Abstract
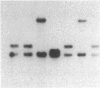
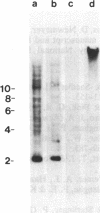
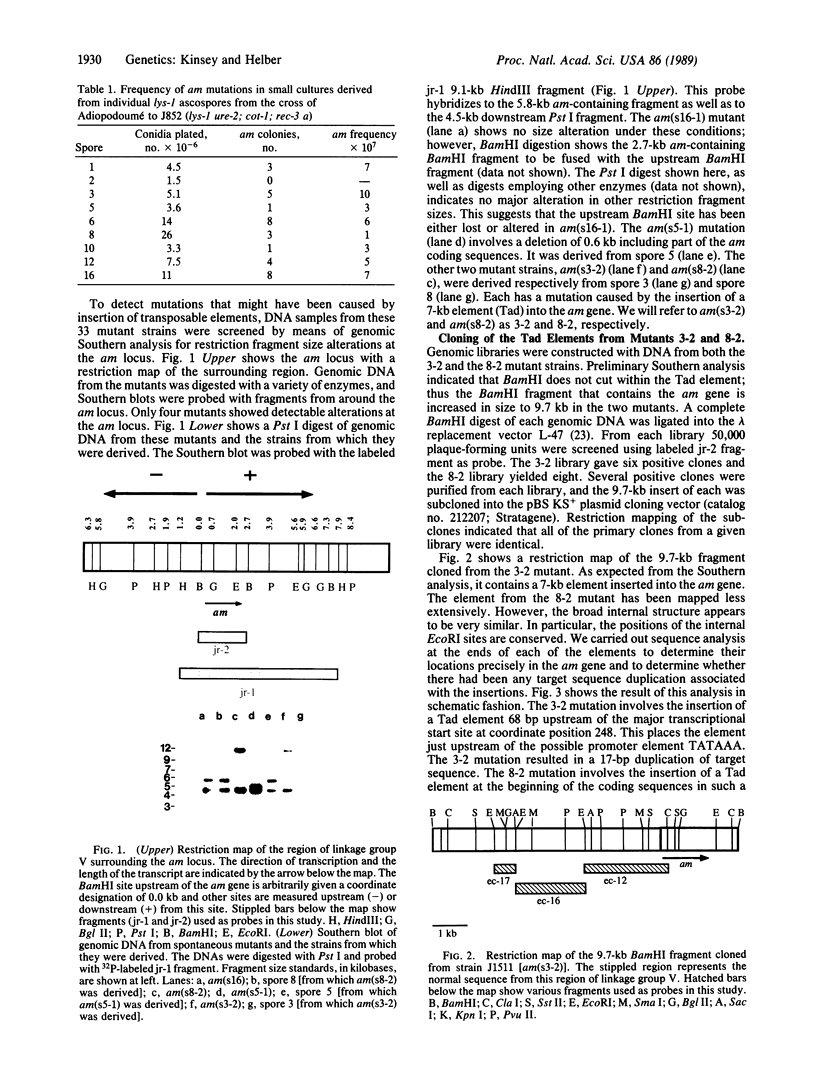
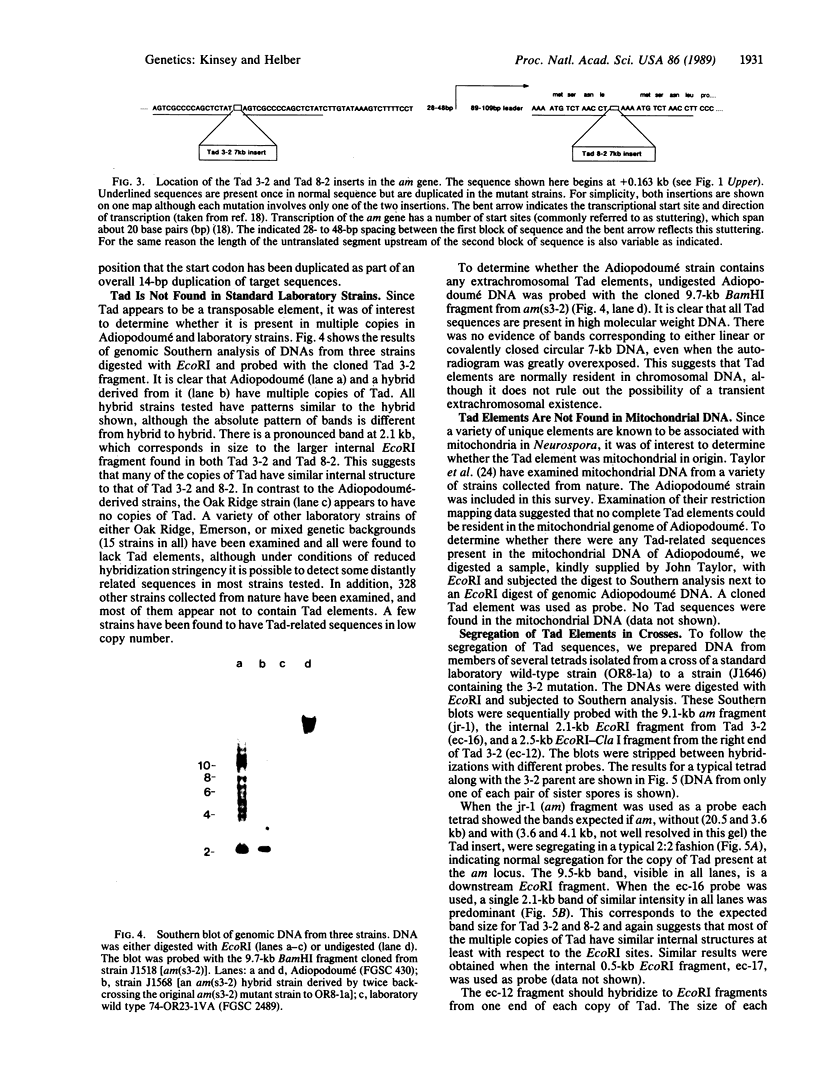
A Neurospora crassa strain from Adiopodoumé, Ivory Coast, contains multiple copies of a transposable element, Tad. The element was detected as a 7-kilobase insertion in two independently isolated spontaneous forward mutants of the am (glutamate dehydrogenase) gene. Laboratory strains do not contain Tad. All progeny from crosses of the Adiopodoumé strain to laboratory strains contain multiple copies. When the element was inserted in am, target sequences of 14 and 17 base pairs were duplicated in the two cases analyzed. One mutation, caused by the insertion of Tad at the beginning of the am coding sequence, is genetically stable. The other mutation, caused by insertion upstream of the transcriptional start site, has a reversion frequency of 2.5 x 10(-3). Precise excisions of Tad have not been found.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Kelley R. L., Lambowitz A. M. Mitochondrial plasmids of Neurospora: integration into mitochondrial DNA and evidence for reverse transcription in mitochondria. Cell. 1986 Nov 21;47(4):505–516. doi: 10.1016/0092-8674(86)90615-x. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Griffiths A. J., Court D. A., Cheng C. K. An extrachromosomal plasmid is the etiological precursor of kalDNA insertion sequences in the mitochondrial chromosome of senescent neurospora. Cell. 1986 Dec 5;47(5):829–837. doi: 10.1016/0092-8674(86)90525-8. [DOI] [PubMed] [Google Scholar]
- Coté B., Bender W., Curtis D., Chovnick A. Molecular mapping of the rosy locus in Drosophila melanogaster. Genetics. 1986 Apr;112(4):769–783. doi: 10.1093/genetics/112.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring H. P., Starlinger P. Molecular genetics of transposable elements in plants. Annu Rev Genet. 1986;20:175–200. doi: 10.1146/annurev.ge.20.120186.001135. [DOI] [PubMed] [Google Scholar]
