Summary
The thyroid gland has long since been known for its self-renewal ability, mainly in cases of hyperplastic disease such as goitre. Recently the amazing improvement in knowledge about stem cells has explained this potentiality. Some stem cell features and their clinical usefulness are summarized here, reviewing data from the literature:
the proven presence of adult stem cells in thyroid tissue, either normal, goitrous or neoplastic, bring with it important implications regarding tissue regeneration and oncogenesis;
modifying culture conditions and micro-environment stem cells have led to mature tissue with specialized functions. This has considerably changed the attitude of regenerative medicine and cancer research;
finally, identification of stem cells and stem cell markers in thyroid cancer, gives hope for the development of new therapeutic approaches in recurrent or treatment-resistant thyroid cancer.
Keywords: Thyroid, Thyroid cancer, Stem cells, Tissue repair
Riassunto
Da tempo i clinici conoscono le capacità rigenerative del tessuto tiroideo, ma solo recentemente sono state identificate cellule staminali adulte nella tiroide, soprattutto nei pazienti con struma. Alcune sorprendenti potenzialità delle cellule staminali e la loro utilità clinica vengono riassunte attraverso l’analisi di dati della letteratura:
la documentata presenza di cellule staminali adulte nel tessuto tiroideo sano, iperplastico, o neoplastico, ha portato a considerazioni di notevole interesse sulle potenzialità rigenerative e sull’eventuale evoluzione tumorale:
la possibilità di orientare, modificando l’ambiente e le condizioni di coltura, la differenziazione delle cellule staminali verso cellule mature diversamente specializzate crea aspettative importanti nel campo della medicina rigenerativa;
infine l’identificazione di cellule staminali e dei loro marcatori biologici nei carcinomi tiroidei, differenziati, indifferenziati o midollari, apre a nuove ipotesi di terapia per le neoplasie non sensibili ai trattamenti usuali.
Introduction
The thyroid gland has long since been known for its self-renewal ability, mainly in cases of hyperplastic disease such as goitre. On the basis of old observations, subtotal thyroidectomy has been considered safe for survival since Professor Kocher’s studies. Only in recent years, have adult stem (AS) cells been documented in the human thyroid 1–4.
Stem cells are usually classified as embryonic or as AS cells. Embryonic stem (ES) cells are primitive totipotent cells derived from inner cell mass that is part of the blastocyst in early embryogenesis. A totipotent ES cell can give rise to any cell type in the body 5 6. AS cells are thought to be multi-potent, i.e., they have self-renewal abilities but can differentiate only into specific cell types according to the organ in which they reside 6 7. AS cells lay undifferentiated in most differentiated adult tissues as quiescent or slow cycling cells, able to make copies of themselves when needed, for tissue turnover or wound repair. Cancer stem cells may originate from these “normal” stem cells, or they may be a consequence of de-differentiation from mature cells. Although their self-renewal ability may be an exciting prospective for their possible use as “spare parts” in replacement therapies for many diseases, ranging from myocardial infarction to diabetes or Parkinson’s disease, this same ability may be an important cancer-initiating risk. The troubling hypothesis that normal stem cells and cancer stem cells might share the same biological aspects and molecular features has led to many studies aiming to identify which signalling pathways are in charge of normal or pathological cell replication. New investigations on cancer treatment procedures currently target selected steps of these signal transduction pathways.
