Abstract
AIM: To investigate the growth inhibitory mechanism of four caged xanthones from Garcinia hanburyi in cholangiocarcinoma (CCA) KKU-100 and KKU-M156 cells.
METHODS: Four caged xanthones, selected on the basis of their anticancer potency and chemical structure diversities (i.e. isomorellin, isomorellinol, forbesione and gambogic acid) were used in this study. Growth inhibition of these caged xanthones was determined using the sulforhodamine B assay. Induction of apoptosis was assessed by observing cell morphology, ethidium bromide and acridine orange staining and DNA fragmentation assay. Levels of apoptotic-related gene and protein expressions were determined by a real-time reverse transcriptase polymerase chain reaction and Western blotting analysis, respectively.
RESULTS: The compounds were found to inhibit growth of both cell lines in a dose-dependent manner and also showed selective cytotoxicity against the cancer cells when compared with normal peripheral blood mononuclear cells. Growth suppression by these compounds was due to apoptosis, as evidenced by the cell morphological changes, chromatin condensation, nuclear fragmentation, and DNA ladder formation. At the molecular level, these compounds induced down-regulation of Bcl-2 and survivin proteins with up-regulation of Bax and apoptosis-inducing factor proteins, leading to the activation of caspase-9 and -3 and DNA fragmentation. The functional group variations did not appear to affect the anticancer activity with regard to the two CCA cell lines; however, at a mechanistic level, isomorellinol exhibited the highest potency in increasing the Bax/Bcl-2 protein expression ratio (120 and 41.4 for KKU-100 and KKU-M156, respectively) and in decreasing survivin protein expression (0.01 fold as compared to control cells in both cell lines). Other activities at the molecular level indicate that functional groups on the prenyl side chain may be important.
CONCLUSION: Our findings for the first time demonstrate that four caged xanthones induce apoptosis in CCA cells which is mediated through a mitochondria-dependent signaling pathway.
Keywords: Garcinia hanburyi, Caged xanthones, Human cholangiocarcinoma cell lines, Apoptosis
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor arising from bile duct epithelial cells[1], characterized by a poor prognosis and unresponsive to conventional chemotherapeutic agents. The increasing global incidence of this tumor underscores the need for novel and effective therapeutic agents for CCA[2,3].
Much attention has been focused on natural products as potential sources of novel anticancer drugs over the decades[4]. An emerging class of compounds, being potent antiproliferatives as well as having anticancer and anti-tumor activities against mammalian cancer cell lines, is caged xanthones. Recent work by our and other research groups has identified a tropical plant, Garcinia hanburyi (G. hanburyi) Hook.f. (family Guttiferae), used as a purgative and externally for infected wounds in Thai traditional medicine[5], as a rich source of these compounds[6-11]. In addition, gambogic acid, derived from the gamboges resin of G. hanburyi, has been shown to induce apoptosis in human gastric carcinoma cells[9]. These data suggest that these caged xanthones might be effective as chemotherapeutic agents and that they warrant further study of the underlying mechanisms of their anticancer activities.
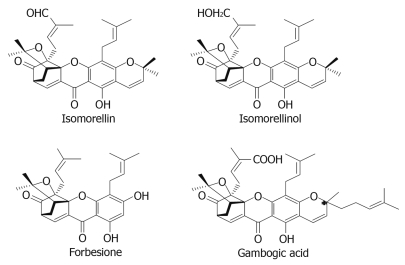
Our interest in searching for agents for the effective treatment of CCA led us to test a series of caged xanthones from G. hanburyi[10] against two human CCA cell lines. Among the compounds tested, isomorellin, isomorellinol, forbesione and gambogic acid (Figure 1) exhibit promising IC50 values against both cell lines in comparison with the standard drug doxorubicin. In view of their anticancer potency and chemical structure diversity, these four caged xanthones were selected for a more in-depth study. In order to further delineate the underlying mechanisms, the apoptotic action of these compounds was investigated in detail. The results are the main focus of this communication.
Figure 1.
Chemical structure of four caged xanthones.
MATERIALS AND METHODS
Reagents and cell culture
Four caged xanthones, i.e. isomorellin, isomorellinol, forbesione and gambogic acid, were isolated from G. hanburyi Hook.f. (family Guttiferae) using bioassay-directed fractionation[10]. The KKU-100 and KKU-M156 cells were isolated from Thai CCA patients and the original characterization of these cell lines has been described previously[12,13]. Human peripheral blood mononuclear cells (PBMCs) were freshly isolated using the standard Ficoll-hypaque gradient centrifugation method and used as normal control cells[14]. Cells were grown in RPMI 1640 (GIBCO BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL of penicillin and 100 μg/mL streptomycin (GIBCO BRL) at 37°C in a humidified incubator containing 5% CO2.
Cell proliferation assay
For the cell proliferation assay, 1.9 × 104 cells/well were seeded in 96-well microtitre plates and incubated for 24 h. After treatment for 72 h with 0-8.8 × 104 μmol/L per well for the caged xanthones, 0.04, 0.4, 4, 40 and 400 μmol/L for doxorubicin (Boryung Pharmaceutic Co. LTD, Korea) as a reference compound and DMSO as the solvent-control cells, cell growth was measured using the sulforhodamine B (SRB) assay[15].
