Abstract
This article reviews the evidence for efficacy and safety of Saccharomyces boulardii (S. boulardii) for various disease indications in adults based on the peer-reviewed, randomized clinical trials and pre-clinical studies from the published medical literature (Medline, Clinical Trial websites and meeting abstracts) between 1976 and 2009. For meta-analysis, only randomized, blinded controlled trials unrestricted by language were included. Pre-clinical studies, volunteer studies and uncontrolled studies were excluded from the review of efficacy and meta-analysis, but included in the systematic review. Of 31 randomized, placebo-controlled treatment arms in 27 trials (encompassing 5029 study patients), S. boulardii was found to be significantly efficacious and safe in 84% of those treatment arms. A meta-analysis found a significant therapeutic efficacy for S. boulardii in the prevention of antibiotic-associated diarrhea (AAD) (RR = 0.47, 95% CI: 0.35-0.63, P < 0.001). In adults, S. boulardii can be strongly recommended for the prevention of AAD and the traveler’s diarrhea. Randomized trials also support the use of this yeast probiotic for prevention of enteral nutrition-related diarrhea and reduction of Heliobacter pylori treatment-related symptoms. S. boulardii shows promise for the prevention of C. difficile disease recurrences; treatment of irritable bowel syndrome, acute adult diarrhea, Crohn’s disease, giardiasis, human immunodeficiency virus-related diarrhea; but more supporting evidence is recommended for these indications. The use of S. boulardii as a therapeutic probiotic is evidence-based for both efficacy and safety for several types of diarrhea.
Keywords: Probiotic, Diarrhea, Saccharomyces boulardii, Adult patients, Meta-analysis
INTRODUCTION
Probiotics have become increasingly popular in the US and are rapidly reaching levels of use in Europe and Asia, which have a longer history of use compared with the US[1-3]. Probiotics are “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host”[4,5]. In one survey, of 435 users of dietary supplements, 50% of women and 33% of men reported use of probiotics[6].
Probiotics are generally recommended to help strengthen host systems and assist in recovery from certain diseases. However, the general public and many health care providers are still confused about how best to utilize probiotics and which ones are effective for specific diseases. There are several challenges in choosing the appropriate probiotic; including the wide diversity of probiotic strains, quality control of commercially-available probiotic products and the degree of evidence-based trials for each disease and probiotic. The ability of an organism to be an effective probiotic has been found to be strain-specific and microbial organisms are defined by their genus, species and strain. For example, Lactobacillus rhamnosus (L. rhamnosus) GG is a specific bacterial strain, which has been shown to be an effective probiotic for several diarrheal diseases, but other strains within the L. rhamnosus species may not be an effective probiotic. Similarly, other species under the same genus (Lactobacillus) may not be effective probiotics[7]. To add to this confusion, probiotic products are available in diverse forms: capsules of freeze-dried or lyophilized cultures, heat-dried culture supernatants, mixed in diary food (such as yogurts, cheese, milks, or ice cream) or other food (kefir, chocolate, wafers)[8-10]. The variety of probiotic products are also regulated under different guidelines according to the ways of use (food, dietary supplement, over-the-counter use or prescription). Different standards of quality assurance also vary with the type of product, indication for use and country.
Despite these challenges, the awareness of probiotics as a therapeutic modality has increased dramatically and the frequency of peer-reviewed randomized clinical trials have kept abreast with the global interest in this innovative method of therapy. Numerous probiotic strains have been investigated for clinical efficacy, including multiple bacterial strains: Lactobacilli, Bifidobacteria, Streptococci, Clostridia and fungal strains: Saccharomyces boulardii (S. boulardii), S. cerevisiae, and Monascus purpureus[11-13]. This review examines evidences for Saccharomyces probiotics and focuses on S. boulardii for the efficacy and safety of different diseases in adult patients. The two goals of this study were: (1) to provide guidance for clinicians and patients on how to appropriately use S. boulardii as a therapeutic probiotic and (2) to review the evidence for efficacy and safety of S. boulardii for various disease indications in adults.
SEARCH STRATEGY FOR SYSTEMATIC REVIEW
PubMed, Medline and Google Scholar were searched for articles unrestricted by language from 1976 to 2009. Three on-line clinical trial registers were searched: Cochrane Central Register of Controlled Trials (www.cochrane.org), metaRegister of Controlled Trials (www.controlled-trials.com/mrct) and National Institutes of Health (www.clinicaltrials.gov). Secondary and hand searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books and meeting abstracts also were performed. Search terms included: Saccharomyces boulardii, probiotics, yeast, diarrhea, risk factors, randomized controlled trials, placebo-controlled, and associated author names. Search strategies were broad-based initially, then narrowed to the disease of interest to increase the search network[14].
META-ANALYSIS
If sufficient numbers of clinical trials (> 5) were found on one type of disease indication, a meta-analysis for the efficacy of S. boulardii was performed; otherwise a systematic review was done. The procedure for the meta-analysis followed standard meta-analysis methodology with clearly delineated parameters, a priori inclusion and exclusion criteria and standardized data extraction[15,16]. Abstracts of all citations and retrieved studies were reviewed and rated for inclusion. In some cases, only published abstracts from meetings were available. Published abstracts from meetings were included to lessen the potential for publication bias due to failure to publish negative findings. Information on study design, methods, interventions, outcomes, adverse effects and treatments was extracted from each article using a standardized extraction table. When necessary, authors were contacted for data not reported in the original article.
The primary objective of the meta-analysis was to determine the overall efficacy of S. boulardii within one specific type of disease indications (antibiotic-associated diarrhea, Clostridium difficile disease, traveller’s diarrhea, irritable bowel syndrome, inflammatory bowel disease or other types of diarrhea). Inclusion criteria were: randomized, controlled, blinded efficacy trials in humans published as full articles or meeting abstracts in peer-reviewed journals. Exclusion criteria included: use of other strains of Saccharomyces (such as Saccharomyces cerevisiae) or other strains of probiotics, pre-clinical studies, safety studies, case reports or case series, phase I studies in volunteers, reviews, duplicate reports, trials of unspecified treatments, uncontrolled studies, prebiotic treatments only (no living organisms) or insufficient data in article.
To estimate pooled relative risks across studies, heterogeneity between and within trials was evaluated using the Cochrane Q and I2 tests[17]. Relative risks (RR) and 95% confidence intervals (CI) were calculated using the Mantel-Haenszel method. The relative risks of responding to S. boulardii therapy were pooled using a random-effect model if significant heterogeneity was found or a fixed-effect model if the studies were homogenous. P values less than 0.05 were considered significant. Analyses were performed using Stata software version 9.2 (Stata Corporation, College Station, Texas). A funnel scatterplot and Begg’s test were used to assess publication bias[18].
HISTORY
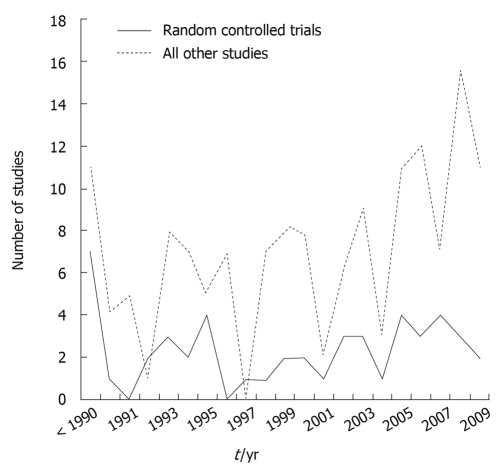
Saccharomyces cerevisiae strains have a long history of use in baking and brewing preparation, but have only infrequently been investigated for probiotic properties[19,20]. Another closely related strain, S. boulardii, was discovered by a French microbiologist, Henri Boulard in 1920 when he was in IndoChina searching for new strains of yeast that could be used in fermenting processes. He was a visitor during a cholera outbreak and noticed that some people who did not develop cholera were drinking a special tea. This tea was made by taking the outer skin from a tropical fruit (lychee and mangosteens) and cooking them down to make tea. He succeeded in isolating the agent responsible. It was a special strain of yeast he named “Saccharomyces boulardii”. The patent for this yeast was bought by Laboratories Biocodex in 1947, which began researching and manufacturing protocols. As shown in Figure 1, interest in this strain has increased, as reflected by the increasing number of scientific publications, encompassing both pre-clinical papers in mechanisms of action, animal models of efficacy, pharmacokinetics, early studies in safety and dose-ranging. In addition, there are currently 53 randomized controlled clinical trials, encompassing 8475 subjects, investigating the safety and efficacy of S. boulardii in pediatric and adult patients spanning several disease indications, of which 43 (81%) found significant efficacy for S. boulardii. As a recent review article on the efficacy of S. boulardii was published for pediatric indications[21], this study will focus on S. boulardii use in adult patients. There have been 27 randomized controlled trials, with 31 treatment arms testing S. boulardii in adult patients for a variety of diseases and most (84%) found a significantly protective efficacy of this probiotic. There have been only a few randomized clinical trials using other strains of Saccharomyces probiotics, which will be discussed later in this paper.
Figure 1.
Frequency of peer-reviewed publications on Saccharomyces boulardii from 1976 to 2009.
TAXONOMY
Probiotics may be either bacterial or yeast microbes. Yeast probiotics, such as S. boulardii, are different from bacterial probiotics (different physiologic structures, large in size, do not acquire antibiotic-resistant genes and are not affected by antibiotics)[12]. Not only is there a confusing array of probiotic products on the global market, but the taxonomy of Saccharomyces strains has been debated[22]. One strain has received considerable discussion about its valid nomenclature[23,24]. It was originally named S. boulardii in the 1950’s. Advances in typing methods opened a debate as to whether this strain should be reclassified as a strain of S. cerevisiae or remain a separate species. Early work using PCR electrophoretic karotyping or rRNA sequencing methods reported that S. boulardii was indistinguishable from other strains of S. cerevisiae[24,25]. Newer metabolomic tools (microsatellite polymorphism analysis and retrotransposon hybridization analyses) show that S. boulardii has a unique clustering different from other strains of S. cerevisiae[26-28]. In addition, S. boulardii differs from other strains of S. cerevisiae by several metabolic and genetic characteristics[29,30]. S. boulardii persists longer in gnotobiotic mice models (10 d) compared with rapid clearance of other strains of S. cerevisiae (< 1 d)[31]. S. boulardii is also different physiologically and metabolically and its optimum growth temperature is 37°C and it is resistant to low pH and is tolerant to bile acids; whereas other strains of S. cerevisiae prefer cooler temperatures (30-33°C) and do not survive well in acid pH ranges[32-34]. S. boulardii can be distinguished from other strains of S. cerevisiae by advanced typing methods, by differences in metabolism and physiology and by the ability to have anti-pathogen effects (as discussed in the mechanisms of action section).
GUIDELINES FOR CHOOSING APPROPRIATE PROBIOTICS
Challenges in providing guidance to patients regarding the appropriate choice of probiotic include the wide diversity of available products on the market, variances in quality control, stability and formulations in those products, and the requirement to match the type of probiotic with the disease indication. The efficacy of probiotics have been shown to be both strain-specific and disease-specific[11,35,36].
Product to product variation
There are many different Saccharomyces products available commercially. Table 1 lists some examples of products that contain S. boulardii, sold as probiotics either as lyophilized or heat-dried powders in capsules, or as one of several strains in a probiotic mixture in capsules or in liquid beverages[12,13,21,37-66]. The quality of these products from different sources have been found to vary. Choosing a probiotic product from a manufacturer with a regulated quality control program is a sound policy. Unfortunately, many of the products available commercially may lack regulated quality control programs. Studies of other probiotics have found a wide diversity in both quality and contamination in products available on the Internet[67]. Marcobal et al[68] tested 14 commercial probiotics in the US and found 93% were incorrectly labeled (57% had contaminants and 36% did not list strains on the label). Masco et al[69] tested 58 different probiotic products from Europe, UK, Asia, Japan and Canada and found only 38% had the dose stated on the label and 29% did not contain strains listed on the label. Not all products were found to have high standards of manufacture and quality control, as only some conform to Good Manufacturing Practices. Although most products state they contain at least 1 × 109 S. boulardii/mg, independent assays have determined 50% of the products contained a dose less than on the label. In one study comparing six S. boulardii products, all had identical PCR typing profiles, but only 50% [Floratil (Merck), Flomicin (NeoChemical), and Florazin (Herald’s)], had the same concentration identified on their label. One product [Lactipan (Sigma Pharm)] had 2 × 104 fewer S. boulardii than stated on its label. One product [Floratil (Merck)], had the highest concentration (1 × 109/100 mL) and maintained high levels (9.5 × 108) six months later[70]. Even if the label states it contains S. boulardii, a variation in efficacy may occur due to lower than stated dose or inaccurate strain composition. Four S. boulardii products were tested (along with one S. cerevisiae product) in Brazil. Only two (50%) of the S. boulardii products were protective in a Salmonella typhimurum mouse model (two S. boulardii products were ineffective, as well as the one S. cerevisiae product)[71]. Without access to specific quality control assays for commercially available probiotic products, the choice of a high quality product can be difficult. One method of selecting a probiotic product is to find a product in which the manufacturing company has sponsored original clinical trials, as this indicates a degree of commitment that may not be present in companies that do not sponsor original research. As shown in Table 1, only a few S. boulardii probiotic products are supported by original research. Although at present, most probiotic clinical trials are sponsored by private companies, as more national funding becomes available, this situation may change.
Table 1.