Thyroid stem cells
The existence of thyroid stem cell populations, within the mature thyroid, was first advanced by Dumont et al. 8. Few reports on the potential proliferation of thyroid solid cell nets (solid ultimo-branchial body remnants: SCN) suggested that the main cells of solid nets could be multi-potent cells, involved in self-renewal of follicles and C-cells 9. In primary cultures, isolated from human goitres and in goitrous tissue specimens, Thomas et al. 2 detected biochemical markers for adult stem and precursor cells of endodermal origin. It has now been proven that adult stem cells do exist within the human goitrous thyroid 1 2. About 1-2% of nodular goitre cells are stem cells; on the other hand, in normal thyroid tissue, stem cells are estimated to be maximum 0.1% 2 4 8, they retain thyroid stimulating hormone (TSH) dependence although their growth is strictly linked to micro-environmental cells (niche cells) that interact with stem cells, regulating differentiation, proliferation and apoptosis 10. Adult stem cells, maintaining both their proliferation and differentiation ability, can be detected in the thyroid gland at any age 1–3. Physiological and pathological stimuli, such as growth factors or malnutrition, iodide deficiency, thyroid tissue destruction or necrosis, can overcome the control of niche cells leading to stem cell hyper-proliferation. A higher growth rate may cause nodular transformation of the thyroid tissue.
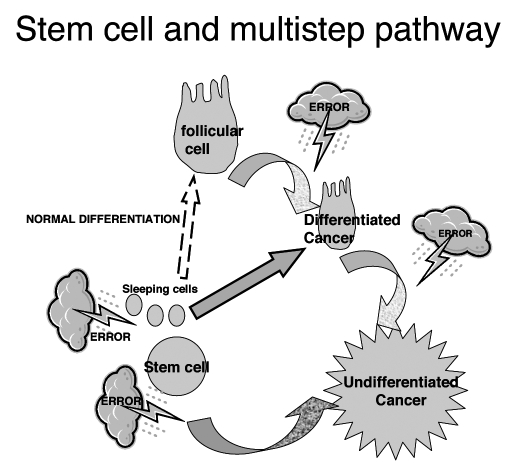
A low but significant proliferation has been shown in the thyroid, even in adults. The thyroid is renewed rather slowly, only 6 to 8 renewals during adult life 4 11. However, most thyrocytes, in the adult thyroid, are able to respond to proliferative stimuli. An extraordinary regeneration of thyrocytes occurs when follicles are severely destroyed, such as in thyroiditis. Regenerating thyrocytes actively reconstruct thyroid follicles together with endothelial cells and fibroblasts. Follicle regeneration and homeostasis are maintained by a pool of stem cells “sleeping” in the adult thyroid gland 11–14. Factors released by damaged tissues, such as cytokines, may direct stem cells to the lesion site, where they meet growth and differentiation factors as well as micro-environmental cells controlling specific progression until the mature tissue. There are several known sources of stem cells. At least three different types of stem cells have been described in the thyroid gland: A) the progenitor of follicular cells, derived from the floor of the foregut (endodermal origin); B) the progenitor of C cells, originating in the ultimo-branchial bodies (neural crest origin); and, C) a bipotential progenitor of both follicular cells and C cells, the existence of which has been suggested by immuno-fluorescence studies 4 11. Alternatively, stem cell may not originate in the thyroid gland itself, but from the migration of some universal stem cells, as occurs in many other tissues: bone marrow stroma cells replenish the blood, but they also contribute to muscle, brain, liver, heart, and endothelium 15–17, while muscle and nerve stem cells can contribute to blood 18. Growth factors and micro-environment, in each recipient tissue, modulate the differentiation to mature cells. Both thyroid stem cells and universal stem cells could undergo genetic alteration triggering tumours.
Many culture conditions have been described to select enriched thyrocyte population from ES, either from animal tissues or from human goitres 2 3. Recently thyrocyte-like cells have been obtained from murine CCE Es cell lines, after 2 weeks, treatment with TSH, the main regulating factor for thyroid tissue 5 6 19. After TSH treatment ES-derived cells express molecular aspects and biological functions specific to follicular cells: thyroglobulin (Tg) and Tg iodination, sodium-iodide symporter (NIS), thyroperoxidase (TPO) and TSH receptors. They are able to generate cAMP in response to TSH 5. These studies suggest that development of thyrocytes is partly possible in cultures of ES cells. Moreover, according to these studies, effects of growth factors and signalling pathways can be investigated with culture model. Further studies on niche and micro-environmental control could help to direct and modulate ES differentiation into mature follicle structures.