Morphological examination
KKU-100 and KKU-M156 cells (1 × 106) were grown in a 25 cm2 flask at 37°C for 24 h, and treated with 2 × IC50 concentration of each caged xanthone for 48 h. Morphological changes occurring in the cells were observed under bright field inverted Nikon microscope. For nuclear staining, cells (1.9 × 103 cells/well) were grown in 96-well microtitre plates at 37°C for 24 h, and treated with 2 × IC50 concentration of each caged xanthone for 24, 36 and 48 h. The treated cells were stained with 14 μL of 100 μg/mL ethidium bromide/acridine orange (EB/AO) mixture (Sigma Chemical, St. Louis, MO) and observed under a Nikon fluorescent microscope. Apoptotic cells with condensed chromatin or fragmented chromatin were counted and expressed as a percentage from a total of 500 cells each[16].
DNA fragmentation assay
The isolation of fragmented DNA was carried out according to the procedure of Herrmann et al[17] with some modifications. Briefly, after culturing for 24 h and starving in medium containing 0.5% FBS for 24 h, cells (1 × 106) were treated with DMSO or 2 × IC50 concentrations of the caged xanthones for 24 and 36 h. After extraction, DNA in cell lysate was purified by QIAamp DNA Blood Mini Kit (QIAGEN, Germany) according to the manufacturer’s protocol. The DNA fragments were precipitated with ethanol, re-suspended in 50 μL of TE buffer, and analyzed by electrophoresis.
RNA extraction, reverse transcription and quantitative real-time polymerase chain reaction
Cells were treated with 2 × IC50 concentrations of the caged xanthones for 0, 6, 12, 24 and 48 h. Total RNA was isolated from the treated and control cells using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The reverse transcription reaction was performed as described[18].
Real-time polymerase chain reaction (PCR) was performed according to the procedure of Namwat et al[18]. Real-time PCR of Bcl-2, Bax, survivin and GAPDH were performed in a 20 μL PCR reaction mixture containing first strand cDNA, 5 pmol of each primer, and 10 μL of 2 × SYBR Green PCR Master Mix (Gene Systems Co., Ltd, USA), employing an ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA). The PCR primers (Bioservice Unit, Bangkok, Thailand) are shown in Table 1. The sequences of these PCR products were obtained by direct sequencing. Each amplicon was then cloned into the pGEM®-T vector (Promega, Madison, WI, USA) in order to generate standard curves for target cDNA. The mRNA level is reported as the ratios of the copy numbers of target cDNA to GAPDH cDNA. The ratio of fold change in gene expression level between the treated and control cells was determined to identify the up- or down-regulation of gene expression.
Table 1.
Primer sequences used for target gene amplification
| GenBank accession No. | Gene | Forward primer | Reverse primer |
| M14745.1 | Bcl-2 | TGGATGACTGAGTACCTGA | TGAGCAGAGTCTTCAGAGA |
| L22473.1 | Bax | AACCATCATGGGCTGGA | CGCCACAAAGATGGTCA |
| AF077350.1 | Survivin | AAGGCTGGGAGCCAGA | TGGCTCTTTCTCTGTCCA |
| BC083511 | GAPDH | TCATCAGCAATGCCTCCTGCA | TGGGTGGCAGTGATGGCA |
Protein extraction and Western blotting analysis
After treatment with DMSO or 2 × IC50 concentrations of the caged xanthones for 0, 12, 24 and 48 h, protein extraction and Western blotting were performed as described previously[19]. After blocking, the membranes were incubated at 4°C overnight with the following antibodies: Bcl-2, Bax, survivin, apoptosis-inducing factor (AIF) (Santa Cruz Biotechnology, CA) or procaspase-9 and -3, activated caspase-9 (Cell Signaling, Beverly, MA) or activated caspase-3, β-actin (Sigma Chemical, St. Louis, MO). The secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG antibodies (Santa Cruz Biotechnology). Protein bands were detected by an enhanced chemiluminescence kit (Pierce Biotechnology, Rockford). The intensity of the protein bands was quantified using Scion Image software.
Statistical analysis
Data were expressed as mean ± SE. Comparisons between untreated control cells and the caged xanthone-treated cells were made using Student’s t test. Differences were considered significant at P < 0.05. All analyses were performed using SPSS version 10.0 (SPSS Inc., USA).
RESULTS
Anti-proliferative effects of four caged xanthones on CCA cell lines
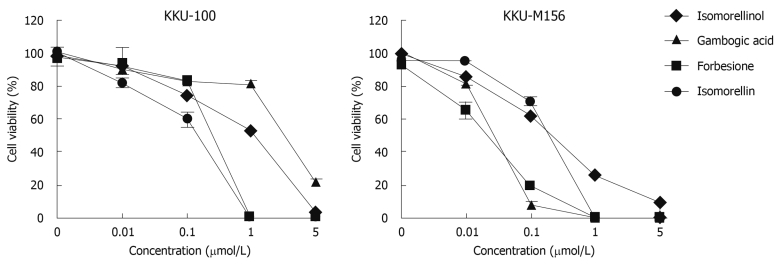
The treatment of KKU-100 and KKU-M156 cells with four caged xanthones markedly inhibited cell growth in a dose-dependent manner (Figure 2). The IC50 values of these caged xanthones and doxorubicin are shown in Table 2. The anticancer potencies of these compounds are comparable to doxorubicin in both cell lines. These caged xanthones induced growth inhibition in the CCA cell lines about 4 to > 8 × 106 times greater than that for normal PBMCs (Table 2). These results indicate that these compounds possess considerable potential for further development.