Examples of commercially available probiotics containing “Saccharomyces boulardii”
| Product name | Probiotic strain | Manufacturer | Stable at room temp1 | Facility certified | Strain confirmed | Colony forming unit (cfu) per mg capsule or mL | Original strain-specific studies | Degree of clinical evidence2 |
| Single strain | ||||||||
| Florastor® (US) Perenterol® (Germany) Reflor® (Turkey) Ultra-Levure® (Asia)3 | S. boulardii lyo | Biocodex (France) | Yes | EU GMP | Microsatellite sequency poly-morphism [28,29] | 5 × 109/250 mg | Multiple[37-62] | A |
| SB+MOS | S. boulardii | Jarrow Formulas (Los Angeles, CA) and Gnosis (Italy) | No | EU GMP | Not stated | 1.5 × 109 | One study[63] | C |
| Saccharomyces boulardii | S. boulardii | Kirkman (Oregon) | No | GMP | DNA finger-print | 3 × 109/150 mg | None | F |
| Saccharomyces boulardii | S. boulardii | Allergy Research Group (CA)/NutriCology (CA) | No | GMP | DNA finger-print | 3 × 109/150 mg | None | F |
| Saccharomyces boulardii | S. boulardii | Klaire Labs (Reno Nevada) | No | GMP ISO 9001 | Ribotyping | 3 × 109/150 mg | None | F |
| In mixtures of probiotics | ||||||||
| Protecflor® (Canada) Erce Flora® (Belgium) | S. cerevisiae boulardii CNCM I-1077) + L. rhamnosus + L. acidophilus + Bifido. strain | Institut Rosell-Lallemand (Quebec, Canada) | Not stated | Canadian GMP | Not stated | 109/2 mL | Two studies [64,65] | D |
| MitoMix® | S. boulardii and Pediococcus acidilactici | Imagilin Technology (Maryland) | Yes | Not stated | Not stated | 2.3 × 109/capsule | One study[66] | D |
| Primal Defense™ | S. boulardii with 13 other strains4 | Garden of Life (FL) | Yes | Not stated | No (only certified “organic”) | Total 1 × 109 per 410 mg | None | F |
| Pro-Bio Defense™ | S. boulardii + 7 other strains5 | Kirkman (OR) | No | GMP | DNA finger-print | Total: 2 × 1010/cap, no data on SB cfu | None | F |
| ABX Support™ | S. boulardii + L. rhamnosus + Bifido. bifidum + Bifido. breve | Klaire Labs (NEV) | No | GMP ISO 9001 | Ribotyping | 5 × 109/300 mg | None | F |
| Kombucha fermented tea | S. boulardii + L. bacterium + blue-green algae | Millennium Products, Inc. | No | No | Not stated | 1 × 109 per 16 oz. | None | F |
Products not stable at room temperature require refrigeration, probably heat-dried. Products stable at room temperature are typically packaged in blister packs and are lyophilized;
Degree of evidence: A = at least two randomized, controlled clinical trials in patients; B = one randomized controlled clinical trial in patients, C = case reports, uncontrolled randomized trials or open safety/kinetic studies in patients or volunteers, D = in vivo animal model or in vitro studies only, E = expert opinion only, F = no direct evidence;
A few examples, Biocodex has over 40 brand names worldwide;
Other strains in Primal Defense include: 11 strains of Lactobacilli (L. acidophilus, L. bulgaricus, L. lactis, L. plantarum, L. casei, L. lactis, L. leichmannii, L. brevis, L. caucasicus, L. fermenti, L. helveticus and 1 strain each: Bifidobacterium bifidum, Bacillus subtilis, Bacillus lichenformis);
Other strains in Pro-Bio Defense include: 5 strains of Lactobacilli (L. plantarum, L. rhamnosus, L. acidophilus, L. casei, L. bulgaricus) and Bifidobacterium lactis and Streptococcus thermophilus. GMP: Good manufacturing practices.
Stability
How the probiotic product is manufactured may significantly affect its potency over time (shelf-life). Probiotics may be available as lyophilized preparations, heat-dried preparations or contained in diary or drink food products. S. boulardii is usually available in capsules of either lyophilized or heat-dried preparations. Heat-dried capsule products may be identified by their labels, which usually state that the products should be refrigerated after opening and they lose their potency rapidly. Lyophilized preparations of S. boulardii are stable over one year at room temperature, as long as it is protected from moisture[72]. Lyophilized products are stable at room temperature and have the advantage of portability and convenience and maintain high viability counts over prolonged periods. Heat-dried preparations are not stable at room temperature and must be refrigerated. A study of four S. boulardii products in Germany found a lyophilized product [Perenterol Forte (Thiemann)] outperformed three heat-killed S. boulardii preparations in terms of higher viable cells and quicker start-up times[73].
Single strain or probiotic “cocktails”
As shown in this review, all the randomized controlled trials using S. boulardii probiotics have utilized a single strain preparation. Although mixtures of probiotics, which may contain S. boulardii, are available on the market, no randomized controlled trials have been done showing that these mixtures are superior to the single stain preparations. Pre-clinical studies in animal models have found promising results in a probiotic mixture (L. rhamnosus, L. acidophilus, Bifidobacterium and S. boulardii) for reducing E. coli diarrhea in rats, but no human clinical trials have been performed with this mixture[65]. Another potential limitation to probiotic mixtures is antagonism between the different probiotic strains and conflicting mechanisms of actions that may tend to attenuate the therapeutic responses of the probiotic strains[74].
Mechanisms of action
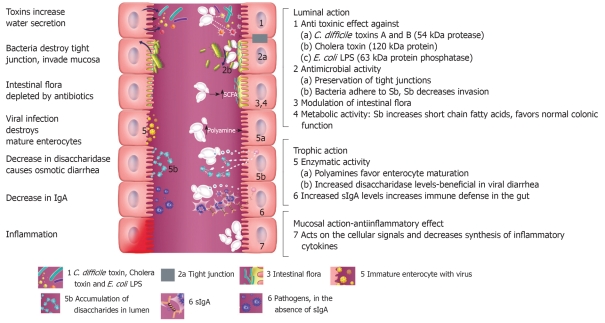
An advantage of probiotics is that they are living organisms incorporating a delivery system (most probiotics survive to the target organ) bringing an arsenal of anti-pathogenic strategies into play. S. boulardii has several different types of mechanisms of action (Figure 2) which may be classified into three main areas: luminal action, trophic action and mucosal-anti-inflammatory signaling effects[8,75-77]. Within the intestinal lumen, S. boulardii may interfere with pathogenic toxins, preserve cellular physiology, interfere with pathogen attachment, interact with normal microbiota or assist in reestablishing short chain fatty acid levels. S. boulardii also may act as an immune regulator, both within the lumen and systemically.
Figure 2.
Schematic of intestinal tract, illustrating the different potential mechanisms of action of Saccharomyces boulardii (Sb). On the left, effects of different pathogenic microbes are depicted. On the right, seven different protective effects of S. boulardii are depicted. Within the lumin of the intestine, S. boulardii may degrade toxins of pathogens, interfere with pathogenic adherence, modulate normal microbiota and preserve normal intestinal physiology. S. boulardii may also indirectly restore normal short chain fatty acid (SCFA) balance. S. boulardii may also increase secretory IgA (sIgA) levels or act as an immune regulator by influencing cytokine levels.
Anti-toxin effects: S. boulardii may interfere with pathogenesis within the intestinal lumen by several mechanisms: either by blocking pathogen toxin receptor sites[78], or acting as a decoy receptor for the pathogenic toxin[79] or by direct destruction of the pathogenic toxin. Castagliuolo et al[80] found a 54 kDa serine protease produced by S. boulardii directly degrades C. difficile toxin A and B. The efficacy of other strains of Saccharomyces has also been investigated. Only S. boulardii produces a protease capable of degrading Clostridium difficile toxins and receptors sites on the enterocyte cell surface, unlike other strains of Saccharomyces[78,81]. Buts et al[82] found a 63 kDa phosphatase produced by S. boulardii destroys the endotoxin of pathogenic E. coli. Several investigators showed that S. boulardii could reduce the effects of cholera toxin and this may be due to a 120 kDa protein produced by S. boulardii[83,84].
Antimicrobial activity: S. boulardii is capable of directly or indirectly interfering with intestinal pathogens. S. boulardii may directly inhibit the growth of pathogens (such as Candida albicans, Salmonella typhimurum, Yersinia enterocolitium, Aeromonas hemolysin[43,85,86]). In animal models testing for the ability to inhibit pathogen growth, several studies using non-S. boulardii strains of S. cerevisiae did not find any effect unlike the protective effects of S. boulardii[31,70]. A few studies have tested other strains of S. cerevisiae and found promising results. Brandao et al[79] tested S. cerevisiae strain W303 in rats and found less histologic damage due to cholera toxin compared with saline controls, but no clinical trials have been done using this strain. S. boulardii may also act by enhancing the integrity of the tight junction between enterocytes, thus preserving intestinal integrity and function[87,88]. Wu et al[88] found less crypt hyperplasia and cell damage in a Citrobacter rodentium-induced mice model of colitis when mice were treated with 1 × 109 S. boulardii per day for 7 d. Garcia Vilela et al[62] found decreased intestinal permeability when patients with Crohn’s disease were given S. boulardii (1.6 × 109/d for 4 mo) compared with placebo. S. boulardii has also been shown to reduce the translocation of pathogens in rat and pig animal models[64,89,90]. S. boulardii can also interfere with pathogenic attachment to intestinal receptor sites[88,91,92]. Gedek et al[93] also found that S. boulardii acts as a decoy by causing EPEC cells to directly bind to the surface of S. boulardii cells rather than enterocytes.
Cross-talk with normal microbiota: Newer techniques, including metagenomics and PCR probes have documented that a typical human may carry over 40 000 bacterial species in the collective intestinal microbiome[94]. The normal intestinal flora has many functions, including digestion of food, but the one that is most germane for this discussion is called “colonization resistance”[77,95,96]. This involves the interaction of many bacterial microflora and results in a barrier effect against colonization of pathogenic organisms. Normal microflora may act by competitive exclusion of nutrients or attachment sites, produce bacteriocins, or produce enzymes detrimental to pathogenic growth. Factors that disrupt this protective barrier, for example antibiotic use or surgery, results in host susceptibility to pathogen colonization until such time as the normal microflora can become re-established. Typically, it takes six to eight weeks for normal microbiota to recover after antibiotic exposure or disease resolution[97]. Probiotics are uniquely qualified to fit into this window of susceptibility and may act as surrogate normal microflora until recovery is achieved. S. boulardii has no effect on normal microbiota in healthy human controls[98,99]. In contrast, when S. boulardii is given to antibiotic-shocked mice or patients with diarrhea, normal microbiota is re-established rapidly[99,100].
Restoration of metabolic activities: S. boulardii has been shown to be able to increase short chain fatty acids (SCFA), which are depressed during disease, indicating altered colonic fermentation[98,101,102].
Trophic effects: S. boulardii can reduce mucositis[103], restore fluid transport pathways[84,101,104], stimulate protein and energy production[105], or act through a trophic effect by releasing spermine and spermidine or other brush border enzymes that aid in the maturation of enterocytes[106,107].
Immune response: S. boulardii may also regulate immune responses, either acting as an immune stimulant or by reducing pro-inflammatory responses. S. boulardii may cause an increase in secretory IgA levels in the intestine[56,108-110]. It has also been found associated with higher levels of serum IgG to C. difficile toxins A and B[111]. S. boulardii may also interfere with NF-κB-mediated signal transduction pathways, which stimulate pro-inflammatory cytokine production[76,112,113]. Chen et al[114] found that S. boulardii blocks activation of ERK1/2 and MAP kinases, which typically stimulate IL-8 production and cell necrosis in mice ileal loop models and in in vitro models. S. boulardii has also been shown to cause the trapping of T helper cells into mesenteric lymph nodes, thereby reducing inflammation[115].
Properties of S. boulardii
To be an effective probiotic, it must survive passage to its target organ (most commonly the colon). Organisms need to survive at body temperature (37°C), be resistant to stomach acids and bile acids, and exist in the competitive milieu of the intestinal tract. Probiotic strains of Saccharomyces have been shown to have these abilities. Although the optimal temperature for most strains of Saccharomyces range from 22-30°C, S. boulardii survives best at 37°C, giving it a unique advantage of being one of the few yeasts that do best at human body temperatures.
Survival to target sites: Although much of the oral dose is destroyed (usually stool levels are 100-1000 times lower than the oral dose), surviving oral doses have been found to be effective (usually at levels over 108 organisms/g stool)[116]. S. boulardii is resistant to antibacterial agents and survives gastric acidity[34,117]. In human volunteers, the concentration in the colon was found to be dose-dependent[118]. When S. boulardii was given to healthy volunteers at doses typically used therapeutically (1-2 × 1010/d), colonic levels were 2 × 108/g stool[118]. Few clinical trials using probiotics have documented the level of organisms present in the terminal site (colonic lumen in their study subjects). However, in one trial of patients with recurrent C. difficile infection (CDI) given S. boulardii (2 × 1010/d) for 28 d, patients who had a subsequent CDI recurrence were found to have significantly lower numbers of S. boulardii (2 × 104/g stool) compared with those without recurrence (1 × 106/g stool)[119].
Pharmocokinetics: S. boulardii, when given orally, achieves steady-state concentrations within three days and is cleared within 3-5 d after it is discontinued[34,119,120]. Blehaut et al[120] gave eight healthy human volunteers S. boulardii (oral dose of 5 × 109) for six days and followed them for time-to-clearance. They determined S. boulardii has half-life of 6 h, fecal steady-state concentrations (2 × 107/g) were reached by day 3 and the yeast was cleared after four days after administration. Klein et al[118] confirmed these findings in their studies of human volunteers and also found S. boulardii levels were 23 times higher in volunteers with disturbed intestinal microbiota (due to ampicillin exposure) than volunteers who were not given ampicillin. Elmer et al[121] found that some types of fiber (psyllium) increased S. boulardii levels by 22%, while other types of fiber (pectin) showed no effect.
CLINICAL EFFICACY: ACUTE DISEASES
S. boulardii probiotics have been tested for clinical efficacy in several types of acute diseases including antibiotic-associated diarrhea, C. difficile infections, Helicobacter pylori (H. pylori) disease, acute adult diarrhea, enteral nutrition-related diarrhea, and traveler’s diarrhea.
