in kidneys. Kidneys have much higher Ang II levels than the heart,25 and changes in renal Ang II content exactly parallel changes in the Ang II content of other tissues (including the heart).25–27 In other words, the renal Ang II levels can be used as a reflection of what happens at the tissue level in general. Renal Ang II levels were compared with the plasma Ang II levels (in the same animals) reported previously by our group.3 For the sake of clarity, Figure 6 shows the geometric mean and range of the Ang II levels in both kidney (A) and plasma (B). Regression analysis revealed that AT1a deletion alone, independently of the presence or absence of the AT1b and/or AT2, diminished renal Ang II by ≈50% (P = 0.002; Figure 7A, left panel) and increased plasma Ang II ≈5-fold (P < 0.0001; Figure 7B, left panel).
Figure 6.
Ang II levels in kidney (A) and blood plasma (B) of wild-type (+/+//+/+//+/+), AT1a- (−/−//+/+//+/+), AT1b- (+/+//−/−//+/+), and AT2-single-knockout mice, mice exclusively expressing AT1a (+/+//−/−//−/−), AT1b (−/−//+/+//−/−), or AT2 (−/−//−/−//+/+), and triple-knockout mice (−/−//−/−//−/−). Data (n = 2–9) are represented as scatter dot plot. The horizontal bar represents the geometric mean. Plasma levels were redrawn from Gembardt et al.3
Figure 7.
Ang II levels in kidney (A) and blood plasma (B) following deletion of AT1a, independently of the presence or absence of AT1b or AT2 (left panels), deletion of AT1b on top of AT1a deletion, independently of the presence of AT2 (middle panels), or deletion of AT2 on top of AT1a deletion, independently of the presence of AT1b (right panels). *P < 0.05; Data (n = 12–29) are represented as scatter dot plot. The horizontal bar represents the geometric mean. The use of ‘X’ implies that for that specific receptor, both +/+ and −/− animals were included, i.e. that the comparison occurred independently of the presence or absence of that receptor. Plasma levels were obtained from Gembardt et al.3
AT1b deletion alone did not affect plasma and renal Ang II (Figure 6). However, on top of AT1a deletion, independently of the presence or absence of the AT2, AT1b deletion further diminished renal Ang II by a factor of 4 (P < 0.0001; Figure 7A, middle panel) and doubled plasma Ang II (P = 0.003; Figure 7B, middle panel).
AT2 deletion alone did not affect plasma and renal Ang II. However, on top of AT1a deletion, independently of the presence or absence of the AT1b, AT2 deletion reduced plasma (but not renal) Ang II (P = 0.018; Figure 7A and B, right panels).
We have previously shown that tissue Ang II is protected against metabolism by binding to AT1 and subsequent internalization.7 To verify this concept, kidneys of two mice lacking all three Ang II receptors were kept at room temperature for 30 min before freezing them in liquid nitrogen. Ang II levels in these kidneys were 2 and 8 fmol/g, whereas the (geometric) mean Ang II level in the kidneys of AT1a/AT1b/AT2 triple knockout mice that had been frozen immediately was 35 fmol/g.
4. Discussion
We have previously shown that AT1a deletion impaired the in vivo pressor response to Ang II bolus injections, whereas deficiency for AT1b and/or AT2 had impact only on top of AT1a deletion.3 The importance of AT1a is also apparent in our current studies using the mouse heart Langendorff model. Deletion of one, two, or all three Ang II receptors did not affect baseline CF or LVSP in comparison to wild-type. The dose-dependent decrease of both CF and LVSP in response to Ang II was similar in all genotypes expressing AT1a independently of the presence or absence of AT1b and/or AT2. These effects were completely abolished in all genotypes deficient for the AT1a. Thus, the AT1a exclusively mediates the Ang II-induced negative inotropy and vasoconstriction in the mouse heart, and AT1b does not exert such effects, possibly because it is not expressed in the heart in sufficient amounts. Whether the negative inotropic and vasoconstrictor effects of Ang II occurred independently of each other could not be determined in the present study. Negative inotropic effects of Ang II in the mouse heart have been described before,28,29 and contrast with the positive inotropic effects of Ang II in human trabeculae.30 Yet, it is also possible that the decrease in LVSP simply is the consequence of the Ang II-induced decrease in CF, i.e. that Ang II has no direct effect on the mouse cardiomyocyte.