- Fawcett D. H., Lister C. K., Kellett E., Finnegan D. J. Transposable elements controlling I-R hybrid dysgenesis in D. melanogaster are similar to mammalian LINEs. Cell. 1986 Dec 26;47(6):1007–1015. doi: 10.1016/0092-8674(86)90815-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Kinnaird J. H., Fincham J. R. The complete nucleotide sequence of the Neurospora crassa am (NADP-specific glutamate dehydrogenase) gene. Gene. 1983 Dec;26(2-3):253–260. doi: 10.1016/0378-1119(83)90195-6. [DOI] [PubMed] [Google Scholar]
- Kinsey J. A. Direct selective procedure for isolating Neurospora mutants defective in nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase. J Bacteriol. 1977 Dec;132(3):751–756. doi: 10.1128/jb.132.3.751-756.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Newmeyer D., Galeazzi D. R. The Instability of Neurospora Duplication Dp(IL-->IR)H4250 , and Its Genetic Control. Genetics. 1977 Mar;85(3):461–487. doi: 10.1093/genetics/85.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A., Hamza H., Mekki-Berrada A., Kalogeropoulos A., Rossignol J. L. Premeiotic and Meiotic Instability Generates Numerous b2 Mutation Derivatives in Ascobolus. Genetics. 1987 May;116(1):33–43. doi: 10.1093/genetics/116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Schechtman M. G. Isolation of telomere DNA from Neurospora crassa. Mol Cell Biol. 1987 Sep;7(9):3168–3177. doi: 10.1128/mcb.7.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J., Hazelrigg T. I., Polisky B. A., Pirrotta V., Scalenghe F., Kaufman T. C. The molecular organization of the Antennapedia locus of Drosophila. Cell. 1983 Dec;35(3 Pt 2):763–776. doi: 10.1016/0092-8674(83)90109-5. [DOI] [PubMed] [Google Scholar]
- Starlinger P., Saedler H. IS-elements in microorganisms. Curr Top Microbiol Immunol. 1976;75:111–152. doi: 10.1007/978-3-642-66530-1_4. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Bingham P. M. Regulation of white locus expression: the structure of mutant alleles at the white locus of Drosophila melanogaster. Cell. 1982 Sep;30(2):529–541. doi: 10.1016/0092-8674(82)90250-1. [DOI] [PubMed] [Google Scholar]