Antibiotic-associated diarrhea
Epidemiology of antibiotic-associated diarrhea: The reported incidence of antibiotic-associated diarrhea (AAD) ranges from 12/100 000 to 34/100[122] depending upon the type of antibiotic, host factors (age, health status, etc.), etiology, hospitalization status and presence of a nosocomial outbreak. The highest frequency of AAD is found (26%-60%) during healthcare associated outbreaks, when susceptible patients are clustered by time, exposure and proximity[123]. Healthcare associated (hospital, long-term care facilities, nursing homes) outbreaks of AAD are to be expected because inciting agents (antibiotics), infectious agents and a susceptible patient population are intermixed. Historically, most cases of AAD were reported in hospitalized patients[122]. More recently, although AAD still occurs in hospital settings, rates of 6-33/100 have been reported in outpatient populations[124,125] and lower rates (12/100 000 to 14/100) in non-hospitalized adults[126,127]. Lower rates observed in outpatients may be due to their generally higher health status compared with hospitalized patients and also to the lack of exposure to nosocomial pathogens that commonly contaminate hospital environments. Higher incidences are still found in healthcare associated pediatric and adult patients (ranging from 5-34/100 patients)[128-130].
The clinical presentation of AAD may be mild (uncomplicated diarrhea) or more severe (colitis), or result in toxic megacolon or death[122]. The onset of AAD may occur while the patient is on antibiotics, but delayed-onset AAD is more common[122]. In addition, the onset of AAD may vary according to the type of antibiotic. Elmer et al[131] found a variable time of onset using the hamster model of AAD and different types of antibiotics. Early onset of AAD was associated with clindamycin, amoxicillin and ampicillin, while delayed-onset AAD was associated with erythromycin, ciprofloxacin and clarithromycin. Consequences of AAD may result in extended hospital stays, increased medical care costs and increased diagnostic procedures[122,132]. Prevention of AAD has rested on discontinuing the inciting antibiotic or switching to an antibiotic with a narrower spectrum of action, but there are no other current effective preventive measures for AAD.
Efficacy evidence for AAD: There have been ten randomized controlled trials in adults using S. boulardii for the prevention of AAD (Table 2)[37,42,44,54,55,58,133-136]. These studies have used a variety of daily doses, durations of treatment, length of follow-up and types of patient population. Of the ten controlled trials, 8 (80%) showed significant efficacy for the prevention of AAD compared with controls. One of the earliest trials by Adam et al[37], randomized 388 hospitalized patients to 200 mg of S. boulardii [4 × 109 cfu (colony forming units)/d] or placebo for seven days and found a significant reduction in AAD in the S. boulardii group (4.5%) compared with the controls (17.5%, P < 0.05). The protective effect of S. boulardii was confirmed in later studies. Monteiro et al[55] enrolled 240 patients receiving oral antibiotics and found significantly fewer patients randomized to S. boulardii developed AAD (15.7%) compared with placebo (27.7%, P < 0.05). McFarland et al[54] enrolled 193 hospitalized patients receiving beta-lactam antibiotics and randomized them to either 1 g of S. boulardii or placebo for the duration of the antibiotic treatment plus three additional days. Significantly fewer patients developed AAD while on the beta-lactam antibiotics or in the seven week follow-up period when given S. boulardii (7.2%, P < 0.05) compared with 14.6% of those given placebo. This study confirmed an earlier study by Surawicz et al[58] of 180 hospitalized adults randomized to either S. boulardii or placebo for the duration of their antibiotic treatment plus an additional two weeks. Of the 23% patients who were contacted 2-3 wk after the study, only one patient given placebo developed delayed-onset AAD. Only 9.5% of those randomized to S. boulardii developed AAD compared with 21.8% of those on placebo (P < 0.05). Can et al[135] enrolled 151 adult inpatients receiving various types of antibiotics, who were then randomized to S. boulardii or placebo for the duration of the antibiotic treatment. Significantly fewer patients (1.4%, P < 0.05) of those given S. boulardii developed AAD compared with the control group (9.0%). Three trials have been in conducted in patients with H. pylori infections receiving triple therapy (usually two antibiotics and an acid suppressor), which is associated with a high rate of AAD development. Duman et al[44] enrolled 389 patients with peptic ulcer or non-ulcer dyspepsia in nine hospitals in Turkey to observe if lower rates of side-effects could be achieved. This study compared 204 patients who received 1 g of S. boulardii (2 × 1010/d for 2 wk) and triple therapy with 185 controls, who received only the triple therapy. Of the 389 patients, 376 completed the treatment phase and the four-week follow-up. Significantly fewer patients given S. boulardii (6.9%) developed AAD compared with the control group (15.6%, P = 0.007). Two other randomized controlled trials in adult patients receiving triple therapy for H. pylori infections were conducted and both showed a significant reduction in AAD for those treated with S. boulardii. Cremonini et al[134] randomized 85 H. pylori carriers to placebo, L. rhamnosus GG, S. boulardii or a mix of L. acidophilus and Bifidobacterium lactis and found significantly fewer patients developed AAD in only the S. boulardii group (5%, P = 0.05) compared with placebo (30%). Cindoruk et al[42] also found a significant reduction in AAD in adult patients treated with triple therapy for H. pylori infections for those randomized to S. boulardii (14.5%, P < 0.05) compared with placebo (30.6%). None of these trials reported any adverse reactions associated with S. boulardii.
Table 2.
Randomized, controlled trials for the prevention of antibiotic associated diarrhea (AAD) using S. boulardii
| Ref. | Treatment groups | Population | Daily dose: cfu/d (mg/d) | Duration (d) | Follow-up (wk) | AAD in S. boularii (%) | AAD in controls (%) |
| Adam et al[37] | S. boulardii vs placebo | 388 hospitalized adults, multisite | 4 × 109 (200 mg) | 7 | 0 | 9/199 (4.5)a | 33/189 (17.5) |
| Monteiro et al[55] | S. boulardii vs placebo | 240 adults, Portugal | (nr) 4 caps/d | 6 | 0 | 19/121 (15.7)a | 33/119 (27.7) |
| Surawicz et al[58] | S. boulardii vs placebo | 180 hospitalized adults at one hospital, Washington | 2 × 1010 (1000 mg) | abx + 14 d | 2-3 | 11/116 (9.5)a | 14/64 (21.8) |
| McFarland et al[54] | S. boulardii vs placebo | 193 hospitalized adults (1 or more beta-lactam antibiotics), 4 hospitals | 2 × 1010 (1000 mg) | abx + 3 d | 7 | 7/97 (7.2)a | 14/96 (14.6) |
| Lewis et al[133] | S. boulardii vs placebo | 72 enrolled, 69 done; elderly patients | 4.5 × 109 (226 mg) | 14 | 0 | 7/33 (21) | 5/36 (13.9) |
| Cremonini et al[134] | S. boulardii vs placebo | 43 H. pylori + adults on triple therapy | 5 × 109 (nr) | 14 | 2 | 1/22 (5)a | 6/21 (30) |
| Duman et al[44] | S. boulardii vs placebo | 389 adults in Turkey with H. pylori + peptic ulcers, all received triple therapy | 2 × 1010 (1000 mg) | 14 | 4 | 14/204 (6.9)a | 28/180 (15.6) |
| Can et al[135] | S. boulardii vs placebo | 151 adult inpatients | 1 × 1010 (500 mg) | abx | 4 | 1/73 (1.4)a | 7/78 (9.0) |
| Cindoruk et al[42] | S. boulardiivs placebo | 124 adults with H. pylori + dyspepsia | 2 × 1010 (1000 mg) | 14 | 6 | 9/62 (14.5)a | 19/62 (30.6) |
| Bravo et al[136] | S. boulardiivs placebo | 89 adult outpatients on amoxicillin | 1 × 1010 (500 mg) | 12 | 9 | 3/41 (7.3) | 5/45 (11.1) |
P < 0.05, probiotic vs controls. cfu: Colony forming unit; nr: Not reported; abx: Antibiotics.
It is important to have a sufficiently long follow-up period (usually 4-6 wk after cessation of antibiotics) to capture delayed-onset AAD. Several studies have shown that AAD may occur in patients on antibiotics, but AAD may also be delayed for up to two months (in up to 38% of patients) after antibiotics are discontinued[54,129]. Most studies of AAD followed patients for 4-6 wk after antibiotic treatment to document delayed-onset AAD[42,44,135]. In contrast, Lewis et al[133] found no significant reduction in AAD in a study of 69 elderly patients randomized to 226 mg of S. boulardii (4.5 × 109 cfu/d) or placebo for 14 d. This failure may have been due to a flawed study design, in that patients were only followed while on antibiotics and no follow-up was done after antibiotics were discontinued. The study by Lewis et al[133] therefore, may have missed a significant number of AAD cases due to a too short follow-up period. Short or no follow-up after antibiotic exposure may explain why some studies[133,136] did not find a significant protective effect of S. boulardii[137]. No adverse reactions associated with S. boulardii were noted in any of these studies. No other strains of Saccharomyces have been found to be effective to prevent AAD.
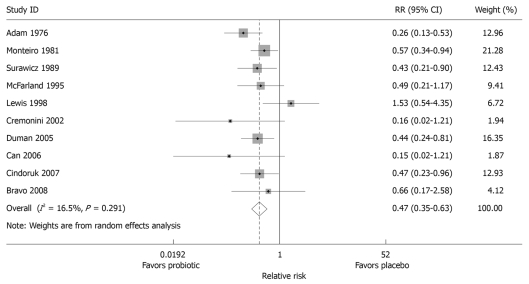
Meta-analyses for AAD: The literature search yielded 322 unique citations on S. boulardii, of which 278 were excluded: non-S. boulardii studies (n = 19), reviews (n = 84), pre-clinical studies including animal models of efficacy, mechanism of action, in vitro assays or strain characterization (n = 130), case reports or case series (n = 15), and phase II safety or pharmacokinetic studies (n = 30). Of the 44 randomized controlled trials with S. boulardii screened, 34 were excluded (pediatric populations, n = 17; or non-AAD indications in adults, n = 17) and 10 were included (AAD in adult populations). As significant heterogeneity was found among studies, a random effect model was used. The random effect model gave a χ2 = 10.8, P = 0.29, indicating that the heterogeneity was well controlled. A meta-analysis of the ten randomized, controlled trials in adults presented in this review found that S. boulardii was significantly protective for AAD (Figure 3), with a pooled relative risk of 0.47 (95% confidence interval 0.35-0.63, P < 0.001). Begg’s test did not find any significant publication bias (P = 0.93), nor did the funnel plot (data not shown). The number needed-to-treat (NTT) to prevent one case of AAD was 10.2.
Figure 3.
Forest plot of meta-analysis of ten randomized controlled trials measuring protective effect after treatment with S. boulardii or placebo for the prevention of antibiotic-associated diarrhea in adult patients. The x-axis depicts effect size, with black dot denoting the relative risk and the line indicating 95% CI, with the size of the grey box proportional to the study size. Relative risks to the left of RR = 1 denote study favored the probiotic, RR to the right denotes the study favors the placebo. Overall, pooled RR was 0.47 (0.35-0.63).
In the established medical literature, meta-analysis has been used to combine different probiotic strains and studies to obtain a pooled estimate of the efficacy of probiotics for the prevention of AAD. A note of caution, however, while most meta-analyses have concluded that probiotics are effective for preventing AAD[123,138], it is inappropriate to conclude that all probiotic strains are effective. The effectiveness of probiotics is strain-specific and disease-specific, so each probiotic strain must be linked to the disease. Most probiotic meta-analyses have focused on one type of disease indication with a variety of probiotic strains (e.g. antibiotic associated diarrhea)[123,138,139]. When there are sufficient numbers of clinical trials, a meta-analysis limited to one disease indication and one type of probiotic strain may reduce the heterogeneity of studies[140]. Szajewska et al[141] limited her meta-analysis to randomized controlled trials of one type of probiotic (S. boulardii) in adults and children. Pooling the results from five trials (involving 1076 subjects), a significantly protective effect of S. boulardii was found (pooled RR = 0.43, 95% CI: 0.23-0.78). As an alternative, some meta-analyses have done sensitivity analysis, separating out sub-groups by patient type (e.g. just adults or just children) or by type of probiotic strain[123,142]. One sensitivity analysis found with sufficient evidence that only two probiotic strains had significant efficacy for the prevention of AAD, i.e. S. boulardii and L. rhamnosus GG. However, L. rhamnosus GG probiotics may be contraindicated that this strain is susceptible when the patient is prescribed a type of antibiotic[123]. Although many meta-analyses have been done, pooling studies must be performed with these limitations in mind.
CDI
Epidemiology of C. difficile disease: For over two decades, Clostridium difficile infections continue to persist as a leading cause of nosocomial gastrointestinal illness[143-146]. The incidence rates of CDI have been increasing globally. In the US, CDI doubled to 301 200 cases from 2001 to 2005[147]. It is estimated that 450 000-750 000 CDI cases will occur per year by 2010 in the US[148,149]. Outbreaks of an emergent strain, BI/NAP1/027, caused large outbreaks of severe CDI with a high mortality rate in Canada between 2003-2005[150]. Studies have documented that CDI extends patients’ hospital stays from 4 to 36 d[151-154]. In a study of 1034 CDI cases in Massachusetts in 2000, the average cost ranged from $10 212-$13 675/patient[155], leading to a nation-wide cost of $3.2 billion/year for CDI. There are only two standard antibiotics for CDI (vancomycin and metronidazole) and the response rate of metronidazole has been declining[152]. In addition, 20%-60% of patients may develop recurrent episodes of CDI despite additional antibiotic treatment. Although other investigational antibiotics are under development, no new antibiotics are superior to the two standard antibiotics.
Efficacy evidence for C. difficile disease: Probiotics may offer promise as an adjunctive therapy (given along with standard antibiotics vancomycin or metronidazole) for CDI. A meta-analysis of six randomized controlled trials of different probiotics (S. boulardii, Lactobacillus rhamnosus GG, L. planatarum 299v, and a mix of L. acidophilus and Bifidobacterium bifidum showed probiotics, in general, had a overall significant efficacy to prevent subsequent recurrences of C. difficile disease (RR = 0.59, 95% CI: 0.41-0.85, P = 0.005)[123]. However, due to limited number of trials, no meta-analysis was conducted for one probiotic strain.