Unexpectedly, absence of the AT2 did not enhance the AT1-mediated effects in the heart. This contrasts with other studies in the heart where AT2 blockade potentiated the effects of Ang II.12,31–33 On the basis of these enhanced effects, it has been concluded that AT2 counteract the AT1-mediated effects. An explanation for the absence of such enhanced cardiac effects in the AT2 knockout mice in the present study could be that chronic absence (unlike acute blockade) of AT2 affects AT1a signalling modifying pathways. Alternatively, life-long absence of AT2 may have resulted in structural changes. However, histological examination did not reveal such alterations, and baseline CF and LVSP were identical in all genotypes. Moreover, the vascular and cardiac effects of other agonists (phenylephrine and endothelin-1) in these mice are unaltered.3 Thus, the discrepancy between pharmacological blockade and the AT2 knockout related to specific parameters could be also a result of ectopic effects of the AT2 blocker as significant ectopic effects have been described for a variety of AT1 blockers.34,35 The AT2 blocker used in these studies, PD123319, has been originally described as an AT2 agonist.36 Taken this in consideration, the genetically receptor-deficient mice are a superior experimental model compared with the pharmacological blockade of AT2.
Furthermore, we also did not observe direct AT2-mediated effects in AT1 knockout mice. This is in line with our previous inability to observe cardiac AT2-mediated effects during AT1 blockade in wild-type mice.12 Apparently therefore, AT2-mediated effects in the mouse heart can only be demonstrated during simultaneous AT1 stimulation, i.e. they occur in conjunction with AT1a activation. However, our findings also show that this concept does not account for all tissues or Ang II-mediated effects. Our data on plasma Ang II levels implicate AT2-mediated effects independent of AT1, because AT2 deficiency led to lower Ang II concentrations in plasma of AT1-deficient animals.
Recently, we reported reduced heart weights for mice lacking AT1a in all combinations, in AT2-deficient mice, and mice lacking Agt.3 In this study, we found a similar, genotype-specific atrophy in the myocardium of AT1a/AT1b double-, AT1a/AT1b/AT2 triple-, and Agt-deficient mice. Interestingly, mice only deficient for AT1a and not for AT1b (−/−//+/+//+/+ and −/−//+/+//−/−) did not show the described atrophic changes. Despite the low AT1b expression in the heart, our data suggest a rescue of AT1a function by AT1b in the maintenance of normal cardiac morphology, whereas AT1b cannot compensate for the lack of growth stimulation in the heart due to the absence of AT1a. The reduction in baseline CF and LVSP in mice deficient for both AT1a and AT1b may be the consequence of these atrophic changes. Thus, atrophy alone cannot explain the reduced heart weight in the five genotypes where lower organ weight has been observed, because mice deficient for AT1a and/or AT2 (−/−//+/+//+/+, +/+//+/+//−/−, and −/−//+/+//−/−) do not show the atrophic changes seen in mice lacking both AT1 subtypes. Therefore, further experiments have to investigate both phenomena; the reduced heart weight and the atrophy in the heart of mice with lacking AT1a/AT1b stimulation.
