Abstract
AIM: To elucidate the role of insulin resistance (IR) and serum adiponectin level in hepatocellular carcinoma (HCC) associated with chronic hepatitis C.
METHODS: Clinical and biochemical characteristics were collected from 165 consecutive patients with newly diagnosed HCC. Homeostasis model assessment of IR (HOMA-IR) and serum adiponectin level were investigated in 188 patients with different stages of hepatitis C virus (HCV) infection.
RESULTS: Among HCC patients, type 2 diabetics (DM) was more prevalent in HCV subjects (35.6%, n = 59) compared to hepatitis B virus (HBV; 12.7%, n = 63) or non-HBV, non-HCV cases (7.1%, n = 28). In patients with chronic hepatitis C, HCC subjects had higher blood sugar (P < 0.001), insulin level (P = 0.003) and HOMA-IR (P = 0.018) than those with chronic hepatitis and advanced fibrosis. Age, male sex and body mass index were significantly associated with serum adiponectin level, whereas HOMA-IR was not. Based on stepwise logistic regression analysis, age (OR: 1.124, P < 0.001), serum insulin level (OR: 1.585, P < 0.001), HOMA-IR (OR: 0.495, P = 0.001), DM (OR: 11.601, P = 0.002) and male sex (OR: 3.877, P = 0.016) were independently associated with HCC. This result was similar even if the diabetic subjects were excluded for analysis.
CONCLUSION: Insulin resistance measured by HOMA-IR, regardless of the presence of diabetes, is significantly associated with HCC development in patients with chronic HCV infection.
Keywords: Hepatitis C virus, Hepatocellular carcinoma, Insulin resistance, Diabetes, Adiponectin
INTRODUCTION
Hepatitis C virus (HCV) infects hundreds of millions of people persistently, and induces a spectrum of chronic liver disease worldwide[1]. Chronic HCV infection causes progressive hepatic fibrosis and cirrhosis in up to 20% of patients, and approximately 10%-20% of cirrhotic patients may develop hepatocellular carcinoma (HCC) within 5 years[2-4]. Recent cohort studies have indicated that HCC is the most frequent cause of death in patients infected with HCV, and epidemiological trends suggest that the mortality rate is rising[5]. Thus, understanding the risk factors for HCC development in patients infected with HCV is of great importance.
HCV may contribute to carcinogenesis by causing advanced fibrosis or cirrhosis, which represents a precancerous state accompanied by increased DNA synthesis[6,7]. Nevertheless, several factors associated with HCC development in chronic hepatitis C have been reported, such as male sex, older age at infection, excessive alcohol consumption, coinfection with hepatitis B virus (HBV) and some viral variability in HCV itself[8-11]. Recently, epidemiological studies have demonstrated that diabetes mellitus (DM) is associated with a 2-4-fold increase in the risk of HCC, regardless of the presence of other major HCC risk factors (HBV, HCV, and alcoholic liver disease)[12-15]. In particular, two large cohort studies have shown that DM is associated with a higher risk of HCC development in patients with chronic hepatitis C compared to HBV-infected subjects or those without HBV and HCV infections[12,13].
The mechanisms that may link DM with carcinogenesis in chronic HCV infection remain unclear. Insulin resistance (IR), which correlates inversely with circulating adiponectin concentration, is a consistent finding in patients with type 2 DM[16,17]. Previous studies have shown that patients infected with HCV have significantly higher IR than healthy controls matched for age, sex and body mass index (BMI)[18,19]. Recent studies have suggested that IR plays a crucial role in fibrosis progression, and has been demonstrated to have a negative impact on treatment responses to antiviral therapy in patients with chronic hepatitis C[18,20,21]. IR has emerged as a risk factor for a wide variety of cancers, including endometrial and breast (especially after menopause), colon and rectal, esophageal, kidney, pancreatic, biliary, ovarian and cervical cancer[22-24]. To the best of our knowledge, the role of IR and serum adiponectin level in the development of HCC associated with chronic HCV infection has not been established. In this present study, we prospectively investigated the IR assessed by the homeostasis model (HOMA-IR) and serum adiponectin level in patents with HCC and those with other clinical stages of chronic HCV infection.