S. boulardii shows promise for C. difficile infection control at several levels: it produces a 54-kDa serine protease that directly degrades Clostridium difficile toxins and also directly destroys the colonic receptor site for C. difficile[76], S. boulardii increases the immune response to Clostridium difficile toxins A and B[108], and animal models of C. difficile disease respond to this yeast. In addition, case reports or small case series of patients with recurrent C. difficile diarrhea treated with S. boulardii showed improvement. Toothaker and Elmer found that significantly fewer (51%) hamsters challenged with C. difficile died if given S. boulardii (5% solution) compared with the saline controls (80%)[156]. Subsequent studies confirmed this efficacy in other animal models: gnotobiotic mice[41,157,158], rats[89,103], and turkeys[159]. Since 1989, case reports or small case series have reported that patients with recurrent CDAD were either cured or improved by S. boulardii treatment[40,59,160,161].
There was a case report[162] and two small case series with 3-4 patients[163,164] with recurrent C. difficile disease who seemed to have responded to S. cerevisiae administration. However, since no comparison groups or controls were used, there is no foundation to claim the efficacy of other strains of Saccharomyces for C. difficile disease.
There have been two randomized, double blinded, controlled trials for the use of S. boulardii and antibiotics for patients with recurrent C. difficile disease (Table 3)[53,60]. In a multisite, randomized, double-blinded, placebo-controlled trial of 168 patients with recurrent C. difficile disease, standard antibiotics were combined with Saccharomyces boulardii or placebo[60]. Three antibiotic regimens were used for 10 d: either high-dose vancomycin (2 g/d), low-dose vancomycin (500 mg/d) or metronidazole (1 g/d). Then either S. boulardii or placebo (1 g/d for 28 d) was added to the antibiotic treatment. The patients were followed up for two months for subsequent Clostridium difficile recurrences. A significant decrease in recurrences was observed only in patients treated with the high-dose vancomycin and S. boulardii treatment (16.7%) compared with patients who received high-dose vancomycin and placebo (50%, P = 0.05). There were no significant reductions in recurrence rates in either the low-dose vancomycin or metronidazole treatment group, regardless if S. boulardii was used. No serious adverse reactions were noted in any of these patients. By the end of the antibiotic treatment, only high-dose vancomycin completely cleared C. difficile toxin from the colon, low-dose vancomycin and metronidazole could not clear the toxin. A subsequent investigation of 163 patients with recurrent CDI confirmed this finding[165]. In this study, stool samples were assayed at the end of antibiotic treatment to determine if there was a difference in toxin clearance by the type or dose of antibiotic. Treatment with high-dose (2 g/d) of vancomycin completely cleared C. difficile toxin, but 11% of those treated with lower doses of vancomycin and 41% treated with metronidazole were positive for C. difficile at the end of antibiotic treatment. S. boulardii may be more effective if complete C. difficile toxin clearance is achieved before sole reliance upon the yeast probiotic is required.
Table 3.
Randomized controlled trials for the treatment of C. difficile disease using S. boulardii
| Ref. | Treatment groups | Study population | Daily dose: cfu/d (mg/d) | Duration of treatment (wk) | Follow-up(wk) | C. difficile recurrence in probiotic group | C. difficile recurrence in placebo group |
| McFarland et al[53] | S. boulardii vs placebo | 124 adult patients on varied doses of vancomycin or metronidazole; recurrent and initial CDAD cases; 3 referral sites, US | 3 × 1010 (1000 mg) | 4 | 4 | 15/57 (26.3%)a | 30/67 (44.8%) |
| Surawicz et al[60] | S. boulardii vs placebo | 168 adult patients recurrent CDAD; on vancomycin (2 g/d, n = 32) or V (500 mg/d, n = 83) or M (1 g/d, n = 53); 4 referral sites, US | 2 × 1010 (1000 mg) | 4 | 4 | V (2 g/d) 3/18 (17%)a; V (500 mg/d) 23/45 (51%); M (1 g/d) 13/27 (48.1%) | V (2 g/d) 7/14 (50%); V (500 mg/d) 17/38 (44.7%); M (1 g/d) 13/26 (50%) |
P < 0.05, probiotic vs controls. V: Vancomycin; M: Metronidazole.
An earlier randomized, controlled double-blinded study had also found efficacy of the combination treatment using a standard antibiotic (vancomycin or metronidazole) and S. boulardii for patients with C. difficile disease[53]. This study did not control the dose or duration of either vancomycin or metronidazole (it was under the physician’s discretion), but randomized patients to either S. boulardii (1 g/d) or placebo for 28 d. All patients were followed up for two months for subsequent recurrences. Approximately half of the enrolled patients had their first episode and half had recurrent C. difficile disease. Twenty-six percent of the 57 patients given S. boulardii had a recurrence of C. difficile disease, which was significantly fewer as compared with 45% of the 67 given placebo (P < 0.05). The strongest effect was found in the 60 patients with recurrent C. difficile disease; significantly fewer patients (35%) given S. boulardii had a recurrence compared with 65% of patients given placebo (P = 0.04). Two adverse reactions were associated with S. boulardii (thirst and constipation). No randomized controlled trials using stains of S. cerevisiae for this disease were found in the literature.
H. pylori
Epidemiology of H. pylori: H. pylori colonizes the gastric mucosa and usually induces a chronic, asymptomatic carrier state, but some people may develop gastroduodenal ulcers as a result. Carriage of H. pylori is a risk factor for developing gastric lymphoma or adenocarcinoma in later life. The prevalence of H. pylori carriage is 50%-80% worldwide[166,167]. Standard treatment for H. pylori involves a two-week course of triple therapy (usually clarithromycin, amoxicillin and an acid suppressor such as lansoprazole or omeprazole), that also has gastrointestinal side effects.
Efficacy for H. pylori: S. boulardii induces morphologic changes in H. pylori cells consistant with cellular damage[21] and was shown to reduce 12% of H. pylori colonization in infected children in one trial[46]. Of four randomized controlled trials testing S. boulardii in H. pylori infections, two were in children[46,168] and two (Table 4) were in adults[42,134]. Cremonini et al[134] first explored the efficacy of probiotics to eradicate H. pylori in 85 asymptomatic carriers. They randomized patients to one of three probiotic treatments (S. boulardii, L. rhamnosus GG or a mixture of L. acidophius and Bifidobacterium lactis) or placebo for 14 d. All patients were treated with the triple therapy for the first week. By the end of the second week, eradication rates were similar for all groups (81% for S. boulardii vs 80% of placebo). A promising result of this study was a lower rate of antibiotic-associated diarrhea (5%) in all the probiotic groups compared with 30% of the placebo group. A subsequent study by Cindoruk et al[42] assessed S. boulardii for both eradication of H. pylori and the reduction of side-effects of the standard triple treatment. This study enrolled 124 adults with H. pylori dyspepsia in Turkey who were receiving the triple therapy and randomized to either 1 g of S. boulardii (2 × 1010/d for 2 wk) or placebo. Patients were followed up for six weeks for side-effects and H. pylori clearance. Although there was no significant difference in H. pylori eradication (71% in S. boulardii vs 60% in placebo), significantly fewer patients randomized to S. boulardii reported epigastric distress (14.5%, P < 0.05) compared with placebo (43.5%), as well as lower global dyspepsia symptom scores. The frequency of AAD was also significantly reduced in those receiving S. boulardii (14.5%) compared with the placebo group (30.6%). These studies indicate that S. boulardii may not be effective in eradicating H. pylori itself, but it is effective in reducing the side-effects of the standard triple therapy.
Table 4.
Randomized, controlled trials for acute disease conditions using S. boulardii
| Ref. | Treatment groups | Study population | Daily dose: cfu/d (mg/d) | Duration of treatment | Follow-up(wk) | Outcome in probiotic | Outcome in controls |
| Helicobacter pylori | |||||||
| Cremonini et al[134] | S. boulardii (Codex, SmithKine Beecham, Italy) vs placebo vs LGG vs mix (L acid and Bifid lactis) | 85 asymptomatic carriers H. pylori 22 SB and 21 placebo; all received triple therapy | 5 × 109 (nr) | 2 wk | 2 | Eradicated in: 17/21 (81%) same for LGG and mix | Placebo, 16/20 (80%) |
| Any symptoms: 4/21 (19%)a | Any symptoms: 12/20 (60%) | ||||||
| Cindoruk et al[42] | S. boulardii (Reflor, Turkey) vs placebo | 124 adults with H. pylori dyspepsia, all received triple therapy | 2 × 1010 (1000 mg) | 2 wk | 6 | Eradication 44/62 (71%) | Eradication 37/62 (59.7%) |
| Epigastric distress: 9 (14.5%)a | Distress: 27 (43.5%) | ||||||
| Acute adult diarrheas | |||||||
| Hochter et al[169] | S. boulardii vs placebo | 92 German outpatient adults | 8 × 109 (300-600 mg) | 8 d | 0 | Diarrhea score reduction: -17.2 + 6.8a | Score: -13.6 + 8.7 |
| Mansour- ghanaei et al[51] | S. boulardii (UltraLevure, France) vs placebo. Both groups got metro and lodoquinol | 57 enrolled, 54 done; adults with E. histolytica amoebic dysentery | 1.5 × 1010 (750 mg) | 10 d | 4 | Cured: 27/27 (100%)a | Cured: 5/27 (19%) |
| Enteral feeding-related diarrhea | |||||||
| Tempe et al[61] | S. boulardii vs placebo | 40 adults in ICU | 1 × 1010 (nr) | 11-21 d | 0 | 34/389 days of diarrhea (8.7%)a | 63/373 days of diarrhea (16.9%) |
| Schlotterer et al[171] | S. boulardiivs placebo | 20 enrolled, 18 done, burnt adults 18-70 yr | 4 × 1010 (2000 mg) | 8-28 d | 0 | 3/204 days of diarrhea (1.5%)a | 19/208 days of diarrhea (9.1%) |
| Bleichner et al[39] | S. boulardii vs placebo | 131 enrolled, 128 done, adults in ICU | 4 × 1010 (2000 mg) | 3 wk | 0 | 50/648 days of diarrhea (7.7%)a | 87/683 days of diarrhea (12.7%) |
| Traveler’s diarrhea | |||||||
| Kollaritsch et al[175] | S. boulardii vs placebo | 832 Austrian tourists to hot climates | 2 × 109 (250 mg) | 5 d before trip and mean 21 d trip | 0 | 143/426 (34%)a | 173/406 (43%) |
| Kollaritsch et al[175] | S. boulardii vs placebo | 805 Austrian tourists to hot climates | 5 × 109 (500 mg) | 5 d before trip and mean 21 d trip | 0 | 127/399 (32%)a | 173/406 (43%) |
| Kollaritsch et al[49] | S. boulardii vs placebo | 713 Austrian tourists to hot climates | 2 × 109 (250 mg) | 5 d before trip and mean 21 d trip | 0 | 121/352 (34%)a | 141/361 (39%) |
| Kollaritsch et al[49] | S. boulardii vs placebo | 664 Austrian tourists to hot climates | 2 × 1010 (1000 mg) | 5 d before trip and mean 21 d trip | 0 | 87/303 (29%)a | 141/361 (39%) |
| Bruns et al[176] | S. cerevisiae Hansen (CBS 5926) vs active treatment1 | 60 tourists in Tunisia with traveler’s diarrhea, 43 done | 1.0 × 1010 (600 mg) | 5 d | 0 | Duration diarrhea 2.1 d | 1.4 days of diarrheaa |
P < 0.05, probiotic vs controls;
Strain currently known as S. boulardii.
Acute adult diarrheas
Epidemiology of acute adult diarrhea: Acute adult diarrhea is a broad classification for diarrhea that includes illnesses that may develop quickly, but are typically short-lived and are sporadic. The etiologies of acute adult diarrhea may include infectious agents (Entamoeba histolytica, E. coli, or Salmonella) or may be idiopathic. Diarrhea in adults occurring in outbreaks or due to other situations (antibiotic-associated diarrhea, C. difficile-associated diarrhea, traveler’s diarrhea, inflammatory bowel disease or irritable bowel disease) are not included in this classification.
Clinical efficacy for acute adult diarrhea: Two randomized controlled trials using S. boulardii showed that this probiotic may be effective in treating acute diarrhea due to a variety of causes. Hochter et al[169] enrolled 92 German adult outpatients with acute diarrhea and randomized them to S. boulardii (8 × 109/d) or placebo for eight days. By the third day, the 43 given S. boulardii had a significantly lower diarrhea severity score (5.5 ± 6.8, P = 0.04) compared with the 49 given placebo (6.7 ± 8.7). Mansour-Ghanaei et al[51] enrolled 57 patients with acute Entamoeba histolytic dysentery and treated them with S. boulardii (1.5 × 1010/d for 10 d) or nothing (control). Both groups also received metronidazole and iodoquinol for 10 d. At the end of four weeks, all the patients given S. boulardii were cured compared with 19% of those not given the yeast. Unfortunately, the number of trials in this area is small and the etiologies were different in the two trials, so conclusions are limited. No randomized controlled trials using stains of S. cerevisiae for this disease were found in the literature.
Enteral nutrition-related diarrhea
Epidemiology of enteral nutrition-related diarrhea: Diarrhea is a common complication associated with enteral tube feeding and may result in a loss of nutrition in an already seriously ill patient. The frequency of diarrhea in enteral tube fed patients has been reported as high as 50%-60%[170] and complications may include life-threatening acidosis, increased morbidity and mortality and increased healthcare costs. Schneider et al[102] reported a significant increase in short-chain fatty acids in 10 enteral-fed patients receiving S. boulardii (1 g/d for 6 d) compared with 15 healthy controls.
Clinical efficacy for enteral nutrition related diarrhea: Three randomized, controlled trials have assessed the ability of S. boulardii to reduce diarrhea in patients receiving this type of nutritional intake (Table 4). Tempe et al[61] compared 40 enteral-fed patients randomized to either S. boulardii (1 × 1010/d) or placebo for 11-21 d and found significantly fewer patients (8.7%) given S. boulardii developed diarrhea compared with placebo (16.9%). Schlotterer et al[171] randomized 18 patients with burns who were receiving enteral nutrition to S. boulardii (4 × 1010/d) or placebo for 8-28 d. Those given S. boulardii suffered fewer diarrhea days (3/204 d, 1.5%) than those given placebo (19/208 d, 9.1%, P < 0.001). The efficacy of S. boulardii was confirmed in a later study of 128 intensive care unit (ICU) patients randomized to either S. boulardii (4 × 1010/d) or placebo for 21 d[39]. Those given S. boulardii reported significantly fewer diarrheal days (7.7%) than those given placebo (12.7%). No adverse reactions associated with S. boulardii were reported in any of these three trials.