Finally, the present study yielded important information on the regulation of tissue Ang II levels. Normally, circulating Ang II is sequestered by multiple tissues via AT1-mediated endocytosis.7 Yet, the majority of tissue Ang II is formed at tissue sites.37,38 Studies with 125I-labelled Ang I have revealed that this production occurs extracellularly and is followed by rapid AT1-mediated endocytosis.4–8 Therefore, despite its extracellular synthesis, e.g. in the interstitial fluid compartment or on the cell surface, the majority of tissue Ang II is located intracellularly.8 Its levels are particularly high in endosomes.39 The intracellular accumulation of Ang II may either facilitate its activation of nuclear receptors40,41 and/or results in its destruction.7 The half-life of AT1-bound tissue Ang II is 20–30 times longer than that of extracellular Ang II,7 thereby explaining, at least in part, why tissue Ang II levels far exceed plasma Ang II levels. Clearly, receptor-binding protects Ang II against rapid metabolism by angiotensinases.
As AT2 does not internalize after ligand binding,42,43 the intracellular accumulation of Ang II exclusively depends on AT1. The current study now shows that both AT1 subtypes contribute to this accumulation and that the contribution of the AT1a exceeds that of the AT1b. Making use of single AT1a knockout mice, a similar conclusion was already drawn by Li and Zhuo.44
Not having AT1a caused a five-fold rise in plasma Ang II. Despite this rise in extracellular Ang II, the renal tissue level of Ang II decreased by 50%. Not having AT1b does not have an effect by itself on either plasma or tissue Ang II, thereby demonstrating that AT1a can make up for the consequences of the deletion of AT1b. However, missing AT1b on top of AT1a deletion further increased plasma Ang II (by a factor of 2) and lowered tissue Ang II by a factor of 4. Therefore, in mice not expressing AT1, the tissue/plasma Ang II concentration ratio is far below that in wild-type mice. Additional AT2 deletion did not alter renal Ang II, in agreement with the above concept of non-internalizing AT2.
Renal Ang II levels in the absence of its three receptors are not zero. To verify whether the measured Ang II is putatively extracellular in AT1a/AT1b/AT2-triple knockout mice, we determined the Ang II content of kidneys that were kept at room temperature for 30 min. Normally, over this time period, the level of AT1-bound Ang II does not change,7 whereas the level of extracellular Ang II rapidly decreases.6 Indeed, tissue Ang II levels in mice deficient for all three Ang II receptors following this procedure decreased by more than 70%, confirming the rapid metabolism of non-receptor bound, extracellular Ang II by angiotensinases. Furthermore, given that the extracellular fluid content of renal tissue consists of blood plasma and interstitial fluid (accounting for ≈5% and ≈10% of tissue weight, respectively), and assuming that the renal interstitial Ang II levels resemble those in blood (≈320 fmol/mL in triple knockout mice), it can be estimated that the renal tissue Ang II levels in triple knockout mice should be in the order of ≈50 fmol/g, if Ang II is limited to the extracellular fluid compartment. This is within the range of the tissue levels that were found (35 fmol/g), thereby again confirming that tissue Ang II in triple knockout mice is extracellular. Consequently, the experimental data in our triple knockout mice clearly argue against the formerly expressed hypothesis of intracellular Ang II generation.45
On the basis of our new findings using animals with different combinations of Ang II receptor deficiencies, we can draw the following conclusions: (i) the lack of both AT1-subtypes led to reduced baseline CF and LVSP, (ii) the coronary constrictor and negative inotropic effects of Ang II are exclusively mediated via AT1a, (iii) deficiency of only one AT1 subtype has no effect on development or maintenance of normal cardiac morphology, whereas the lack of both receptors led to atrophic changes in the heart, (iv) tissue Ang II levels largely depend on AT1-mediated endocytosis of extracellular Ang II; (v) in the absence of the three Ang II receptors, tissue Ang II is only located extracellularly.
Conflict of interest: none declared.
Funding
F.G. was paid by a grant from the ‘National Institute of Health’ (NIH; 5R01HL082722-02).