Pathogenetic mechanism in thyroid cancer
Differentiated thyroid cancer is generally thought to have a very good prognosis. In spite of this, a small percentage of tumours rapidly progress unexpectedly and is sometimes devastating. Although prognostic algorithms are available to identify patients at high risk, based on their clinical evaluation and histology, there are still many unsolved problems in identifying molecular mechanism underlying these uncontrolled progressions and their lack of responsiveness to routine treatment.
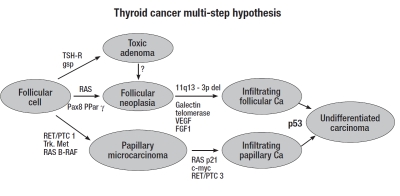
According to classical multistep carcinogenesis model (Fig. 1), thyroid cancer cells are generally thought to originate from the sequential accumulation of genetic alterations during the life cycle of well-differentiated pre-malignant thyrocytes, until the overt expression of the neoplastic phenotype, followed by clonal overgrowth 20. There are two classes of genes, in which mutations are of particular importance in carcinogenesis: 1) the tumour suppressor genes, the mutations of which are important events in tumour progression, and, 2) the oncogenes, abnormal genes generated by mutation/activation of “normal” proto-oncogenes. Chromosomal rearrangements leading to expression of oncogenes in the tyrosine kinase domain are common findings in differentiated thyroid cancers 21.
Fig. 1.
Multi-step pathogenesis of differentiated thyroid cancer.
The first deregulated receptor-tyrosine kinase identified in thyroid cancer, the RET proto-oncogene, is located on chromosome 10 and encodes a trans-membrane receptor-tyrosine kinase involved in early stages of both papillary and medullary thyroid cancer pathogenesis. RET activation mechanisms are different: a point mutation for medullary carcinoma and an intra- or inter-chromosomal rearrangement (RET/PTC) for papillary cancer, with higher prevalence in radiation induced PTCs and much lower prevalence in sporadic tumours.
The second alteration is due to a point mutation in the B-RAF proto-oncogene, located on chromosome 7 and involved in the MAPK signal transduction pathway, closely linked to early events of papillary cancer, more frequent in sporadic tumours, and rare in radiation-induced PTCs 22.
Another chromosomal rearrangement in papillary cancers involves the NTRK1 proto-oncogene (neutrophic receptor tyrosine kinase) located on chromosome 1, which forms with different fusion patterns chimeric oncogenes, involved in several signal-transduction cascades.
Finally, the RAS oncogene is involved in the early steps of follicular thyroid cell transformation, and RAS point mutations are found both in adenomas and follicular carcinomas. A crucial role in the progression of follicular adenoma to follicular carcinoma seems to be a translocation which generates a chimeric gene, PAX8-PPAR 23 24.
The current concept of the pathogenesis of undifferentiated thyroid cancer is that it may evolve through further dedifferentiation of follicular or papillary cancers, and the most frequent genetic alteration found in the late transition to undifferentiated thyroid cancer involves inactivating point mutation of p53 20 21 25.
These multistep models of molecular determination of thyroid cancer do not explain all types of known thyroid tumours, actually it accounts for less than 85% of differentiated tumours and 22-82% of undifferentiated tumours, thus some phenotypic variances of thyroid cancer follow a molecular model, at present not yet fully understood.
The histological aspects and biological behaviours of thyroid cancers are extremely variable, and, even in the same tissue, trabeculae, nests, sheets, follicles and papillary structures may coexist, but in front of different aspects these cells have often a monoclonal origin.
Recently, besides the classical multi-step theories, a different view of thyroid tumourigenesis has been proposed: the foetal-stem cell carcinogenesis hypothesis (Fig. 2). In this model, the cellular target for malignant transformation are normal stem/precursor cells, thus the different histological subtypes and the different biological aggressiveness could reflect different stages of aberrant biological pathway 11 26.