Traveler’s diarrhea
Epidemiology of traveler’s diarrhea: Traveler’s diarrhea (TD) is a common health complaint among travelers; globally 12 million cases are reported every year[172]. Rates of TD range from 5% to 50%, depending upon the destination, with equatorial countries having the highest rates of TD. TD usually occurs as sporadic cases, but outbreaks of TD may occur in large groups traveling or located together (tourists on cruise ships, group tours, military personnel, disaster-relief groups). The etiologies of TD vary widely, but commonly include enterotoxigenic and entroaggregative E. coli, Campylobacter jejuni, Salmonella typhimurum, viruses (Norwalk or Rotavirus) or parasites (Entamoeba histolytica, Giardia lamblia). Traditional preventive measures including frequent handwashing, avoiding high-risk foods and water and ingestion of bismuth subsalicyclate showed only modest protection[173].
Clinical efficacy for TD: A meta-analysis of 12 randomized, controlled trials of various probiotics (including S. boulardii, Lactobacilli and mixtures of different probiotic strains) for the prevention of TD found a significant reduction in the risk of TD if probiotics were used (RR = 0.85, 95% confidence interval 0.79-0.91)[174]. Two randomized controlled trials have been done with S. boulardii that included a total of four different probiotic treatment arms (Table 4). Kollaritsch et al[175] enrolled 1231 Austrian tourists traveling to hot climates and randomized travelers to either of two doses of S. boulardii (250 or 500 mg/d) or placebo for 3 wk. The treatment was started 5 d prior to the trip and continued through the duration of the trip. Traveler’s diarrhea developed in 43% given placebo and significantly fewer in those given the low-dose S. boulardii (34%) and the higher dose S. boulardii (32%). A second study by Kollaritsch et al[49] was done with 3000 Austrian tourists traveling to northern African, the Middle East and the Far East. Travelers were randomized to either 250 mg/d (5 × 109) S. boulardii, 1000 mg/d (2 × 1010) S. boulardii or placebo, starting five days before leaving and throughout the duration of their trip (median of 3 wk). Only 1016 (34%) completed the study, but S. boulardii significantly reduced the incidence of traveler’s diarrhea in a dose-dependent manner. Of the travelers given placebo, 39% developed diarrhea whereas only 34% of those given the lower dose and 29% of those given the higher dose of S. boulardii developed traveler’s diarrhea (P < 0.05). No adverse reaction was noted by the travelers in either of these studies.
Bruns et al[176] tested S. cerevisiae Hansen CBS 5926 (Perenterol®, Germany) to treat travelers who had developed TD in Tunisia. Travelers with TD were randomized to 600 mg of S. cerevisiae (1 × 1010/d) for five days or an active control treatment and the duration of their TD tracked. Of 60 enrolled, 43 completed the trial, but the duration of diarrhea was not significantly different between those given S. cerevisiae (2.1 d) and those given ethacridine-lactate and tannalbuminate (1.4 d). This strain is known as S. boulardii outside of Germany. No other randomized controlled trials have been done for the treatment of TD using S. boulardii. These limited numbers of studies indicate that probiotics may be more effective in preventing TD, rather than treating the diarrhea once it becomes symptomatic.
CLINICAL EFFICACY: CHRONIC DISEASES
S. boulardii has been tested for clinical efficacy in several types of chronic diseases including Crohn’s disease, irritable bowel syndrome, giardiasis and human immunodeficiency virus (HIV)-related diarrhea.
Inflammatory bowel disease
Epidemiology of inflammatory bowel disease: inflammatory bowel diseases (IBDs) are chronic, immune-related inflammatory diarrheas, which include ulcerative colitis, pouchitis and Crohn’s disease. The incidence of Crohn’s disease varies by countries, with the highest incidences (8-66/100 000) found in Wales, New Zealand, Canada, Scotland, France, Netherlands, intermediate rates (4-7/100 000) in UK, USA, Europe, Australia, and the lowest rates (0.2-3/100 000) in South America, China, Korea, Japan[177]. In Crohn’s disease, typically the lower small intestine and colon are the most affected organs. Symptoms include diarrhea, abdominal pain or cramping and loss of appetite. Unlike ulcerative colitis, lesions of Crohn’s disease are deep and patchy and often involve thickened areas resulting in intestinal obstruction, which can be life threatening. Disruption of the intestinal wall also allows intestinal bacteria to translocate and incite the immune system. Complications of Crohn’s disease are common, in that 40% of Crohn’s patients report lower GI bleeding, intestinal obstruction, or perforation[178]. Lifetime risk for surgery in Crohn’s patients is extremely high (70%-80%)[179]. Unfortunately, the cause of Crohn’s disease is not known, so current therapies are directed at symptom relief. But 10%-60% suffer recurrences of Crohn’s disease after treatment is completed[180].
Clinical efficacy for IBD: Guslandi et al[48] enrolled 25 adults with mild to moderate ulcerative colitis in a pilot study and treated them with a combination of mesalazine (3 g/d) and S. boulardii (1.5 × 1010/d) for 4 wk. All of these patients had histories of poorly tolerated steroid courses. Most (68%) of the patients responded to the probiotic treatment. This study had a promising result, but the implications were uncertain as patients treated for only a short time, were not followed up for subsequent flare-ups of their disease and there was no control group in this pilot study. Garcia Vilelea et al[62] enrolled 31 patients with Crohn’s disease in remission (at the time of enrollment). All patients continued their maintenance medications during the trial (mesalamine, azathioprine or others). Patients were randomized to either S. boulardii (2 × 109/d) for three months or placebo. Those treated with S. boulardii were found to have a significant reduction in colonic permeability compared with those given placebo, thus reducing the risk of translocation in these patients.
Two randomized controlled clinical trials have been done testing S. boulardii for patients with Crohn’s disease (Table 5)[47,57]. Plein et al[57] randomized 20 patients with Crohn’s disease to either 750 mg of S. boulardii (1.5 × 1010/d) or placebo for seven weeks. All patients continued their maintenance medications during the trial. At the end of seven weeks, patients treated with S. boulardii were significantly improved (mean = 3.3 ± 1.2 stools/d, P < 0.05) compared with the placebo group (mean = 4.6 ± 1.9 stools/d). Guslandi et al[47] studied the effect of S. boulardii on the rate of Crohn’s disease relapses in a study of 32 patients with Crohn’s disease in Italy. Adults (23-49 years old) who were in remission were randomized to either 1 g of S. boulardii (2 × 1010/d) and mesalamine (2 g/d) or mesalamine alone (3 g/d) for 6 mo. Significantly fewer patients treated with S. boulardii (6%, P = 0.04) relapsed than the control group (38%). No adverse reactions associated with S. boulardii were reported in any of these trials.
Table 5.
Randomized, controlled trials for chronic disease conditions using S. boulardii
| Ref. | Treatment groups | Study population | Daily dose: cfu/d (mg/d) | Duration of treatment and follow-up | Outcome in probiotic | Outcome in controls |
| Crohn’s disease | ||||||
| Plein et al[57] | S. boulardii (Perenterol®) vs placebo | 20 enrolled with Crohn’s, 17 done, all on maintenance medications | 1.5 × 1010 (750 mg) | 7 wk | 3.3 ± 1.2 stools/d at week 9a | 4.6 ± 1.9 stools/d at week 9 |
| Guslandi et al[47] | S. boulardii (Perenterol®) + mesalamine (2 g/d) vs mesalamine alone (3 g/d) | 32 with Crohn’s in remission in Italy (23-49 yr old) | 2 × 1010 (1000 mg) | 6 mo | 1/16 (6%) relapsea | 6/16 (38%) relapse |
| Irritable bowel syndrome | ||||||
| Maupas et al[52] | S. boulardii vs placebo | 34 with IBS | 9 × 109 (nr) | 4 wk | Mean decrease in #stools/d: -2.2/da | Mean decrease -0.5/d |
| Improved: 14/16 (87.5%)a | Improved: 13/18 (72%) | |||||
| Giardiasis | ||||||
| Besirbellioglu et al[38] | S. boulardii + metro (2250 mg/d) vs placebo + metro | 65 adults with giardiasis | 1 × 1010 (500 mg) | 10 d with 4 wk f/up | 100% cured, 0% giardia cystsa | 100% cured 6/35 (17%) cysts present |
| HIV-related diarrhea | ||||||
| Saint- Marc et al[189] | S. boulardii vs placebo | 35 French AIDS patients with chronic diarrhea | 6 × 1010 (3000 mg) | 7 d | 11/18 (61%) cureda | 2/17 (12%) |
P < 0.05, probiotic vs controls. IBS: Irritable bowel syndrome; HIV: Human immunodeficiency virus; AIDS: Acquired immune deficiency syndrome; f/up: Follow-up time.
Crohn’s disease is a challenging condition for clinical trials in that it is sporadic, has no defined etiology, has multiple measurable disease outcome and requires lengthy treatment and follow-up period. S. boulardii offers promise to this group of patients who are in need of a safe and effective therapy for this chronic condition. The probiotic protection seems to be strain specific. A study tested another strain of S. cerevisiae (Baker’s yeast) in 19 adults with Crohn’s disease, but found the disease was worse in those receiving this strain than in controls[181].
Irritable bowel syndrome
Epidemiology of irritable bowel syndrome: Irritable bowel syndrome (IBS) is a frequent disorder characterized by a triad of symptoms (bloating, abdominal pain, and intestinal transit disturbances). The prevalence of IBS is high (3%-20%) in the general population and IBS may account for 25%-50% of gastroenterologist practice[182]. Consequences of IBS include worse quality of life[183], higher health care costs ($1.7 billion in health care costs)[184], and higher incidences of depression[185].
Clinical efficacy for IBS: Standard treatments target symptom relief, but have not been more effective than placebo. IBS is a recent focus of probiotic clinical trials. A meta-analysis of 20 randomized, controlled trials (with 23 treatment arms) including 1404 subjects found a pooled relative risk (RR) for improvement in global IBS symptoms in 14 probiotic treatment arms (RR = 0.77, 95% CI: 0.62-0.94)[36]. One of those trials (Table 5) randomized 34 patients with IBS to S. boulardii (9 × 109/d) or placebo for four weeks[52]. A significant decrease in the daily number of stools was found in the S. boulardii group and 87.5% of this group reported their IBS had improved compared with 72% of the placebo group (P < 0.05). No adverse reactions were noted in this trial. More trials using S. boulardii for IBS are required.
Giardiasis
Epidemiology of giardiasis: This condition is characterized by long lasting diarrhea with symptoms ranging from mild to severe diarrhea, weight loss, abdominal pain and weakness. The incidence may be high in developing countries such as Colombia (13%) and lower in developed countries such as Germany (12/100 000)[186,187]. It is also found in people enjoying outdoor hiking and camping who drink contaminated untreated water that appears clean.
Clinical efficacy for giardiasis: Besirbellioglu et al[38] randomized 65 adults with giardiasis in Turkey to either S. boulardii (1 × 1010/d) or placebo for 10 d. Both groups also received metronidazole for the same duration. Two weeks later, both groups reported a resolution of their diarrhea, but none of those on S. boulardii had detectable giardia cysts, while significantly more (17%) on placebo still carried giardia cysts.
HIV diarrhea
Epidemiology of HIV-related diarrhea: Patients infected with HIV are susceptible to a variety of diseases and may also develop chronic life-threatening diarrhea. The prevalence of diarrhea in HIV patients ranges from 50%-60% in developed countries and nearly 100% in developing countries[188].
Clinical efficacy for HIV-related diarrhea: A blinded, placebo-controlled dose-ranging study was done in 11 HIV positive patients who had chronic diarrhea[45]. S. boulardii was given at 1-3 g/d and six patients reported diarrhea was controlled while taking 3 g/d after one month, while lower doses of S. boulardii were not as effective. Patients with HIV-associated diarrhea seem to be one group that requires a higher than typical dose of S. boulardii. Saint-Marc et al[189] randomized 35 Stage IV AIDS adult patients with chronic diarrhea to either S. boulardii (3 g/d or 6 × 1010/d) or placebo for one week (Table 5). Significantly more of those given S. boulardii (61%, P = 0.002) had their diarrhea resolved compared with placebo (12%) patients. S. boulardii was well tolerated by all HIV patients even treated with high doses of 3 g/d in these two studies.
SAFETY OF S. BOULARDII
The use of a living organism as therapy increases the potential risk in four general areas: transfer of antibiotic-resistance genes, translocation of the living organism from the intestine to other areas of the body, persistence in the intestines and the development of adverse reactions. The first three theorical concerns are of minimal impact on S. boulardii. Unlike other bacterial strains of probiotics, such as Enterococcus faecium and Lactobacillus rhamnosus, which have been shown to acquire antibiotic-resistance genes, S. boulardii has not developed any antibiotic or antifungal resistance[190,191]. Data from animal models shows reduced translocation with S. boulardii treatment[64,89], unlike other strains of S. cerevisiae[192]. As pharmacokinetic studies indicate, S. boulardii does not persist 3-5 d after oral ingestion is discontinued, so persistence is not a concern for this probiotic[118].
S. boulardii has been used as a probiotic since the 1950s in Europe and has been investigated in clinical trials worldwide. Safety and adverse event data collected during clinical trials, when patients are closely monitored for problems associated with the investigational treatment, has documented a remarkable safety profile of S. boulardii in adults. A wide diversity of adult patients have been followed up who were either randomized to S. boulardii as part of a clinical trial or were documented in case series or case reports for various disease indications (TD, n = 1596; AAD, n = 958; adult acute diarrhea, 156; enteral tube feeding, n = 103; IBD, n = 66; IBS, n = 16, HIV-related diarrhea, n = 18 and giardia infections, n = 50). These studies provide safety data for a total of 2963 adult patients and the only adverse reactions associated with S. boulardii was thirst (in 5 patients) and constipation (in 8 patients) in a trial of patients with C. difficile infections[53]. No case of S. boulardii fungemia has been reported in these diverse patient populations enrolled in clinical trials.
