Abstract
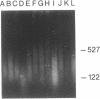
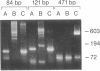
Several chemical and enzymatic properties were examined in the DNA extracted from dry remains of soft tissues that vary in age from 4 to 13,000 years and represent four species, including two extinct animals (the marsupial wolf and giant ground sloth). The DNA obtained was invariably of a low average molecular size and damaged by oxidative processes, which primarily manifest themselves as modifications of pyrimidines and sugar residues as well as baseless sites and intermolecular cross-links. This renders molecular cloning difficult. However, the polymerase chain reaction can be used to amplify and study short mitochondrial DNA sequences that are of anthropological and evolutionary significance. This opens up the prospect of performing diachronical studies of molecular evolutionary genetics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985 Jul 16;24(15):4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Doran G. H., Dickel D. N., Ballinger W. E., Jr, Agee O. F., Laipis P. J., Hauswirth W. W. Anatomical, cellular and molecular analysis of 8,000-yr-old human brain tissue from the Windover archaeological site. 1986 Oct 30-Nov 5Nature. 323(6091):803–806. doi: 10.1038/323803a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R. G., Wrischnik L. A., Oakes E., George M., Tong B., Wilson A. C. Mitochondrial DNA of the extinct quagga: relatedness and extent of postmortem change. J Mol Evol. 1987;25(4):283–287. doi: 10.1007/BF02603111. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Bowman B., Freiberger M., Ryder O. A., Wilson A. C. DNA sequences from the quagga, an extinct member of the horse family. Nature. 1984 Nov 15;312(5991):282–284. doi: 10.1038/312282a0. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Revision of consensus sequence of human Alu repeats--a review. Gene. 1987;53(1):1–10. doi: 10.1016/0378-1119(87)90087-4. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Servenius B., Rask L., Peterson P. A. Characterization of an HLA DR beta pseudogene. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1475–1479. doi: 10.1073/pnas.82.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Ljungquist S. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J Biol Chem. 1977 May 10;252(9):2808–2814. [PubMed] [Google Scholar]
- Ljungquist S., Andersson A., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974 Mar 10;249(5):1536–1540. [PubMed] [Google Scholar]
- Päbo S., Gifford J. A., Wilson A. C. Mitochondrial DNA sequences from a 7000-year old brain. Nucleic Acids Res. 1988 Oct 25;16(20):9775–9787. doi: 10.1093/nar/16.20.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S. Molecular cloning of Ancient Egyptian mummy DNA. Nature. 1985 Apr 18;314(6012):644–645. doi: 10.1038/314644a0. [DOI] [PubMed] [Google Scholar]
- Päbo S. Molecular genetic investigations of ancient human remains. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):441–446. [PubMed] [Google Scholar]
- Päbo S., Wilson A. C. Polymerase chain reaction reveals cloning artefacts. Nature. 1988 Aug 4;334(6181):387–388. doi: 10.1038/334387b0. [DOI] [PubMed] [Google Scholar]
- Rebrov L. B., Kozel'tsev V. L., Shishkin S. S., Debov S. S. Nekotorye énzimaticheskie aspekty posmertnogo autoliza. Vestn Akad Med Nauk SSSR. 1983;(10):82–89. [PubMed] [Google Scholar]
- Reynolds T. M. Chemistry of nonenzymic browning. II. Adv Food Res. 1965;14:167–283. doi: 10.1016/s0065-2628(08)60149-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak A., Di Capua E., Koller T. Elongation of duplex DNA by recA protein. J Mol Biol. 1981 Sep 25;151(3):557–564. doi: 10.1016/0022-2836(81)90010-3. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Kuo I. Radiation damage to DNA in aqueous solution: a comparison of the response of the single-stranded form with that of the double-stranded form. Radiat Res. 1978 Aug;75(2):278–285. [PubMed] [Google Scholar]
- Wick G., Haller M., Timpl R., Cleve H., Ziegelmayer G. Mummies from Peru. Demonstration of antigenic determinants of collagen in the skin. Int Arch Allergy Appl Immunol. 1980;62(1):76–80. [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]