Fig. 2.
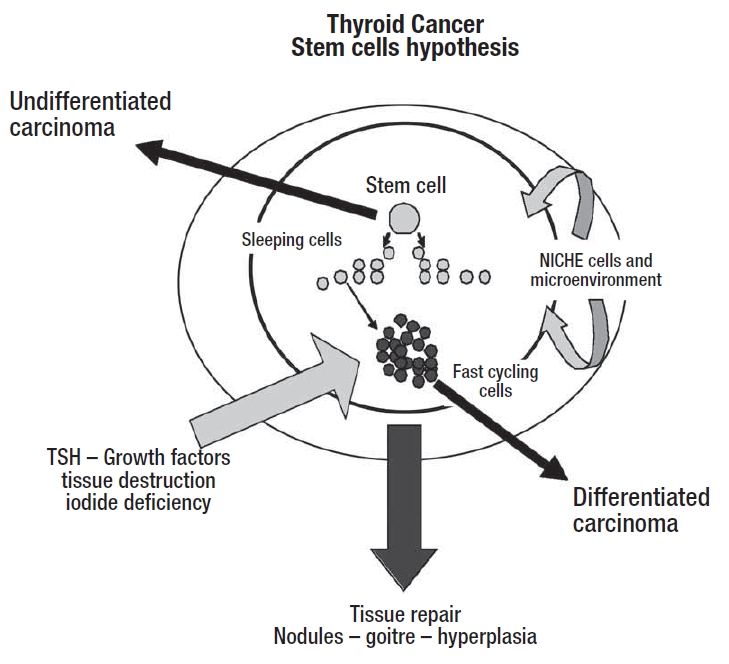
Stem cell hypothesis for thyroid cancer.
Due to their pluripotency and undifferentiated state, stem cells have been thought to be involved in the pathogenesis of human tumours in many different body tissues, since stem cells share many properties with cancer cells, such as self-renewal and indefinite growth 1 27 28. The hypothesis that also thyroid cancer could be a stem cell disease has been proposed by several Authors 11 29 30 and cancer stem cells have been reported in some thyroid carcinoma cell lines 30 31. Theoretically, cancer stem cells may originate or from “normal” stem cells for a wrong differentiation, either from “normal” mature cells for dedifferentiation 11, both pathways are probably involved, but the time and the mutations needed for complete trip to cancer are less for stem cells.
Certainly, not all cancers are due to a stem cell “gone bad”, but some cancer-initiating cells are probably stem cells, and the degree of differentiation may depend on the stage at which the error occurs.
The classical hypothesis of multistep disease together with the stem cells hypothesis do not exclude each other, but, on the contrary, they may contribute to explain the very different behaviour of some thyroid cancers and their different molecular aspects.
The cancer stem cell model brings with it important implications, both for the diagnosis and for the treatment of thyroid cancer: to identify the first step or the first clonal error in stem cell proliferation and dedifferentiation shows the way for target therapies. Future findings regarding genetic determination of thyroid tumours and cancer stem cells would possibly redirect therapeutic choices in the management of recurrent or rapidly spreading diseases, not responding to conventional 13,1I therapy.
Stem cells and therapy
Over the last few years, the use of embryonic stem (ES) cells has strongly impressed researchers due to their potential contribution to regenerative medicine. ES cells isolated from the inner cell mass of blastocyst-stage embryos are totipotent cells, able to produce all cell types in the body both in vitro and in vivo 19 32 33. By manipulating culture conditions, ES cells may differentiate into cells of all lineage. Protocols are currently available according to which hepatocytes and neural, haemopoietic, cardiac or pancreatic cells can be generated 34 35.