References
- 1.Elton TS, Stephan CC, Taylor GR, Kimball MG, Martin MM, Durand JN, et al. Isolation of two distinct type I angiotensin II receptor genes. Biochem Biophys Res Commun. 1992;184:1067–1073. doi: 10.1016/0006-291x(92)90700-u. [DOI] [PubMed] [Google Scholar]
- 2.Iwai N, Inagami T, Ohmichi N, Nakamura Y, Saeki Y, Kinoshita M. Differential regulation of rat AT1a and AT1b receptor mRNA. Biochem Biophys Res Commun. 1992;188:298–303. doi: 10.1016/0006-291x(92)92384-a. [DOI] [PubMed] [Google Scholar]
- 3.Gembardt F, Heringer-Walther S, van Esch JHM, Sterner-Kock Dvm A, van Veghel R, Le TH, et al. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. FASEB J. 2008;22:3068–3077. doi: 10.1096/fj.08-108316. [DOI] [PubMed] [Google Scholar]
- 4.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–662. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 5.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 6.Schuijt MP, van Kats JP, de Zeeuw S, Duncker DJ, Verdouw PD, Schalekamp MADH, et al. Cardiac interstitial fluid levels of angiotensin I and II in the pig. J Hypertens. 1999;17:1885–1891. doi: 10.1097/00004872-199917121-00017. [DOI] [PubMed] [Google Scholar]
- 7.van Kats JP, de Lannoy LM, Danser AHJ, van Meegen JR, Verdouw PD, Schalekamp MADH. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30:42–49. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 8.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MADH, Danser AHJ. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens. 2001;19:583–589. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 9.Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 10.Gustafson LA, Van Beek JH. Measurement of the activation time of oxidative phosphorylation in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H3118–H3123. doi: 10.1152/ajpheart.2000.279.6.H3118. [DOI] [PubMed] [Google Scholar]
- 11.Wang QD, Tokuno S, Valen G, Sjoquist PO, Thoren P. Cyclic fluctuations in the cardiac performance of the isolated Langendorff-perfused mouse heart: pyruvate abolishes the fluctuations and has an anti-ischaemic effect. Acta Physiol Scand. 2002;175:279–287. doi: 10.1046/j.1365-201X.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- 12.van Esch JHM, Schuijt MP, Sayed J, Choudhry Y, Walther T, Danser AHJ. AT(2) receptor-mediated vasodilation in the mouse heart depends on AT(1A) receptor activation. Br J Pharmacol. 2006;148:452–458. doi: 10.1038/sj.bjp.0706762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol. 2003;30:867–878. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 14.Curtis MJ, Macleod BA, Tabrizchi R, Walker MJ. An improved perfusion apparatus for small animal hearts. J Pharmacol Methods. 1986;15:87–94. doi: 10.1016/0160-5402(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 15.Stepan H, Walther D, Faber R, Walther T. Detection of C-type natriuretic peptide in fetal circulation. J Perinat Med. 2000;28:118–121. doi: 10.1515/JPM.2000.015. [DOI] [PubMed] [Google Scholar]
- 16.Walther T, Klostermann K, Heringer-Walther S, Schultheiss HP, Tschope C, Stepan H. Fibrosis rather than blood pressure determines cardiac BNP expression in mice. Regul Pept. 2003;116:95–100. doi: 10.1016/j.regpep.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, et al. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–344. doi: 10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
- 19.Padi SS, Chopra K. Selective angiotensin II type 1 receptor blockade ameliorates cyclosporine nephrotoxicity. Pharmacol Res. 2002;45:413–420. doi: 10.1006/phrs.2002.0959. [DOI] [PubMed] [Google Scholar]
- 20.Danser AHJ, van Kats JP, Admiraal PJJ, Derkx FHM, Lamers JMJ, Verdouw PD, et al. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- 21.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 22.Abdellatif MM, Neubauer CF, Lederer WJ, Rogers TB. Angiotensin-induced desensitization of the phosphoinositide pathway in cardiac cells occurs at the level of the receptor. Circ Res. 1991;69:800–809. doi: 10.1161/01.res.69.3.800. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias AG, Suarez C, Feierstein C, Diaz-Torga G, Becu-Villalobos D. Desensitization of angiotensin II: effect on [Ca2+]i, inositol triphosphate, and prolactin in pituitary cells. Am J Physiol Endocrinol Metab. 2001;280:E462–E470. doi: 10.1152/ajpendo.2001.280.3.E462. [DOI] [PubMed] [Google Scholar]