MATERIALS AND METHODS
Patients
From January 2007 to August 2008, a total of 165 newly diagnosed patients with HCC (122 men and 43 women; median age: 60.1 ± 12.4 years) who fulfilled all criteria outlined below were consecutively collected in a single center. The diagnosis of HCC was based on either the histopathological findings in tumor tissue, one typical HCC feature on a dynamic image or alpha-fetoprotein (AFP) > 200 ng/mL if the nodule was > 2 cm in cirrhotic liver, or two typical HCC features of dynamic images if the nodule was between 1 and 2 cm in a cirrhotic liver[25]. Patients with concurrent human immunodeficiency virus infection, significant change of body weight (≥ 3 kg within 3 mo), previous history of interferon-based antiviral therapy, and current treatment with any dosage of insulin therapy were excluded.
Of these patients, 63 were positive for hepatitis B surface antigen (HBsAg) (Abbott Laboratories, North Chicago, IL, USA), 59 were positive for anti-HCV antibody (third-generation ELISA; AxSYM HCV 3.0; Abbott Laboratories), 15 were positive for both HBsAg and anti-HCV, and 28 were negative for HBsAg and anti-HCV, in whom alcoholic liver disease (n = 11, 39%) was the major cause of HCC.
During the same period, 129 consecutive patients (61 men and 68 women, 23-77 years old; median age: 53.0 ± 11.5 years) with chronic HCV infection who consulted our clinics were studied, including 86 with chronic hepatitis (F0-2) and 43 with advanced fibrosis (F3-4). All patients had positive anti-HCV antibody and detectable HCV RNA (Amplicor™; Roche Diagnostics, Branchburg, NJ, USA). Pathological diagnosis of chronic hepatitis or advanced fibrosis was made by percutaneous liver biopsies according to the modified Knodell histological activity index[26], which were analyzed by pathologists who were blind to the patients’ characteristics. The Human Research and Ethics Committee (Institutional Review Board) approved the study, and informed consent was obtained from each patient involved in the study.
Clinical and laboratory assessments
Patients with a BMI of 18.5-24.9 kg/m2 were classified as normal, 25-29.9 as overweight, and ≥ 30 as obese. The diagnosis of type 2 DM was based on the American Diabetes Association revised criteria, using a value of fasting blood glucose of ≥ 126 mg/dL on at least two occasions[27], or ongoing treatment with hypoglycemic agents.
Blood glucose, serum insulin level and stored serum samples for adiponectin were collected after 12 h of overnight fasting from each individual. For HCC patients, serum samples were collected before any treatment for tumor. Serum insulin was measured by radioimmunoassay (Coat-A-Count insulin kit; Diagnostic Products Corp., Los Angeles, CA, USA). IR was calculated by the HOMA-IR using the following formula: HOMA-IR = fasting insulin (μU/mL) × plasma glucose (mmol/L)/22.5. Circulating level of adiponectin was measured in duplicate by sandwich ELISA using commercial kits according to the manufacturer’s instructions (Quantikine ELISA kits; R&D Systems, Inc., Minneapolis, MN, USA). The differences between duplicate wells were consistently less than 10% of the mean values. The mean values of duplicate measurements were used in the analyses.
Statistical analysis
Continuous data are expressed as the median (interquartile range), and the categorical data are expressed as a number (percentage). Comparisons of differences in the categorical date between groups were performed using the χ2 test. Distributions of continuous variables were analyzed by the Mann-Whitney U test or one-way ANOVA test with least significant difference (LSD) post-hoc correction between groups where appropriate. Spearman’s correlation coefficient analysis was used to evaluate the factors associated with HOMA-IR and adiponectin level. Multiple linear regression analysis with stepwise variable selection was performed to assess the independent factors. Stepwise logistic regression analysis was performed to assess the influence of each factor on the risk of developing HCC. All analyses were carried out using SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA). All tests were two-tailed, and P < 0.05 was considered statistically significant.