Figure 2.
Antiproliferative activities of the four caged xanthones against KKU-100 and KKU-M156 cells. Cells were treated with DMSO or indicated amounts of isomorellinol, forbesione, gambogic acid and isomorellin for 72 h. The percent cell viability was determined by SRB assay. Data are expressed as mean ± SE (n = 3).
Table 2.
Concentrations of four caged xanthones and doxorubicin causing a 50% inhibitory effect (IC50) in cell proliferation
| Cells |
IC50 values (μmol/L) |
||||
| Isomorellin | Isomorellinol | Forbesione | Gambogic acid | Doxorubicin | |
| KKU-100 | 0.11 ± 0.004 | 2.2 ± 0.33 | 0.15 ± 0.007 | 2.64 ± 1.29 | 0.66 ± 0.001 |
| KKU-M156 | 0.12 ± 0.005 | 0.43 ± 0.06 | 0.02 ± 0.002 | 0.03 ± 0.004 | 0.36 ± 0.001 |
| PBMC | > 88 × 104 | 59 ± 2.9 | 59.5 ± 1.0 | 11 ± 3 | ND |
Data are mean ± SE of three independent experiments. ND: Not detected.
Apoptosis induction by four caged xanthones in CCA cell lines
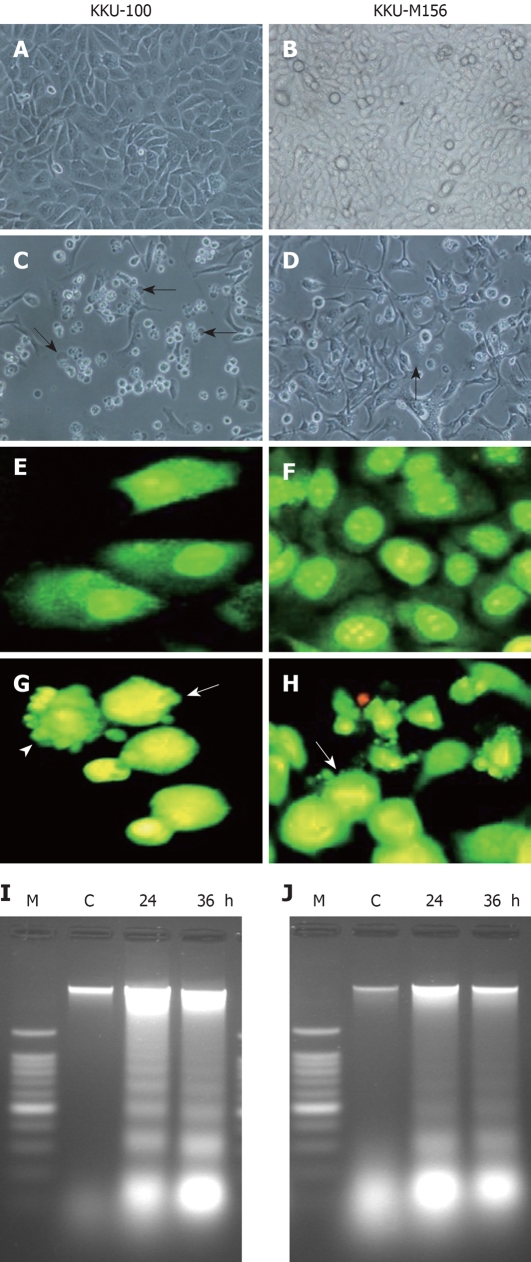
In both cell lines, treatment with four caged xanthones resulted in cell shrinkage, rounding and membrane blebbing, thus taking on the typical characteristics of apoptotic cells (Figure 3C and D); whereas no effect was observed with the vehicle control cells (Figure 3A and B). Using EB/AO staining, both cell lines showed nuclei with homogeneous chromatin distribution under vehicle-control conditions (Figure 3E and F). In the treated cells, characteristic apoptotic features such as chromatin condensation and nuclear fragmentation were observed (Figure 3G and H). The number of apoptotic cells in both cell lines treated with four caged xanthones was significantly increased in a time-dependent manner (Table 3). Using an agarose gel electrophoresis, a typical ladder pattern of internucleosomal DNA fragmentation, a hallmark of apoptotic cell death[20], was observed in the treated cells (Figure 3I and J).
Figure 3.