Infrequent cases of fungemia have been reported in case reports or case series in the literature. Most of the adult cases of S. boulardii fungemia are with serious co-morbidities and have central venous catheters. Most cases responded well to treatment with fluconazole or amphotericin B[193]. Some cases developed fungemia, not from the direct ingestion of S. boulardii probiotics, but acquired the yeast from contaminated environmental fomites[194]. Fungemia from S. cerevisiae (non-boulardii strains) have also been reported and are similar to S. boulardii cases, but have poorer prognosis[195,196]. A challenge to determining valid incidence of fungemia is the lack of available advanced yeast identification assays which can distinguish S. cerevisiae from S. boulardii.
CONTRAINDICATIONS/PRECAUTIONS
Even though fungemia with S. boulardii is infrequent, it may be prudent to closely follow up the adult inpatients who are severely ill or in intensive care units and have central catheter for episodes of unexplained fever. Some studies have recommended not to give S. boulardii to immunosuppressed patients or those with central catheters to reduce this risk[108].
Unlike many bacterial probiotics, there are few drug or antibiotic interactions with S. boulardii. However, if patients are on anti-fungal medications, a staggered dose regime (at least 4 h apart) is suggested[45].
CONCLUSION
The use of S. boulardii as a therapeutic probiotic is supported by its mechanisms of action, pharmacokinetics, and efficacy from animal models and clinical trials. The overall safety profile for S. boulardii is beneficial. S. boulardii can be recommended for several diseases. Other strains of Saccharomyces may have probiotic properties, but clinical efficacy evidence for other strains are still deficient. Guidelines for the most effective use of S. boulardii are given in Table 6 and generally, the daily dose is typically > 109/d, the duration of treatment can vary from 7 d to 6 mo and it may be given alone or as an adjunctive treatment, depending upon the disease indication. S. boulardii is significantly effective for the prevention of AAD and prevention of TD. Trials also show evidence for S. boulardii in the reduction of side-effects of H. pylori treatment and the prevention of enteral nutrition-related diarrhea. More clinical trials are encouraged for the treatment of chronic diseases (Crohn’s disease, irritable bowel syndrome and HIV-related diarrhea) and the prevention of C. difficile disease recurrences.
Table 6.
Summary of recommendations for clinical use of S. boulardii in adults
| Use for disease | Dose (mg/d) | Duration | Adjunct to | Strength of evidence1 |
| Prevention of antibiotic associated diarrhea | 500-1000 | During antibiotics with additional 3 d to 2 wk after | Nothing | ++++ |
| Prevention of Traveler’s diarrhea | 250-1000 | Duration of trip (3 wk) | Nothing | +++ |
| Enteral nutrition-related diarrhea | 2000 | 8-28 d | Nothing | ++ |
| H. pylori symptoms | 1000 | 2 wk | Standard triple therapy | ++ |
| Treatment of Clostridium difficile infections | 1000 | 4 wk | Vancomycin or metronidazole | + |
| Acute adult diarrhea | 500-750 | 8-10 d | Nothing | + |
| Inflammatory bowel disease | 750-1000 | 7 wk to 6 mo | Mesalamine | + |
| Irritable bowel syndrome | 500 | 4 wk | Nothing | + |
| Giardiasis | 500 | 4 wk | Metronidazole | + |
| HIV-related diarrhea | 3000 | 7 d | Nothing | + |
Strength of evidence, + (weak, needs more randomized controlled trials) to ++++ (strong, efficacy and safety are evidence based from numerous large randomized controlled trials).
Acknowledgments
The author had full access to all of the data in the Meta-nalysis and takes responsibility for the integrity of the data and accuracy of the data analysis. The author has been a paid speaker/consultant for the following companies: Acambis, Biocodex, Lallemand, Massachusetts Biologic Laboratories and Osel, Inc.. The author is not currently employed nor owns stock or equity in any of these companies. The views expressed in this article are those of the author and do not represent the views of the Department of Veterans Affairs.
Footnotes
Peer reviewer: Andrew S Day, MB, ChB, MD, FRACP, AGAF, Associate Professor, Department of Paediatrics, University of Otago, Christchurch, PO Box 4345, Christchurch 8140, New Zealand
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Vanderhoof JA, Young R. Probiotics in the United States. Clin Infect Dis. 2008;46 Suppl 2:S67–S72; discussion S144-S151. doi: 10.1086/523339. [DOI] [PubMed] [Google Scholar]
- 2.Amagase H. Current marketplace for probiotics: a Japanese perspective. Clin Infect Dis. 2008;46 Suppl 2:S73–S75; discussion S144-S151. doi: 10.1086/523338. [DOI] [PubMed] [Google Scholar]
- 3.Saxelin M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin Infect Dis. 2008;46 Suppl 2:S76–S79; discussion S144-S151. doi: 10.1086/523337. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman FA, Heimbach JT, Sanders ME, Hibberd PL. Executive summary: scientific and regulatory challenges of development of probiotics as foods and drugs. Clin Infect Dis. 2008;46 Suppl 2:S53–S57. doi: 10.1086/523342. [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization. Joint FAO/WHO Working Group Report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada. May 1, 2002. Accessed November 25, 2009. Available from: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. [Google Scholar]
- 6.Block G, Jensen CD, Norkus EP, Dalvi TB, Wong LG, McManus JF, Hudes ML. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: a cross-sectional study. Nutr J. 2007;6:30. doi: 10.1186/1475-2891-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Møller PL, Michaelsen KF, Paerregaard A, Sandström B, Tvede M, Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmer GW, McFarland LV, McFarland M. Chapter 1. Introduction. In: The power of probiotics: improving your health with beneficial microbes., editor. Binghamton, NY: Haworth Press; 2007. pp. 1–24. [Google Scholar]
- 9.Homayouni A, Azizi A, Ehsani M, Yarmand M, Razavi S. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. [Google Scholar]
- 10.Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. 2008;42:239–243. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009;15:274–280. doi: 10.1016/j.anaerobe.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 13.Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870–876. doi: 10.1001/jama.275.11.870. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RL, Booth A, Sutton AJ, Miller T, Smith JA, Young B, Jones DR, Dixon-Woods M. Finding qualitative research: an evaluation of search strategies. BMC Med Res Methodol. 2004;4:5. doi: 10.1186/1471-2288-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448–1454. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 17.Clarke M, Oxman AD. Analyzing and Presenting Results: Cochrane Reviewers’ Handbook 4.2 (updated November 2002- Section 8.) In: the Cochrane Library., editor. Oxford: Update Software; 2003, issue 1. Accessed November 25; 2009. Available from: http://www.cochrane.org/resources/handbook/index.htm. [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Moyad MA, Robinson LE, Kittelsrud JM, Reeves SG, Weaver SE, Guzman AI, Bubak ME. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo-controlled trial. Adv Ther. 2009;26:795–804. doi: 10.1007/s12325-009-0057-y. [DOI] [PubMed] [Google Scholar]
- 20.Pennacchia C, Blaiotta G, Pepe O, Villani F. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J Appl Microbiol. 2008;105:1919–1928. doi: 10.1111/j.1365-2672.2008.03968.x. [DOI] [PubMed] [Google Scholar]
- 21.Vandenplas Y, Brunser O, Szajewska H. Saccharomyces boulardii in childhood. Eur J Pediatr. 2009;168:253–265. doi: 10.1007/s00431-008-0879-7. [DOI] [PubMed] [Google Scholar]
- 22.Sankoff D. Reconstructing the history of yeast genomes. PLoS Genet. 2009;5:e1000483. doi: 10.1371/journal.pgen.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarland LV. Saccharomyces boulardii is not Saccharomyces cerevisiae. Clin Infect Dis. 1996;22:200–201. doi: 10.1093/clinids/22.1.200. [DOI] [PubMed] [Google Scholar]
- 24.McCullough MJ, Clemons KV, McCusker JH, Stevens DA. Species identification and virulence attributes of Saccharomyces boulardii (nom. inval.) J Clin Microbiol. 1998;36:2613–2617. doi: 10.1128/jcm.36.9.2613-2617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitterdorfer G, Mayer HK, Kneifel W, Viernstein H. Clustering of Saccharomyces boulardii strains within the species S. cerevisiae using molecular typing techniques. J Appl Microbiol. 2002;93:521–530. doi: 10.1046/j.1365-2672.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie DA, Defernez M, Dunn WB, Brown M, Fuller LJ, de Herrera SR, Günther A, James SA, Eagles J, Philo M, et al. Relatedness of medically important strains of Saccharomyces cerevisiae as revealed by phylogenetics and metabolomics. Yeast. 2008;25:501–512. doi: 10.1002/yea.1601. [DOI] [PubMed] [Google Scholar]
- 27.Posteraro B, Sanguinetti M, Romano L, Torelli R, Novarese L, Fadda G. Molecular tools for differentiating probiotic and clinical strains of Saccharomyces cerevisiae. Int J Food Microbiol. 2005;103:295–304. doi: 10.1016/j.ijfoodmicro.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Malgoire JY, Bertout S, Renaud F, Bastide JM, Mallié M. Typing of Saccharomyces cerevisiae clinical strains by using microsatellite sequence polymorphism. J Clin Microbiol. 2005;43:1133–1137. doi: 10.1128/JCM.43.3.1133-1137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennequin C, Thierry A, Richard GF, Lecointre G, Nguyen HV, Gaillardin C, Dujon B. Microsatellite typing as a new tool for identification of Saccharomyces cerevisiae strains. J Clin Microbiol. 2001;39:551–559. doi: 10.1128/JCM.39.2.551-559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards-Ingram L, Gitsham P, Burton N, Warhurst G, Clarke I, Hoyle D, Oliver SG, Stateva L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2007;73:2458–2467. doi: 10.1128/AEM.02201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pecquet S, Guillaumin D, Tancrede C, Andremont A. Kinetics of Saccharomyces cerevisiae elimination from the intestines of human volunteers and effect of this yeast on resistance to microbial colonization in gnotobiotic mice. Appl Environ Microbiol. 1991;57:3049–3051. doi: 10.1128/aem.57.10.3049-3051.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fietto JL, Araújo RS, Valadão FN, Fietto LG, Brandão RL, Neves MJ, Gomes FC, Nicoli JR, Castro IM. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol. 2004;50:615–621. doi: 10.1139/w04-050. [DOI] [PubMed] [Google Scholar]
- 33.Pardo S, Galvagno MA, Cerrutti P. [Studies of viability and vitality after freezing of the probiotic yeast Saccharomyces boulardii: physiological preconditioning effect.] Rev Iberoam Micol. 2009;26:155–160. doi: 10.1016/S1130-1406(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 34.Graff S, Chaumeil JC, Boy P, Lai-Kuen R, Charrueau C. Influence of pH conditions on the viability of Saccharomyces boulardii yeast. J Gen Appl Microbiol. 2008;54:221–227. doi: 10.2323/jgam.54.221. [DOI] [PubMed] [Google Scholar]
- 35.Salminen S, Collado MC, Isolauri E, Gueimonde M. Microbial-host interactions: selecting the right probiotics and prebiotics for infants. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:201–213; discussion 213-217, 251-257. doi: 10.1159/000235792. [DOI] [PubMed] [Google Scholar]
- 36.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam J, Barret C, Barret-Bellet A, Benedetti E, Calendini A, Darchen P, Galibert JM, Guerci P, Guiot G, Haechler M, et al. Controlled double-blind clinical trials of Ultra-Levure: multi centre study by 25 physicians in 388 cases] Gazette Medicale de France. 1977;84:2072–2078. [Google Scholar]
- 38.Besirbellioglu BA, Ulcay A, Can M, Erdem H, Tanyuksel M, Avci IY, Araz E, Pahsa A. Saccharomyces boulardii and infection due to Giardia lamblia. Scand J Infect Dis. 2006;38:479–481. doi: 10.1080/00365540600561769. [DOI] [PubMed] [Google Scholar]
- 39.Bleichner G, Bléhaut H, Mentec H, Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. A multicenter, randomized, double-blind placebo-controlled trial. Intensive Care Med. 1997;23:517–523. doi: 10.1007/s001340050367. [DOI] [PubMed] [Google Scholar]
- 40.Buts JP, Corthier G, Delmee M. Saccharomyces boulardii for Clostridium difficile-associated enteropathies in infants. J Pediatr Gastroenterol Nutr. 1993;16:419–425. doi: 10.1097/00005176-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Castex F, Corthier G, Jouvert S, Elmer GW, Lucas F, Bastide M. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: a scanning electron microscopic and microbiological study. J Gen Microbiol. 1990;136:1085–1089. doi: 10.1099/00221287-136-6-1085. [DOI] [PubMed] [Google Scholar]
- 42.Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309–316. doi: 10.1111/j.1523-5378.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 43.Ducluzeau R, Bensaada M. [Comparative effect of a single or continuous administration of "Saccharomyces boulardii" on the establishment of various strains of "candida" in the digestive tract of gnotobiotic mice] Ann Microbiol (Paris) 1982;133:491–501. [PubMed] [Google Scholar]
- 44.Duman DG, Bor S, Ozütemiz O, Sahin T, Oğuz D, Iştan F, Vural T, Sandkci M, Işksal F, Simşek I, et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur J Gastroenterol Hepatol. 2005;17:1357–1361. doi: 10.1097/00042737-200512000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Elmer GW, Moyer KA, Vega R, Surawicz CM, Collier AC, Hooton TM and McFarland LV. Evaluation ofSaccharomyces boulardii for patients with HIV-related chronic diarrhoea and in healthy volunteers receiving antifungals. Microb Ther. 1995;25:23–31. [Google Scholar]