Although many culture conditions and selection strategies have been described to obtain mature thyrocytes from ES cells, these culture procedures produced only transient thyrocyte-like cells, and further genetic manipulation of micro-environment and thyroid specific proteins are necessary to obtain pure, differentiated and perfectly functional thyroid cells 6. The use of stem cells as a basis for reparative processes is indicated not only in degenerative diseases but also in cases of extensive tissue destruction as occurs in burns or following radical surgery.
Follicular reconstruction is not generally thought to be particularly useful for human well-being, due to availability of effective economic and well-tolerated hormone replacement therapy for hypothyroidism. On the other hand, thyroid stem cells, mainly from goitre tissue, could be used for transplantation therapy in various degenerative diseases or in myocardial diseases. The major concern, in reconstructive medicine, could be that the transplanted cells must escape destruction by the recipient’s immune system, mainly in patients with autoimmune disease 19. This problem could be at least partly overcome with the use of autologous stem cells. Bone marrow and blood are two common places where autologous stem cells are obtained; thyroid and lipid tissue could offer an alternative in cases of bone marrow destruction, such as after chemotherapy. Stem cells have also been used as “delivery vehicles” of drugs such as interferon β due to their capacity to reach wounded or cancer tissues, attracted by VEGF to form the stroma of the target tissue or the perycytes for angiogenesis 36 37.
The cancer stem cell theory could really affect cancer treatment, providing both information on aggressiveness and new targets for chemotherapy or biologic treatment. Although the idea of treatments focused on the cancer stem cells may look exciting, targeting the cancer stem cells is not easy at all. Cancer stem cells are relatively quiescent compared to other cancer cells and for that reason are resistant to traditional anti-cancer drugs directed against rapidly dividing cells. They do not appear to have hyper-activation of proliferative signals like tyrosine kinase, so they are probably resistant to anti thyrokinase drugs. If cancer stem cells are quiescent during therapy they may survive treatment directed to dividing cells. In this case, the patient may be apparently “cured” but will relapse when stem cells are reactivated. Anti-VEGF drugs, or biological drugs, are likely to be used in the future, as for the treatment of leukaemia. Drugs targeting cancer stem cells could stabilize and prolong disease-free survival 6 36. Further research is required to differentiate genes and signalling pathways involved in the process of stem cell carcinogenesis and for the development of new targeted forms of treatment.
Fig. 3.
Stem cell and multi-step pathway to thyroid cancer.
References
- 1.Yamada H, Takano T, Ito Y, Matsuzuka F, Miya A, Kobayashi K, et al. Expression of nestin mRNA is a differentiation marker in thyroid tumors. Cancer Lett 2009;280:61-4. [DOI] [PubMed] [Google Scholar]
- 2.Thomas T, Nowka K, Lan L, Derwahl M. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid 2006;16:537-44. [DOI] [PubMed] [Google Scholar]
- 3.Lan L, Cui D, Nowka K, Derwahl M. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require TSH for differentiation into thyrocytes. J Clin Endocrinol Metab 2007;92:3681-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoshi N, Kusakabe T, Taylor BJ. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology 2007;148:4251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin RY, Kubo A, Keller GM, Davies TF. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology 2003;144:2644-9. [DOI] [PubMed] [Google Scholar]
- 6.Thomas D, Friedman S, Lin R. Thyroid stem cells: lessons from normal development and thyroid cancer. Endocr Relat Cancer 2008;15:51-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowry W.E, Richter L. Signaling in adult stem cells. Front Biosci 2007;12:3911-27. [DOI] [PubMed] [Google Scholar]