RESULTS
Comparison of baseline features among HBV, HCV, dual HBV/HCV, and non-HBV, non-HCV-related HCC
Table 1 shows the comparison of baseline features among the 165 patients with HCC related to different etiology. The median age of HCV-related HCC patients (66 years) was significantly higher than that in HCC patients infected with HBV (55 years) or dual HBV/HCV (60 years) (P < 0.001). There was a male predominance among all four groups. The prevalence of DM was higher (35.6%) in patients with HCV-related HCC compared to those infected with HBV (12.7%) (P < 0.005) or non-HBV, non-HCV subjects (7.1%) (P < 0.005). HOMA-IR was higher in HCC patients with HCV (median 4.4) than in those with HBV (median 3.5) (P < 0.05). However, there was no significant difference in BMI and prevalence of overweight and obesity among the four groups. Patients with HCV-related HCC had significantly higher aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and lower platelet counts than those infected with HBV or non-HBV, non-HCV subjects. In addition, patients with HCV-related HCC had smaller tumors and earlier Barcelona clinic liver cancer stage (BCLC) than HBV or non-HBV, non-HCV subjects.
Table 1.
Comparison of baseline features among HBV, HCV, dual HBV and HCV, and non-HBV, non-HCV related hepatocellular carcinoma patients
| HBV n = 63 | HCV n = 59 | HBV + HCV n = 15 | Non-HBV, non-HCV n = 28 | P value | |
| Age (yr)1 | 55 (46-66)ac | 66 (57-75)ad | 60 (50-68)d | 61 (55-67)c | < 0.001 |
| Sex (M/F) | 45/18 | 41/18 | 10/5 | 26/2 | 0.093 |
| DM (%) | 8 (12.7)a | 21 (35.6)ae | 4 (26.7) | 2 (7.1)e | 0.003 |
| BMI (kg/m2)1 | 24.3 (22.2-26.8) | 23.8 (21.8-26.6) | 23.6 (20.9-25.5) | 24.5 (23.3-26.7) | 0.439 |
| Overweight (%) | 24 (38) | 22 (37) | 4 (27) | 10 (36) | 0.797 |
| Obesity (%) | 4 (6) | 5 (8) | 1 (7) | 2 (7) | 0.965 |
| AST (U/L)1 | 48 (38-71)a | 72 (39-123)ae | 72 (47-111) | 38 (29-52)e | 0.002 |
| ALT (U/L)1 | 45 (34-63)a | 63 (29-100)ae | 82 (54-117) | 27 (21-39)e | 0.011 |
| Child–Pugh class (A/B, C) | 47/16 | 46/13 | 11/4 | 23/5 | 0.857 |
| Platelet (104/μL)1 | 16.5 (12.0-25.0)a | 13.2 (8.7-17.1)ae | 12.7 (7.6-18.1)f | 21.1 (15.3-24.1)ef | 0.001 |
| AFP (ng/mL)1 | 15 (6-525) | 28 (7-256) | 38 (11-4388) | 15 (6-8027) | 0.468 |
| Tumor size (≤ 3 cm) (%) | 14 (22.2)a | 29 (49.2)ae | 7 (46.7) | 5 (17.9)e | < 0.001 |
| BCLC (≤ early stage) (%) | 20 (31.7)ab | 31 (52.5)aef | 9 (60.0)b | 4 (14.3)ef | 0.001 |
| HOMA-IR | 3.5 (2.0-8.6)a | 4.4 (2.9-6.6)a | 3.2 (1.7-10.7) | 3.4 (2.0-4.6) | 0.108 |
| Insulin (μU/mL)1 | 12.8 (8.4-25.0) | 14.8 (9.9-21.30) | 11.9 (8.1-23.5) | 9.7 (7.6-14.8) | 0.256 |
| Adiponectin (μg/mL) | 4.7 (2.9-8.1)ac | 7.9 (5.2-11.0)ae | 8.0 (3.8-10.9)f | 3.7 (2.0-6.0)cef | 0.002 |
Median (interquartile range); P value by one-way ANOVA test or χ2 test;
P < 0.05 between HBV and HCV;
P < 0.05 between HBV and HBV + HCV;
P < 0.05 between HBV and non-HBV, non-HCV;
P < 0.05 between HCV and HBV + HCV;
P < 0.05 between HCV and non-HBV, non-HCV;
P < 0.05 between HBV + HCV and non-HBV, non-HCV HCC with LSD post-hoc correction. HBV: Hepatitis B virus; HCV: Hepatitis C virus; DM: Diabetes mellitus; BMI: Body mass index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; AFP: Alpha-fetoprotein; BCLC: Barcelona clinic liver cancer; HOMA-IR: Homeostasis model assessment of insulin resistance.