- 46.Gotteland M, Poliak L, Cruchet S, Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:1747–1751. doi: 10.1111/j.1651-2227.2005.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 47.Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci. 2000;45:1462–1464. doi: 10.1023/a:1005588911207. [DOI] [PubMed] [Google Scholar]
- 48.Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15:697–698. doi: 10.1097/00042737-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Kollaritsch H, Holst H, Grobara P, Wiedermann G. [Prevention of traveler's diarrhea with Saccharomyces boulardii. Results of a placebo controlled double-blind study] Fortschr Med. 1993;111:152–156. [PubMed] [Google Scholar]
- 50.Kotowska M, Albrecht P, Szajewska H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2005;21:583–590. doi: 10.1111/j.1365-2036.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 51.Mansour-Ghanaei F, Dehbashi N, Yazdanparast K, Shafaghi A. Efficacy of saccharomyces boulardii with antibiotics in acute amoebiasis. World J Gastroenterol. 2003;9:1832–1833. doi: 10.3748/wjg.v9.i8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maupas JL, Champemont P, Delforge M. Treatment of irritable bowel syndrome. Double blind trial of Saccharomyces boulardii. Med Chir Dig. 1983;12:77–79. [Google Scholar]
- 53.McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 54.McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439–448. [PubMed] [Google Scholar]
- 55.Monteiro E, Fernandes JP, Vieira MR, Correia JP, Caetano JM, Ribeiro T, Antunes AB, Noronha R, Batista F, Reis L, et al. [Double blind clinical trial on the use of ultra-levure in the prophylaxis of antibiotic induced gastro-intestinal and mucocutaneous disorders] Acta Med Port. 1981;3:143–145. [PubMed] [Google Scholar]
- 56.Ozkan TB, Sahin E, Erdemir G, Budak F. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J Int Med Res. 2007;35:201–212. doi: 10.1177/147323000703500204. [DOI] [PubMed] [Google Scholar]
- 57.Plein K, Hotz J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn's disease with special respect to chronic diarrhea--a pilot study. Z Gastroenterol. 1993;31:129–134. [PubMed] [Google Scholar]
- 58.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 59.Surawicz CM, McFarland LV, Elmer G, Chinn J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol. 1989;84:1285–1287. [PubMed] [Google Scholar]
- 60.Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 61.Tempe JD, Steidel AL, Blehaut H, Hasselmann M, Lutun P, Maurier F. [Prevention of diarrhea administering Saccharomyces boulardii during continuous enteral feeding] Sem Hop. 1983;59:1409–1412. [PubMed] [Google Scholar]
- 62.Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A, Carolina Carneiro Aguirre A, Paiva Martins F, Marcos Andrade Goulart E, Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol. 2008;43:842–848. doi: 10.1080/00365520801943354. [DOI] [PubMed] [Google Scholar]
- 63.Ooi CY, Dilley AV, Day AS. Saccharomyces boulardii in a child with recurrent Clostridium difficile. Pediatr Int. 2009;51:156–158. doi: 10.1111/j.1442-200X.2008.02782.x. [DOI] [PubMed] [Google Scholar]
- 64.Lessard M, Dupuis M, Gagnon N, Nadeau E, Matte JJ, Goulet J, Fairbrother JM. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge. J Anim Sci. 2009;87:922–934. doi: 10.2527/jas.2008-0919. [DOI] [PubMed] [Google Scholar]
- 65.Bisson JF, Hidalgo S, Rozan P, Messaoudi M. Preventive effects of different probiotic formulations on travelers' diarrhea model in wistar rats : preventive effects of probiotics on TD. Dig Dis Sci. 2010;55:911–919. doi: 10.1007/s10620-009-0822-4. [DOI] [PubMed] [Google Scholar]
- 66.Lee S, Lillehoj HS, Park DW, Hong YH, Lin JJ. Effects of Pediococcus- and Saccharomyces-based probiotic (MitoMax) on coccidiosis in broiler chickens. Comp Immunol Microbiol Infect Dis. 2007;30:261–268. doi: 10.1016/j.cimid.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Weese JS. Evaluation of deficiencies in labeling of commercial probiotics. Can Vet J. 2003;44:982–983. [PMC free article] [PubMed] [Google Scholar]
- 68.Marcobal A, Underwood MA, Mills DA. Rapid determination of the bacterial composition of commercial probiotic products by terminal restriction fragment length polymorphism analysis. J Pediatr Gastroenterol Nutr. 2008;46:608–611. doi: 10.1097/MPG.0b013e3181660694. [DOI] [PubMed] [Google Scholar]
- 69.Masco L, Huys G, De Brandt E, Temmerman R, Swings J. Culture-dependent and culture-independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int J Food Microbiol. 2005;102:221–230. doi: 10.1016/j.ijfoodmicro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Martins FS, Nardi RM, Arantes RM, Rosa CA, Neves MJ, Nicoli JR. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol. 2005;51:83–92. doi: 10.2323/jgam.51.83. [DOI] [PubMed] [Google Scholar]
- 71.Martins FS, Veloso LC, Arantes RM, Nicoli JR. Effects of yeast probiotic formulation on viability, revival and protection against infection with Salmonella enterica ssp. enterica serovar Typhimurium in mice. Lett Appl Microbiol. 2009;49:738–744. doi: 10.1111/j.1472-765X.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- 72.Graff S, Chaumeil JC, Boy P, Lai-Kuen R, Charrueau C. Formulations for protecting the probiotic Saccharomyces boulardii from degradation in acidic condition. Biol Pharm Bull. 2008;31:266–272. doi: 10.1248/bpb.31.266. [DOI] [PubMed] [Google Scholar]
- 73.Schwenzer VB. Saccharomyces boulardii. Deutsche Apotheker Zeitung. 1998;138:75–77. [Google Scholar]
- 74.Myllyluoma E, Ahonen AM, Korpela R, Vapaatalo H, Kankuri E. Effects of multispecies probiotic combination on helicobacter pylori infection in vitro. Clin Vaccine Immunol. 2008;15:1472–1482. doi: 10.1128/CVI.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23:679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 76.Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30:826–833. doi: 10.1111/j.1365-2036.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 78.Pothoulakis C, Kelly CP, Joshi MA, Gao N, O’Keane CJ, Castagliuolo I, Lamont JT. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 79.Brandao RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJ, Neves MJ, Dos Santos RG, Gomes NC, Nicoli JR. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64:564–568. doi: 10.1128/aem.64.2.564-568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67:302–307. doi: 10.1128/iai.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buts JP, Dekeyser N, Stilmant C, Delem E, Smets F, Sokal E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr Res. 2006;60:24–29. doi: 10.1203/01.pdr.0000220322.31940.29. [DOI] [PubMed] [Google Scholar]
- 83.Vidon N, Huchet B, Rambaud JC. Effect of S. boulardii on water and sodium secretions induced by cholera toxin. Gastroenterol Clin Biol. 1986;10:1–4. [PubMed] [Google Scholar]
- 84.Czerucka D, Rampal P. Effect of Saccharomyces boulardii on cAMP- and Ca2+ -dependent Cl- secretion in T84 cells. Dig Dis Sci. 1999;44:2359–2368. doi: 10.1023/a:1026689628136. [DOI] [PubMed] [Google Scholar]
- 85.Zbinden R, Bonczi E, Altwegg M. Inhibition of Saccharomyces boulardii (nom. inval.) on cell invasion of Salmonella typhimurium and Yersinia enterocolitica. Micro Ecol Health Dis. 1999;11:158–162. [Google Scholar]
- 86.Altwegg M, Schnack J, Zbinden R. Influence of Saccharomyces boulardii on Aeromonas hemolysin. Med Microbiol Lett. 1995;4:417–425. [Google Scholar]
- 87.Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immun. 2003;71:766–773. doi: 10.1128/IAI.71.2.766-773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O'Kusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294:G295–G306. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- 89.Karen M, Yuksel O, Akyürek N, Ofluoğlu E, Cağlar K, Sahin TT, Paşaoğlu H, Memiş L, Akyürek N, Bostanci H. Probiotic agent Saccharomyces boulardii reduces the incidence of lung injury in acute necrotizing pancreatitis induced rats. J Surg Res. 2010;160:139–144. doi: 10.1016/j.jss.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Zaouche A, Loukil C, De Lagausie P, Peuchmaur M, Macry J, Fitoussi F, Bernasconi P, Bingen E, Cezard JP. Effects of oral Saccharomyces boulardii on bacterial overgrowth, translocation, and intestinal adaptation after small-bowel resection in rats. Scand J Gastroenterol. 2000;35:160–165. doi: 10.1080/003655200750024326. [DOI] [PubMed] [Google Scholar]
- 91.Tasteyre A, Barc MC, Karjalainen T, Bourlioux P, Collignon A. Inhibition of in vitro cell adherence of Clostridium difficile by Saccharomyces boulardii. Microb Pathog. 2002;32:219–225. doi: 10.1006/mpat.2002.0495. [DOI] [PubMed] [Google Scholar]
- 92.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68:5998–6004. doi: 10.1128/iai.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gedek BR. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses. 1999;42:261–264. doi: 10.1046/j.1439-0507.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 94.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 95.McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000;12:193–207. [Google Scholar]
- 96.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oirard-Pipau F, Pompei A, Schneider S, Nano JL, Hebuterne X, Boquet P, Rampal P. Intestinal microflora, short chain and cellular fatty acids, influence ofa probiotic S. boulardii. Microb Ecology Health Dis. 2002;14:220–227. [Google Scholar]
- 99.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 100.Barc MC, Charrin-Sarnel C, Rochet V, Bourlioux F, Sandré C, Boureau H, Doré J, Collignon A. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: Influence of Saccharomyces boulardii. Anaerobe. 2008;14:229–233. doi: 10.1016/j.anaerobe.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Breves G, Walter C, Burmester M, Schröder B. In vitro studies on the effects of Saccharomyces boulardii and Bacillus cereus var. toyoi on nutrient transport in pig jejunum. J Anim Physiol. 2000:84: 9–20. [Google Scholar]
- 102.Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, Pompei A, Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11:6165–6169. doi: 10.3748/wjg.v11.i39.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sezer A, Usta U, Cicin I. The effect of Saccharomyces boulardii on reducing irinotecan-induced intestinal mucositis and diarrhea. Med Oncol. 2009;26:350–357. doi: 10.1007/s12032-008-9128-1. [DOI] [PubMed] [Google Scholar]
- 104.Schroeder B, Winckler C, Failing K, Breves G. Studies on the time course of the effects of the probiotic yeast Saccharomyces boulardii on electrolyte transport in pig jejunum. Dig Dis Sci. 2004;49:1311–1317. doi: 10.1023/b:ddas.0000037828.05100.52. [DOI] [PubMed] [Google Scholar]
- 105.Buts JP, Stilmant C, Bernasconi P, Neirinck C, De Keyser N. Characterization of alpha,alpha-trehalase released in the intestinal lumen by the probiotic Saccharomyces boulardii. Scand J Gastroenterol. 2008;43:1489–1496. doi: 10.1080/00365520802308862. [DOI] [PubMed] [Google Scholar]
- 106.Buts JP, De Keyser N, De Raedemaeker L. Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr Res. 1994;36:522–527. doi: 10.1203/00006450-199410000-00019. [DOI] [PubMed] [Google Scholar]
- 107.Jahn HU, Ullrich R, Schneider T, Liehr RM, Schieferdecker HL, Holst H, Zeitz M. Immunological and trophical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion. 1996;57:95–104. doi: 10.1159/000201320. [DOI] [PubMed] [Google Scholar]
- 108.Buts JP. Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci. 2009;54:15–18. doi: 10.1007/s10620-008-0322-y. [DOI] [PubMed] [Google Scholar]
- 109.Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM, Nicoli JR. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191:623–630. doi: 10.1007/s00203-009-0491-x. [DOI] [PubMed] [Google Scholar]
- 110.Pant N, Marcotte H, Brüssow H, Svensson L, Hammarström L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007;7:86. doi: 10.1186/1471-2180-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 112.Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156:78–87. doi: 10.1111/j.1365-2249.2009.03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fidan I, Kalkanci A, Yesilyurt E, Yalcin B, Erdal B, Kustimur S, Imir T. Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans. Mycoses. 2009;52:29–34. doi: 10.1111/j.1439-0507.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 114.Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O’Brien M, Pothoulakis C, Kelly CP. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281:24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 115.Dalmasso G, Cottrez F, Imbert V, Lagadec P, Peyron JF, Rampal P, Czerucka D, Groux H, Foussat A, Brun V. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131:1812–1825. doi: 10.1053/j.gastro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol. 2000;95:S2–S4. doi: 10.1016/s0002-9270(99)00806-0. [DOI] [PubMed] [Google Scholar]
- 117.Buts JP. Chapter 2, Mechanism of Action of Biotherapeutic Agents. In: Elmer G, McFarland L, Surawicz C, editors. Biotherapeutic Agents and Infectious Diseases. Totwa (NJ): Humana Press; 1999. pp. 27–46. [Google Scholar]
- 118.Klein SM, Elmer GW, McFarland LV, Surawicz CM, Levy RH. Recovery and elimination of the biotherapeutic agent, Saccharomyces boulardii, in healthy human volunteers. Pharm Res. 1993;10:1615–1619. doi: 10.1023/a:1018924820333. [DOI] [PubMed] [Google Scholar]
- 119.Elmer GW, McFarland LV, Surawicz CM, Danko L, Greenberg RN. Behaviour of Saccharomyces boulardii in recurrent Clostridium difficile disease patients. Aliment Pharmacol Ther. 1999;13:1663–1668. doi: 10.1046/j.1365-2036.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- 120.Blehaut H, Massot J, Elmer GW, Levy RH. Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos. 1989;10:353–364. doi: 10.1002/bdd.2510100403. [DOI] [PubMed] [Google Scholar]
- 121.Elmer GW, Martin SW, Horner KL, McFarland LV, Levy RH. Survival of Saccharomyces boulardii in the Rat Gastrointestinal Tract and Effects of Dietary Fiber. Microb Ecology Health Dis. 1999;11:29–34. [Google Scholar]