- 8.Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 1992;72:667-97. [DOI] [PubMed] [Google Scholar]
- 9.Cameselle-Teijeiro J, Febles-Perez C, Sobrinho-Simoes M. Papillary and mucoepidermoid carcinoma of the thyroid with anaplastic transformation: a case report with histologic and immunohistochemical findings that support a provocative histogenetic hypothesis. Pathol Res Pract 1995;191:1214-21. [DOI] [PubMed] [Google Scholar]
- 10.Moore KA, Lemischka IR. Stem cells and their niches. Science 2006;311:1880-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Zuo H, Ozaki T, Nakagomi N, Kakudo K. Cancer stem cell hypothesis in thyroid cancer. Pathol Int 2006;56:485-9. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 2004;6:1082-93. [DOI] [PubMed] [Google Scholar]
- 13.Tsonis PA, Lambris JD, Del Rio-Tsonis K. To regeneration … with complement. Adv Exp Med Biol 2006;586:63-70. [DOI] [PubMed] [Google Scholar]
- 14.Shuji T, Norimasa K, Hajime S. Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: perspective for thyroid tissue engineering. Pathol Int 2001;51:403-17. [DOI] [PubMed] [Google Scholar]
- 15.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991;67:1033-6. [DOI] [PubMed] [Google Scholar]
- 16.Lu SH, Yang AH, Wei CF, Chiang HS, Chancellor MB. Multi-potent differentiation of human purified muscle-derived cells: potential for tissue regeneration. BJU Int 2009; 27 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41-9. Erratum in: Nature 2007;447:879-80. [DOI] [PubMed] [Google Scholar]
- 18.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell 2001;105:829-41. [DOI] [PubMed] [Google Scholar]
- 19.Lin RY, Davies TF. Derivation and characterization of thyrocyte-like cells from embryonic stem cells in vitro. Methods Mol Biol 2006;330:249-61. [DOI] [PubMed] [Google Scholar]
- 20.Kondo T, Ezzat S, Asa S. Pathogenic mechanism in thyroid follicular cell neoplasia. Nat Rev Cancer 2006;6:292-306. [DOI] [PubMed] [Google Scholar]
- 21.Kim DS, McCabe CJ, Buchanan MA, Watkinson JC. Oncogenes in thyroid cancer. Clin Otolaryngol Allied Sci 2003;28:386-95. [DOI] [PubMed] [Google Scholar]
- 22.Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab 2008;22:955-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implication for diagnosis, treatment and prognosis. Expert Rev Mol Diagn 2008;8:83-95. [DOI] [PubMed] [Google Scholar]
- 24.Zhu XG, Cheng SY. Modelling thyroid cancer in the mouse. Horm Metab Res 2009;41:488-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez HG. Molecular basis of epithelial thyroid tumorigenesis. C R Acad Sci III 2000;323:519-28. [DOI] [PubMed] [Google Scholar]
- 26.Takano T. Fetal cell carcinogenesis of the thyroid: theory and practice. Semin Cancer Biol 2007;17:233-40. [DOI] [PubMed] [Google Scholar]
- 27.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem cell biology to cancer. Nat Rev Cancer 2003:3:895-902. [DOI] [PubMed] [Google Scholar]
- 28.Polyak K, Hahan WC. Roots and stems: stem cells in cancer. Nat Med 2006;12:296-300. [DOI] [PubMed] [Google Scholar]
- 29.Takano T, Amino N. Fetal cell carcinogenesis: a new hypothesis for better understanding of thyroid carcinoma. Thyroid 2005;15:432-8. [DOI] [PubMed] [Google Scholar]
- 30.Zito G, Richiusa P, Bommarito A, Carissimi E, Russo L, Coppola A, et al. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS One 2008;3:e3544.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology 2007;148:1797-803. [DOI] [PubMed] [Google Scholar]
- 32.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154-6. [DOI] [PubMed] [Google Scholar]
- 33.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995;7:862-9. [DOI] [PubMed] [Google Scholar]
- 34.Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells 2004;22:925-40. [DOI] [PubMed] [Google Scholar]
- 35.Shirahashi H, Wu J, Yamamoto N, Catana A, Wege H, Wager B, et al. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant 2004;13:197-211. [DOI] [PubMed] [Google Scholar]
- 36.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int 2007;7:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006;355:1253-61. [DOI] [PubMed] [Google Scholar]