Induction of apoptosis in cholangiocarcinoma (CCA) cell lines by four caged xanthones. Photomicrographs (400 ×) of CCA cell lines KKU-100 (A, C) and KKU-M156 (B, D) treated with DMSO and gambogic acid for 48 h. A, B: Treated with DMSO; C, D: Treated with gambogic acid. Cells with membrane blebbing indicated by arrows; Fluorescence photomicrographs (400 ×) of CCA cell lines KKU-100 (E, G) and KKU-M156 (F, H) treated with DMSO and isomorellinol for 36 h followed by EB/AO staining. E, F: Treated with DMSO; G, H: Treated with isomorellinol. Nuclei of treated cells showing chromatin condensation (arrows) and nuclear fragmentation (arrow heads); DNA fragmentation of CCA cell lines treated with forbesione for the indicated times (24 h and 36 h). Genomic DNA was isolated and separated on 1.6% agarose gels containing 0.1 mg/mL ethidium bromide. I: KKU-100 cells; J: KKU-M156 cells. Lane C: DNA band of CCA cell lines treated with DMSO; Lane M: DNA markers. The figures show representative results of three independent experiments.
Table 3.
Percentage of apoptotic cells in the four caged xanthone-treated and non treated CCA cell lines
| Compounds |
% apoptotic cells |
|||
|
KKU-100 (h) |
KKU-M156 (h) |
|||
| 36 | 48 | 24 | 36 | |
| Control | 8 ± 1.45 | 13 ± 0.66 | 8 ± 0.88 | 23 ± 0.66 |
| Isomorellinol | 44 ± 2.31b | 79 ± 1.76b | 34 ± 1.50b | 89 ± 1.20b |
| Forbesione | 34 ± 0.67b | 78 ± 1.15b | 38 ± 3.17b | 83 ± 3.05b |
| Gambogic acid | 32 ± 0.87b | 70 ± 1.45b | 17 ± 1.50a | 72 ± 0.66b |
| Isomorellin | 25 ± 1.21b | 69 ± 1.76b | 31 ± 0.58b | 77 ± 4.16b |
Data are mean ± SE of three independent experiments.
P < 0.05,
P < 0.01 vs control. CCA: Cholangiocarcinoma.
Effect of four caged xanthones on the expression of apoptosis-related genes and proteins
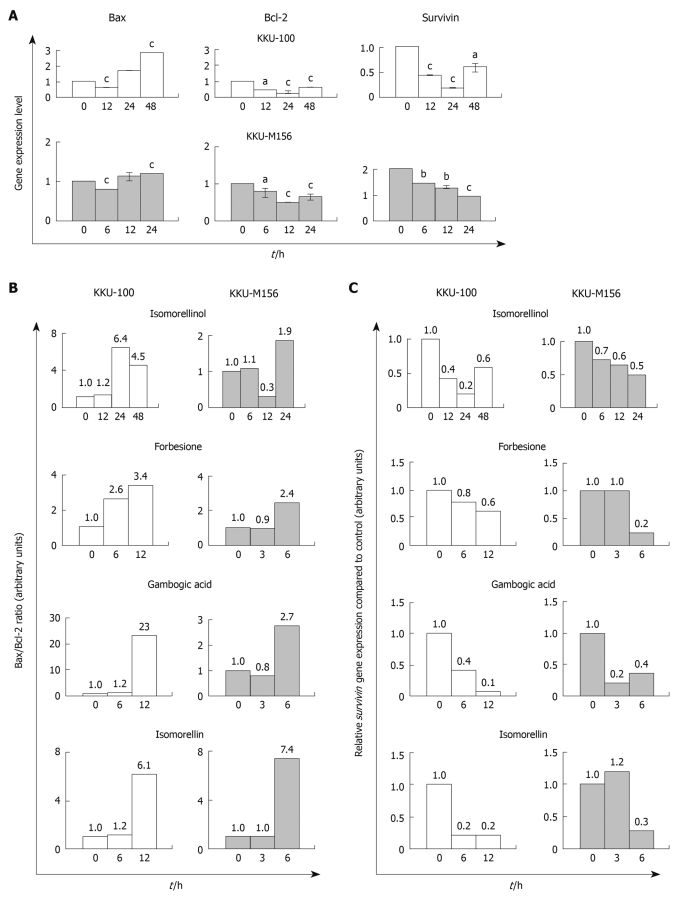
In both CCA cell lines treated with isomorellinol there was a significant increase in both mRNA (Figure 4A) and protein (Figure 5) of pro-apoptotic Bax, but a marked decrease in the anti-apoptotic Bcl-2 (Figure 4A and Figure 5) in a time-dependent manner leading to an enhanced Bax/Bcl-2 ratio (Figure 4B and Table 4). The same results were obtained when both CCA cell lines were treated with the other three compounds (Figure 4B and Table 4). Using Western blotting, isomorellinol was found to have the highest potency in increasing Bax/Bcl-2 ratio. At 48 h of treatment, isomorellinol increased the Bax/Bcl-2 ratio up to 120 and 41.4 in the KKU-100 and KKU-M156 cells, respectively (Table 4).
Figure 4.
The four caged xanthones induce up-regulation of Bax with down-regulation of Bcl-2 and survivin gene expressions in CCA cell lines. KKU-100 and KKU-M156 cells were treated with DMSO or the four caged xanthones for the indicated times. Real-time polymerase chain reaction for each gene was performed as described in Materials and Methods. Data were expressed as mean ± SE fold increase/decrease in mRNA expression compared to control, normalized with GAPDH (n = 3). aP < 0.05, bP < 0.01, cP < 0.001 vs control. All four compounds showed the same result. A: The histograms of the isomorellinol-treated cells were used as a representative; B: Bax/Bcl-2 ratio; C: Relative survivin gene expression compared to control.