- 24.Reagan LP, Ye X, Maretzski CH, Fluharty SJ. Down-regulation of angiotensin II receptor subtypes and desensitization of cyclic GMP production in neuroblastoma N1E-115 cells. J Neurochem. 1993;60:24–31. doi: 10.1111/j.1471-4159.1993.tb05818.x. [DOI] [PubMed] [Google Scholar]
- 25.van Kats JP, Schalekamp MADH, Verdouw PD, Duncker DJ, Danser AHJ. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 26.van Kats JP, Duncker DJ, Haitsma DB, Schuijt MP, Niebuur R, Stubenitsky R, et al. Angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade prevent cardiac remodeling in pigs after myocardial infarction: role of tissue angiotensin II. Circulation. 2000;102:1556–1563. doi: 10.1161/01.cir.102.13.1556. [DOI] [PubMed] [Google Scholar]
- 27.van Kats JP, Chai W, Duncker DJ, Schalekamp MADH, Danser AHJ. Adrenal angiotensin: origin and site of generation. Am J Hypertens. 2005;18:1104–1110. doi: 10.1016/j.amjhyper.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai K, Norota I, Tanaka H, Kubota I, Tomoike H, Endo M. Negative inotropic effects of angiotensin II, endothelin-1 and phenylephrine in indo-1 loaded adult mouse ventricular myocytes. Life Sci. 2002;70:1173–1184. doi: 10.1016/s0024-3205(01)01497-7. [DOI] [PubMed] [Google Scholar]
- 29.Sekine T, Kusano H, Nishimaru K, Tanaka Y, Tanaka H, Shigenobu K. Developmental conversion of inotropism by endothelin I and angiotensin II from positive to negative in mice. Eur J Pharmacol. 1999;374:411–415. doi: 10.1016/s0014-2999(99)00373-8. [DOI] [PubMed] [Google Scholar]
- 30.Chai W, Garrelds IM, de Vries R, Batenburg WW, van Kats JP, Danser AHJ. Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension. 2005;46:701–706. doi: 10.1161/01.HYP.0000182661.98259.4f. [DOI] [PubMed] [Google Scholar]
- 31.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 32.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 33.van Esch JHM, Oosterveer CR, Batenburg WW, van Veghel R, Danser AHJ. Effects of angiotensin II and its metabolites in the rat coronary vascular bed: is angiotensin III the preferred ligand of the angiotensin AT(2) receptor? Eur J Pharmacol. 2008;588:286–293. doi: 10.1016/j.ejphar.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 34.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 35.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 36.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 37.Danser AHJ. Local renin–angiotensin systems: the unanswered questions. Int J Biochem Cell Biol. 2003;35:759–768. doi: 10.1016/s1357-2725(02)00178-4. [DOI] [PubMed] [Google Scholar]
- 38.Schuijt MP, Danser AHJ. Cardiac angiotensin II: an intracrine hormone? Am J Hypertens. 2002;15:1109–1116. doi: 10.1016/s0895-7061(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 40.Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol. 2006;40:696–707. doi: 10.1016/j.yjmcc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol. 2006;291:C995–C1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 42.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 43.Ouali R, Berthelon MC, Begeot M, Saez JM. Angiotensin II receptor subtypes AT1 and AT2 are down-regulated by angiotensin II through AT1 receptor by different mechanisms. Endocrinology. 1997;138:725–733. doi: 10.1210/endo.138.2.4952. [DOI] [PubMed] [Google Scholar]
- 44.Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]Val5-ANG II in the kidneys and adrenals of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2008;294:F293–F302. [Google Scholar]
- 45.Kumar R, Singh VP, Baker KM. The intracellular renin–angiotensin system: a new paradigm. Trends Endocrinol Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]