- 122.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 123.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 124.Damrongmanee A, Ukarapol N. Incidence of antibiotic-associated diarrhea in a pediatric ambulatory care setting. J Med Assoc Thai. 2007;90:513–517. [PubMed] [Google Scholar]
- 125.Turck D, Bernet JP, Marx J, Kempf H, Giard P, Walbaum O, Lacombe A, Rembert F, Toursel F, Bernasconi P, et al. Incidence and risk factors of oral antibiotic-associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22–26. doi: 10.1097/00005176-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Levy DG, Stergachis A, McFarland LV, Van Vorst K, Graham DJ, Johnson ES, Park BJ, Shatin D, Clouse JC, Elmer GW. Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin Ther. 2000;22:91–102. doi: 10.1016/s0149-2918(00)87980-1. [DOI] [PubMed] [Google Scholar]
- 127.Conway S, Hart A, Clark A, Harvey I. Does eating yogurt prevent antibiotic-associated diarrhoea? A placebo-controlled randomised controlled trial in general practice. Br J Gen Pract. 2007;57:953–959. doi: 10.3399/096016407782604811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sugita R, Yamanaka N, Kudo F, Ito R, Kawai M, Ohwaki I, Asano S, Nagata T. [Efficacy and safety of potassium clavulanate/amoxicillin (CLAVAMOX) dry syrup in children with otitis media] Jpn J Antibiot. 2007;60:221–241. [PubMed] [Google Scholar]
- 129.Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corrêa NB, Péret Filho LA, Penna FJ, Lima FM, Nicoli JR. A randomized formula controlled trial of Bifidobacterium lactis and Streptococcus thermophilus for prevention of antibiotic-associated diarrhea in infants. J Clin Gastroenterol. 2005;39:385–389. doi: 10.1097/01.mcg.0000159217.47419.5b. [DOI] [PubMed] [Google Scholar]
- 131.Elmer GW. Vega R, Mohutsky MA and McFarland LV. Variable Time of Onset ofC difjicile Disease Initiated by Antimicrobial Treatment in Hamsters. Microb Ecology Health Dis. 1999;11:163–168. [Google Scholar]
- 132.Dubberke ER, Reske KA, Olsen MA, McDonald LC, Fraser VJ. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46:497–504. doi: 10.1086/526530. [DOI] [PubMed] [Google Scholar]
- 133.Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171–174. doi: 10.1016/s0163-4453(98)80008-x. [DOI] [PubMed] [Google Scholar]
- 134.Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–2749. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 135.Can M, Beşirbellioglu BA, Avci IY, Beker CM, Pahsa A. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study. Med Sci Monit. 2006;12:PI19–PI22. [PubMed] [Google Scholar]
- 136.Bravo MV, Bunout D, Leiva L, de la Maza MP, Barrera G, de la Maza J, Hirsch S. [Effect of probiotic Saccharomyces boulardii on prevention of antibiotic-associated diarrhea in adult outpatients with amoxicillin treatment] Rev Med Chil. 2008;136:981–988. [PubMed] [Google Scholar]
- 137.McFarland LV. Unraveling the causes of negative studies: a case of S boulardii for the prevention of antibiotic-associated diarrhea. Rev Med Chil. 2009;137:719–720. [PubMed] [Google Scholar]
- 138.Meerpohl JJ, Timmer A. [News from the Cochrane Library: probiotics for the prevention of paediatric antibiotic-associated diarrhoea] Z Gastroenterol. 2007;45:715–717. doi: 10.1055/s-2007-963352. [DOI] [PubMed] [Google Scholar]
- 139.Johnston BC, Supina AL, Ospina M, Vohra S. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2007:CD004827. doi: 10.1002/14651858.CD004827.pub2. [DOI] [PubMed] [Google Scholar]
- 140.Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- 141.Szajewska H, Mrukowicz J. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2005;22:365–372. doi: 10.1111/j.1365-2036.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- 142.Johnston BC, Supina AL, Vohra S. Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials. CMAJ. 2006;175:377–383. doi: 10.1503/cmaj.051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 144.Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29:823–828. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 145.McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008;5:40–48. doi: 10.1038/ncpgasthep1029. [DOI] [PubMed] [Google Scholar]
- 146.Shek FW, Stacey BS, Rendell J, Hellier MD, Hanson PJ. The rise of Clostridium difficile: the effect of length of stay, patient age and antibiotic use. J Hosp Infect. 2000;45:235–237. doi: 10.1053/jhin.2000.0770. [DOI] [PubMed] [Google Scholar]
- 147.Elixhauser A, Jhung M. Clostridium difficile-associated disease in U.S. Hospitals, 1993-2005. . Agency for Healthcare Research and Quality, Statistical brief #50. April 2008. Accessed November 25, 2009. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/Sb50.pdf. [PubMed] [Google Scholar]
- 148.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14:929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis. 2007;45:1141–1151. doi: 10.1086/522187. [DOI] [PubMed] [Google Scholar]
- 150.Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678–684. doi: 10.1093/infdis/162.3.678. [DOI] [PubMed] [Google Scholar]
- 152.McFarland LV, Beneda HW, Clarridge JE, Raugi GJ. Implications of the changing face of Clostridium difficile disease for health care practitioners. Am J Infect Control. 2007;35:237–253. doi: 10.1016/j.ajic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 153.Al-Eidan FA, McElnay JC, Scott MG, Kearney MP. Clostridium difficile-associated diarrhoea in hospitalised patients. J Clin Pharm Ther. 2000;25:101–109. doi: 10.1046/j.1365-2710.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 154.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 155.O'Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 156.Toothaker RD, Elmer GW. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984;26:552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elmer GW, Corthier G. Modulation of Clostridium difficile induced mortality as a function of the dose and the viability of the Saccharomyces boulardii used as a preventative agent in gnotobiotic mice. Can J Microbiol. 1991;37:315–317. doi: 10.1139/m91-049. [DOI] [PubMed] [Google Scholar]
- 158.Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81:251–256. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 159.Bradley GL, Savage TF, Timm KI. The effects of supplementing diets with Saccharomyces cerevisiae var. boulardii on male poult performance and ileal morphology. Poult Sci. 1994;73:1766–1770. doi: 10.3382/ps.0731766. [DOI] [PubMed] [Google Scholar]
- 160.Hassett J, Meyers S, McFarland L, Mulligan ME. Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii. Clin Infect Dis. 1995;20 Suppl 2:S266–S268. doi: 10.1093/clinids/20.supplement_2.s266. [DOI] [PubMed] [Google Scholar]
- 161.Popoola J, Swann A, Warwick G. Clostridium difficile in patients with renal failure - management of an outbreak using biotherapy. Nephrol Dial Transplant. 2000;15:571–574. doi: 10.1093/ndt/15.5.571. [DOI] [PubMed] [Google Scholar]
- 162.Kovacs DJ, Berk T. Recurrent Clostridium difficile-associated diarrhea and colitis treated with Saccharomyces cerevisiae (Baker's yeast) in combination with antibiotic therapy: a case report. J Am Board Fam Pract. 2000;13:138–140. doi: 10.3122/15572625-13-2-138. [DOI] [PubMed] [Google Scholar]
- 163.Schellenberg D, Bonington A, Champion CM, Lancaster R, Webb S, Main J. Treatment of Clostridium difficile diarrhoea with brewer's yeast. Lancet. 1994;343:171–172. doi: 10.1016/s0140-6736(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 164.Chia JK, Chan SM, Goldstein H. Baker’s yeast as adjunctive therapy for relapses of Clostridium difficile diarrhea. Clin Infect Dis. 1995;20:1581. doi: 10.1093/clinids/20.6.1581. [DOI] [PubMed] [Google Scholar]
- 165.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 166.Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami SQ. Helicobacter pylori infection in children: population-based age-specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99:279–282. doi: 10.1111/j.1651-2227.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 167.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 168.Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98:127–131. doi: 10.1111/j.1651-2227.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 169.Hochter W, Chase D, Hagenhoff G. Saccharomyces boulardii in acute adult diarrhea: efficacy and tolerability of treatment. Munch Med Wschr. 1990;132:188–192. [Google Scholar]
- 170.Whelan K, Judd PA, Tuohy KM, Gibson GR, Preedy VR, Taylor MA. Fecal microbiota in patients receiving enteral feeding are highly variable and may be altered in those who develop diarrhea. Am J Clin Nutr. 2009;89:240–247. doi: 10.3945/ajcn.2008.26219. [DOI] [PubMed] [Google Scholar]
- 171.Schlotterer M, Bernasconi P, Lebreton F, Wassermann D. Value of Saccharomyces boulardii in the digestive acceptability of continuous-flow enteral nutrition in burnt patients. Nutr Clin Matalbol. 1987;1:31–34. [Google Scholar]
- 172.Cheng AC, Thielman NM. Update on Traveler’s Diarrhea. Curr Infect Dis Rep. 2002;4:70–77. doi: 10.1007/s11908-002-0070-7. [DOI] [PubMed] [Google Scholar]
- 173.Centers for Communicable Diseases, CDC. Travelers’ diarrhea. The Yellow Book. CDC, Atlanta, Georgia. 4-27-2004, 1-8. [Google Scholar]
- 174.McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 175.Kollaritsch H, Kremsner P, Wiedermann , G , Scheiner O. Prevention of traveller’s diarrhea: comparison of different non-antibiotic preparations. Travel Med Internatl. 1989;7:9–18. [Google Scholar]
- 176.Bruns R, Raedsch R. Therapy of traveller’s diarrhea. Medizinische Welt. 1995;46:591–596. [Google Scholar]
- 177.Economou M, Pappas G. New global map of Crohn’s disease: Genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008;14:709–720. doi: 10.1002/ibd.20352. [DOI] [PubMed] [Google Scholar]
- 178.Lok KH, Hung HG, Ng CH, Li KK, Li KF, Szeto ML. The epidemiology and clinical characteristics of Crohn's disease in the Hong Kong Chinese population: experiences from a regional hospital. Hong Kong Med J. 2007;13:436–441. [PubMed] [Google Scholar]
- 179.Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn's disease: record linkage studies. BMJ. 2007;335:1033. doi: 10.1136/bmj.39345.714039.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elmer GW, McFarland LV, McFarland M. Chapter 6. Inflammatory bowel disease, irritable bowel syndrome and digestive problems. In: The power of probiotics: improving your health with beneficial microbes., editor. Binghamton, NY: Haworth Press; 2007. pp. 111–116. [Google Scholar]
- 181.Barclay GR, McKenzie H, Pennington J, Parratt D, Pennington CR. The effect of dietary yeast on the activity of stable chronic Crohn's disease. Scand J Gastroenterol. 1992;27:196–200. doi: 10.3109/00365529208999948. [DOI] [PubMed] [Google Scholar]
- 182.Park KS, Ahn SH, Hwang JS, Cho KB, Chung WJ, Jang BK, Kang YN, Kwon JH, Kim YH. A survey about irritable bowel syndrome in South Korea: prevalence and observable organic abnormalities in IBS patients. Dig Dis Sci. 2008;53:704–711. doi: 10.1007/s10620-007-9930-1. [DOI] [PubMed] [Google Scholar]
- 183.Cain KC, Headstrom P, Jarrett ME, Motzer SA, Park H, Burr RL, Surawicz CM, Heitkemper MM. Abdominal pain impacts quality of life in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:124–132. doi: 10.1111/j.1572-0241.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 184.Foxx-Orenstein A. IBS--review and what's new. MedGenMed. 2006;8:20. [PMC free article] [PubMed] [Google Scholar]
- 185.Ladep NG, Okeke EN, Samaila AA, Agaba EI, Ugoya SO, Puepet FH, Malu AO. Irritable bowel syndrome among patients attending General Outpatients' clinics in Jos, Nigeria. Eur J Gastroenterol Hepatol. 2007;19:795–799. doi: 10.1097/MEG.0b013e3282202ba5. [DOI] [PubMed] [Google Scholar]
- 186.Londoño AL, Mejía S, Gómez-Marín JE. [Prevalence and risk factors associated with intestinal parasitism in preschool children from the urban area of Calarcá, Colombia] Rev Salud Publica (Bogota) 2009;11:72–81. doi: 10.1590/s0124-00642009000100008. [DOI] [PubMed] [Google Scholar]
- 187.Sagebiel D, Weitzel T, Stark K, Leitmeyer K. Giardiasis in kindergartens: prevalence study in Berlin, Germany, 2006. Parasitol Res. 2009;105:681–687. doi: 10.1007/s00436-009-1438-5. [DOI] [PubMed] [Google Scholar]
- 188.Rossit AR, Gonçalves AC, Franco C, Machado RL. Etiological agents of diarrhea in patients infected by the human immunodeficiency virus-1: a review. Rev Inst Med Trop Sao Paulo. 2009;51:59–65. doi: 10.1590/s0036-46652009000200001. [DOI] [PubMed] [Google Scholar]
- 189.Saint-Marc Th, Blehaut H, Musial Ch, Touraine JL. AIDS-related diarrhea: a double-blind trial of Saccharomyces boulardii. Sem Hôp Paris. 1995;71:735–741. [Google Scholar]
- 190.Temmerman R, Pot B, Huys G, Swings J. A quality analysis of commercial probiotic products. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66:535, 537–535, 542. [PubMed] [Google Scholar]
- 191.Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Järvinen A. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis. 2006;42:e35–e44. doi: 10.1086/500214. [DOI] [PubMed] [Google Scholar]
- 192.Byron JK, Clemons KV, McCusker JH, Davis RW, Stevens DA. Pathogenicity of Saccharomyces cerevisiae in complement factor five-deficient mice. Infect Immun. 1995;63:478–485. doi: 10.1128/iai.63.2.478-485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264; quiz 1446-1447. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 194.Hennequin C, Kauffmann-Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, Berche P. Possible role of catheters in Saccharomyces boulardii fungemia. Eur J Clin Microbiol Infect Dis. 2000;19:16–20. doi: 10.1007/s100960050003. [DOI] [PubMed] [Google Scholar]
- 195.Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis. 2005;41:1559–1568. doi: 10.1086/497832. [DOI] [PubMed] [Google Scholar]
- 196.Montineri A, Iacobello C, Larocca L, La Rosa R, Nigro L, Fatuzzo F. [Saccharomyces cerevisiae fungemia associated with multifocal pneumonia in a patient with alcohol-related hepatic cirrhosis] Infez Med. 2008;16:227–229. [PubMed] [Google Scholar]