Figure 5.
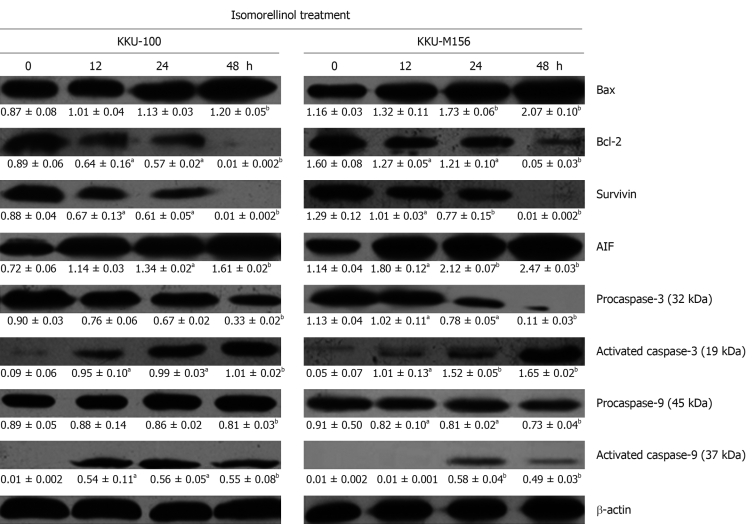
The four caged xanthones induce increase of Bax, AIF, activated caspase-3 and -9 while decreasing Bcl-2, survivin, procaspase-3 and -9 protein levels in CCA cell lines. KKU-100 and KKU-M156 cell lines were treated with DMSO or the four caged xanthones for the indicated times. Western blottings for each protein were performed as described in Materials and Methods. The mean ± SE of density reading of target protein normalized to β-actin was expressed in the respective box (n = 3). aP < 0.05, bP < 0.01 vs control. All four compounds showed the same result. The protein bands of the isomorellinol-treated cells were used as a representative.
Table 4.
Bax/Bcl-2 ratios of the four caged xanthone-treated and non treated CCA cell lines
| Compounds |
Bax/Bcl-2 ratio |
|||||||
|
KKU-100 (h) |
KKU-M156 (h) |
|||||||
| 0 | 12 | 24 | 48 | 0 | 12 | 24 | 48 | |
| Isomorellinol | 0.98 | 1.58 | 1.98 | 120 | 0.73 | 1.04 | 1.43 | 41.40 |
| Forbesione | 1.02 | 1.93 | 2.35 | 2.91 | 1.53 | 2.38 | 5.12 | 7.02 |
| Gambogic acid | 0.81 | 1.51 | 3.42 | 7.00 | 0.43 | 0.72 | 1.83 | 3.14 |
| Isomorellin | 0.98 | 1.98 | 3.12 | 5.20 | 0.56 | 0.70 | 1.00 | 1.90 |
Data are expressed as ratio of mean of protein expression level of Bax to Bcl-2 in control and the caged xanthone-treated CCA cell lines.
Western blotting analysis revealed that treatment of both cell lines with isomorellinol resulted in a significant reduction of procaspase-3 and -9 while increasing activated caspase-3 and -9 in a time-dependent manner (Figure 5). The treatment of both CCA cell lines with the other three compounds also showed the same results (data not shown). When the relative intensities of activated caspase-3 and -9 to β-actin of treated cells were compared to control cells (0 h), there was a multi-fold increase in the activated caspase-3 and -9 (Table 5). The high degree of multi-fold increase in activated caspase-9 was detected in the isomorellinol-treated KKU-100 and KKU-M156 cells (56 × and 58 ×, respectively), in gambogic acid-treated KKU-100 cells (78 ×) and in isomorellin-treated KKU-M156 cells (34 ×) (Table 5). All four compounds were also shown to increase the activated caspase-3 level in treated KKU-M156 cells, from 10 × to 46 × compared with the control cells (0 h), with a high degree of activated caspase-3 for isomorellinol-treated (33 ×) and gambogic acid-treated KKU-M156 cells (46.7 ×) (Table 5).
Table 5.
Fold decrease or increase of apoptotic-related proteins of the four caged xanthone-treated compared to non treated CCA cell lines
| Apoptotic proteins | Compounds |
Fold change in protein of the treated cells compared to control cells |
|||||||
|
KKU-100 (h) |
KKU-M156 (h) |
||||||||
| 0 | 12 | 24 | 48 | 0 | 12 | 24 | 48 | ||
| Activated caspase-9 | Isomorellinol | 1 | 54 | 56 | 55 | 1 | 1 | 58 | 49 |
| Forbesione | 1 | 9.6 | 13.1 | 13 | 1 | 5.7 | 8 | 5.6 | |
| Gambogic acid | 1 | 30 | 78 | 60 | 1 | 2.6 | 4.5 | 3.9 | |
| Isomorellin | 1 | 6.3 | 12.3 | 9.3 | 1 | 1 | 29 | 34 | |
| Activated caspase-3 | Isomorellinol | 1 | 10.6 | 11 | 11.2 | 1 | 20.2 | 30.4 | 33 |
| Forbesione | 1 | 6 | 9.6 | 12 | 1 | 5.7 | 9.6 | 10 | |
| Gambogic acid | 1 | 5.5 | 7.5 | 12.2 | 1 | 28 | 39.3 | 46.7 | |
| Isomorellin | 1 | 8.9 | 10 | 13 | 1 | 5 | 5.7 | 12 | |
| Survivin | Isomorellinol | 1 | 0.8 | 0.7 | 0.01 | 1 | 0.8 | 0.6 | 0.01 |
| Forbesione | 1 | 0.5 | 0.54 | 0.3 | 1 | 0.7 | 0.54 | 0.5 | |
| Gambogic acid | 1 | 0.6 | 0.4 | 0.3 | 1 | 0.7 | 0.6 | 0.4 | |
| Isomorellin | 1 | 0.6 | 0.5 | 0.3 | 1 | 0.8 | 0.5 | 0.3 | |
| AIF | Isomorellinol | 1 | 1.6 | 1.9 | 2.3 | 1 | 1.6 | 1.9 | 2.2 |
| Forbesione | 1 | 1.2 | 1.6 | 2.1 | 1 | 1.6 | 2.8 | 4 | |
| Gambogic acid | 1 | 1.5 | 1.6 | 2.7 | 1 | 1.6 | 2.1 | 3.4 | |
| Isomorellin | 1 | 1.9 | 3.3 | 3.5 | 1 | 1.3 | 1.7 | 2.5 | |
Data are expressed as ratio of mean of protein expression level of the apoptotic-related proteins in the caged xanthone-treated to non treated CCA cell lines.
The real-time PCR and Western blotting analysis exhibited a significant time-dependent decrease in survivin gene (Figure 4A) and protein (Figure 5) expression in the isomorellinol-treated KKU-100 and KKU-M156 cell lines. The same results were obtained when these cell lines were treated with the three other compounds (data not shown). The multi-fold decreases in survivin gene and protein expression are shown in Figure 4C and Table 5, respectively. Using Western blotting, isomorellinol showed the highest potency in suppression of survivin protein expression (0.01 ×) (Table 5).
Western blotting analysis revealed that cells treated with isomorellinol resulted in a significant increase of AIF protein (Figure 5). Similar results were obtained for both CCA cell lines treated with the other three compounds (data not shown). Similar multi-fold increases in AIF protein expression were found for the four compound-treated cells as compared to controls (Table 5).
DISCUSSION
The present study demonstrated that the four caged xanthones; isomorellinol, forbesione, gambogic acid and isomorellin, exerted strong growth inhibition with different IC50 values in both CCA cell lines in a dose-dependent manner (Table 2 and Figure 2). The results indicate that these two CCA cell lines responded differently to different compounds. However, the potencies of these four compounds were very high, indicating their broad spectrum of anti-CCA effects. In addition, the selectivity of these compounds toward cancer cell lines compared to normal PBMCs was demonstrated (Table 2). Previously, these four caged xanthones have been shown to inhibit the growth of various mammalian cancer cell lines[10]. Gambogic acid was reported to be an effective telomerase inhibitor, displaying potent anticancer activity both in vitro and in vivo[8-11], and was shown to kill cancer cells selectively with no influence on normal cells[9]. The anticancer potencies of these caged xanthones were comparable to doxorubicin. Due to their potencies, the anticancer mechanisms of these four compounds deserve further investigation.
The growth inhibitory effect of these caged xanthones in both CCA cell lines was modulated through apoptosis which was evidenced by the typical morphological characteristics of apoptosis, such as cell shrinkage, membrane blebbing, chromatin condensation, nuclear fragmentation and DNA ladder formation (Figure 3). Cleavage of DNA at the inter-nucleosomal linker sites yielding DNA fragments has been used as a hallmark of apoptosis[20]. Consistent with our study, the apoptotic effects of gambogic acid in human breast tumor cells T47D[21] and human gastric carcinoma cells MGC-803[9] have been reported.
Apoptosis is regulated by several different genes in response to various stimuli. The Bcl-2 family of proteins plays major roles in apoptotic regulation through the mitochondrial pathway[22]. Our results show that these caged xanthones down-regulated the expression of the Bcl-2 gene and protein while up-regulating the expression of Bax gene and protein, leading to an increase in the Bax/Bcl-2 ratio (Figure 4B and Table 4). In agreement with our study, gambogic acid-induced apoptosis by regulation of the expression of Bax and Bcl-2 proteins in the human gastric carcinoma cell line MGC-803 has been reported[9]. Bax protein has previously been reported to induce the intrinsic apoptosis pathway by inhibiting the function of Bcl-2[23], and by increasing the release of death-mediated proteins such as cytochrome C, Smac/DIABLO and AIF from the inter-membrane space to the cytosol[24,25]. Our results show a significant increase in the level of AIF protein in response to the four caged xanthone treatments (Figure 5 and Table 5). This result suggests that the AIF, caspase-independent mitochondrial pathway[25] may be involved in apoptosis induction in the caged xanthone-treated cells.
In the intrinsic apoptotic pathway, the release of cytochrome C from mitochondria triggers caspase-9 and -3 activation through formation of the apoptosome complex. Then the activated caspase-3 is able to cleave multiple cellular substrates leading to DNA fragmentation[24]. Our results show that caspase-9 and -3 were activated by the caged xanthones (Figure 5 and Table 5), suggesting that these caged xanthones induced apoptosis in both CCA cell lines through caspase-9 and -3 activation. Previously, mitochondrial destabilization and caspase-3 activation were reportedly involved in the apoptosis of Jurkat cells induced by gaudichaudione A, a cytotoxic xanthone[26]. Gambogic acid reportedly induced apoptosis and activated caspases in T47D cells[21].
The family of human inhibitor of apoptosis proteins (IAP), including XIAP, c-IAP1, c-IAP2, NAIP and survivin, have been reported to be direct inhibitors of activated caspase-3 and -7[27,28]. Our data show that survivin gene and protein expressions were decreased by the four caged xanthones (Figure 4A and C, and Table 5). Since these compounds were found to induce apoptosis in both CCA cell lines by activation of caspase-3, the factor contributing to caspase-3 activation in these treated cells may be a decreased survivin expression. Previously, gambogic acid has been reported to down-regulate IAP, IAP1 and IAP2 in human myeloid leukemia cells[29].
Using Western blotting, isomorellinol was found to have the highest potency in increasing the Bax/Bcl-2 ratio (Table 4) and in decreasing survivin protein expression (Figure 5 and Table 5). This result was also confirmed by the greatly increased amount of activated caspase-3 and -9 in both isomorellinol-treated CCA cell lines (Table 5). Gambogic acid was shown to have lower potency in increasing the Bax/Bcl-2 ratio (Table 4) and decreasing survivin protein expression (Table 5); whereas the increased level of activated caspase-3 was similar to that of isomorellinol (Table 5). These results suggest that the activation of caspase-3 might be stimulated by caspase-12 through endoplasmic reticulum stress-induced apoptosis[30]. Comparing these two CCA cell lines, both isomorellinol and gambogic acid showed higher potency in increasing activated caspase-3 in KKU-M156 cells than KKU-100 cells (Table 5). This may be due to the different histological type of the CCA cell lines. KKU-100 is a poorly differentiated adenocarcinoma which has a higher resistance to chemotherapeutic drugs than KKU-M156, a moderately differentiated adenocarcinoma[13]; whereas forbesione and isomorellin showed the same potency against both CCA cell lines. The latter result may be due to the different functional groups present in these four caged xanthones.
The chemical structure diversity of the four compounds reflects the biological activities. The common feature of all four compounds is the presence of the novel caged structure, which is believed to be a prerequisite for potent anticancer activity in comparison with normal xanthones and chromyl xanthones. The chromene ring is absent in forbesione compared with the other three compounds. Forbesione has only two non-functionalized prenyl side-chains, whereas isomorellin, isomorellinol and gambogic acid are highly prenylated with one of the prenyl methyl groups functionalized as an aldehyde, a primary alcohol and a carboxylic acid functional group, respectively. The functional group variations do not appear to affect the anticancer activity against the two CCA cell lines; however, at a mechanistic level, isomorellinol exhibited the highest potency in increasing the Bax/Bcl-2 ratio and in decreasing survivin protein expression. Other activities at the molecular level indicate that functional groups on the prenyl side chain may be important. It is premature to conclude what the definite structure-activity relationship may be but this preliminary insight provides a basis for further medicinal chemistry studies.
In conclusion, our findings demonstrate for the first time that the four caged xanthones, isolated from G. hanburyi, are capable of inhibiting proliferation and inducing apoptosis in CCA cell lines. This is also the first report which shows the molecular mechanism of the growth inhibitory effects of isomorellinol, forbesione and isomorellin. These compounds induce down-regulation of Bcl-2 protein while up-regulating the Bax and AIF proteins, leading to the activation of caspase-9 and -3 and DNA fragmentation. Moreover, the down-regulation of survivin protein thus maintains caspase-3 in an active state and stimulates the molecular cascade of apoptosis. Based on these results, we conclude that these four caged xanthones stimulate both caspase- and non-caspase mitochondrial-dependent apoptosis in KKU-100 and KKU-M156 cells. Furthermore, it appears the mitochondrial apoptosis pathway is induced by the upstream receptor-ligand mediated apoptosis pathway. The precise target of action of these four caged xanthones therefore remains to be elucidated. There is a potential role for these caged xanthones in cancer therapy.
COMMENTS
Background
Cholangiocarcinoma (CCA) is a malignant tumor, characterized by a poor prognosis and unresponsive to conventional chemotherapeutic agents. Therefore, searching for novel and effective therapeutic agents for CCA is necessary. Several caged xanthones from Garcinia hanburyi (G. hanburyi) have been reported to be potent antiproliferatives, as well as having anticancer and anti-tumor activities, and induce apoptosis in various cancer cell lines.
Research frontiers
The four caged xanthones from G. hanburyi; isomorellin, isomorellinol, forbesione and gambogic acid, exerted strong growth inhibition in both KKU-100 and KKU-M156 cells, indicating their broad spectrum of anti-CCA effects. These compounds were shown to kill cancer cells selectively with no influence on normal peripheral blood mononuclear cells (PBMCs). Growth suppression by these compounds was due to apoptosis which correlated with an increase in Bax/Bcl-2 ratio and apoptosis-inducing factor, activation of caspase-9 and -3, and a decrease in survivin expression. At mechanistic level, isomorellinol exhibited the highest potency.
Innovations and breakthroughs
This is the first report which shows the growth inhibitory effect and molecular mechanisms of the four caged xanthones isolated from G. hanburyi in CCA cell lines. The results suggest that these compounds stimulate both a caspase- and non-caspase mitochondrial-dependent signaling pathway in CCA cell lines.
Applications
The chemical structure diversity of the four compounds reflects the biological activities. At the molecular level, isomorellinol exhibited the highest potency, indicating that functional groups on the prenyl side chain may be important. It is premature to conclude what the definite structure-activity relationship may be, but this preliminary insight provides a basis for further medicinal chemistry studies. Based on these results, The authors suggest that these four caged xanthones are compounds with great promise and may serve as a potential source and lead-structure for the development of a drug for the treatment of CCA.
Peer review
The manuscript by Hahnvajanawong et al evaluates the mechanism by which various caged xanthones exert their antiproliferative activity on cholangiocarcinoma cell lines. The data presented are convincing and the manuscript well written.
Acknowledgments
The authors are grateful to Dr. Napaporn Kaewdoungdee and Mr. Khosit Pinmai for technical assistance. The authors sincerely thank Professor James A Will, University of Wisconsin-Madison, USA for reviewing of the manuscript and Mr. Bryan Roderick Hamman for assistance with the English language presentation of the manuscript.
Footnotes
Supported by Grants from the Center of Excellence for Innovation in Chemistry, Commission on Higher Education, No. 48-03-3-00-144; Faculty of Medicine, No. 51-03-2-00-008 and Khon Kaen University, No. 50-03-1-01-005, Research Funds, Khon Kaen University, Thailand
Peer reviewer: Sharon DeMorrow, Assistant Professor, Division of Research and Education, Scott and White Hospital and The Texas A&M University System, Health Science Center College of Medicine, Temple, TX 76504, United States
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
References
- 1.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR, Fujiwara Y, Pazdur R, Newman DJ, Dagher R, et al. Anticancer drug discovery and development throughout the world. J Clin Oncol. 2002;20:47S–59S. [PubMed] [Google Scholar]
- 5.Saralamp P, Chuakul W, Temsiririrkkul R, Clayton T. Medicinal plants in Thailand. Vol 1. Bangkok: Amarin Printing and Publishing Public Co., Ltd; 1996. pp. 209–211. [Google Scholar]
- 6.Han QB, Wang YL, Yang L, Tso TF, Qiao CF, Song JZ, Xu LJ, Chen SL, Yang DJ, Xu HX. Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. Chem Pharm Bull (Tokyo) 2006;54:265–267. doi: 10.1248/cpb.54.265. [DOI] [PubMed] [Google Scholar]
- 7.Asano J, Chiba K, Tada M, Yoshii T. Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry. 1996;41:815–820. doi: 10.1016/0031-9422(95)00682-6. [DOI] [PubMed] [Google Scholar]
- 8.Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol Sin. 2004;25:769–774. [PubMed] [Google Scholar]
- 9.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27:998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 10.Reutrakul V, Anantachoke N, Pohmakotr M, Jaipetch T, Sophasan S, Yoosook C, Kasisit J, Napaswat C, Santisuk T, Tuchinda P. Cytotoxic and anti-HIV-1 caged xanthones from the resin and fruits of Garcinia hanburyi. Planta Med. 2007;73:33–40. doi: 10.1055/s-2006-951748. [DOI] [PubMed] [Google Scholar]
- 11.Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull. 2004;27:1769–1774. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- 12.Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100) World J Gastroenterol. 2005;11:3392–3397. doi: 10.3748/wjg.v11.i22.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tepsiri N, Chaturat L, Sripa B, Namwat W, Wongkham S, Bhudhisawasdi V, Tassaneeyakul W. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J Gastroenterol. 2005;11:2748–2753. doi: 10.3748/wjg.v11.i18.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 15.Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol. 2008;14:1491–1497. doi: 10.3748/wjg.14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann M, Lorenz HM, Voll R, Grünke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namwat N, Amimanan P, Loilome W, Jearanaikoon P, Sripa B, Bhudhisawasdi V, Tassaneeyakul W. Characterization of 5-fluorouracil-resistant cholangiocarcinoma cell lines. Chemotherapy. 2008;54:343–351. doi: 10.1159/000151541. [DOI] [PubMed] [Google Scholar]
- 19.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–163. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 20.Compton MM. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992;11:105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, Drewe J, Cai SX. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12:309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Tilli CM, Stavast-Koey AJ, Ramaekers FC, Neumann HA. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29:79–87. doi: 10.1034/j.1600-0560.2002.290203.x. [DOI] [PubMed] [Google Scholar]
- 24.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 25.Bröker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Cao S, Goh S, Hsu A, Tan BK. Mitochondrial destabilisation and caspase-3 activation are involved in the apoptosis of Jurkat cells induced by gaudichaudione A, a cytotoxic xanthone. Planta Med. 2002;68:198–203. doi: 10.1055/s-2002-23142. [DOI] [PubMed] [Google Scholar]
- 27.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 28.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 29.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]