Comparison of baseline features, serum insulin, HOMA-IR and adiponectin level among chronic hepatitis, advanced fibrosis and HCC patients
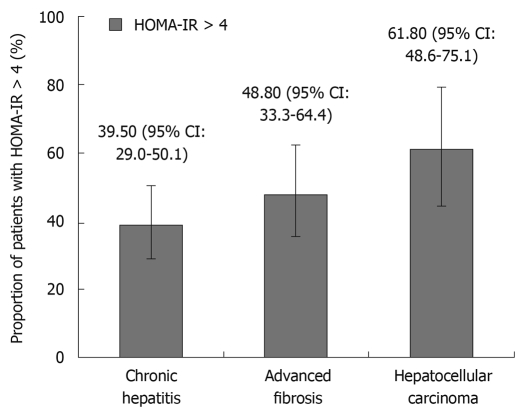
The comparison of baseline features, serum insulin, HOMA-IR and adiponectin level in different clinical stages of chronic HCV infection is shown in Table 2. The median age for HCC patients (66 years) was significantly higher than those with advanced fibrosis (56 years) and chronic hepatitis (53 years) (P < 0.001). Patients with HCC had a higher male-to-female ratio and higher prevalence of DM than those with advanced fibrosis or chronic hepatitis. There was no significant difference in BMI among these three groups. The HCC subjects had lower AST and ALT levels compared to those with advanced fibrosis; however, the platelet count was comparable between these two groups. Patients with HCC had higher blood sugar (P < 0.001), insulin level (P = 0.003) and HOMA-IR (P = 0.018) than those with chronic hepatitis and advanced fibrosis. As shown in Figure 1, patients with HCC had a higher ratio of HOMA-IR > 4 (61.8%, 95% CI: 48.6%-75.1%) than those with chronic hepatitis (39.5%, 95% CI: 29.0%-50.1%) and advanced fibrosis (48.8%, 95% CI: 33.3%-64.4%) (P = 0.036). There was no significant difference in serum adiponectin among the three groups.
Table 2.
Comparison of baseline features, HOMA-IR and adiponectin level among chronic hepatitis, advanced fibrosis and HCC patients with chronic HCV infection
| Chronic hepatitis (F0-2) n = 86 | Advanced fibrosis (F3-4) n = 43 | Hepatocellular carcinoma n = 59 | P value2 | |
| Age (yr)1 | 53 (45-58)ab | 56 (49-63)ac | 66 (57-75)bc | < 0.001 |
| Male sex (%) | 44 (51)b | 17 (40)c | 41 (69)bc | 0.008 |
| DM (%) | 13 (15)b | 12 (28) | 21 (36)b | 0.016 |
| BMI (kg/m2)1 | 24.3 (21.8-26.3) | 25.0 (22.0-29.4) | 23.8 (21.8-26.6) | 0.227 |
| AST (U/L)1 | 90 (64-122) | 114 (87-178)c | 72 (39-123)c | 0.085 |
| ALT (U/L)1 | 127 (92-187)b | 140 (102-199)c | 63 (29-100)bc | 0.001 |
| Platelet (104/μL)1 | 20.2 (15.8-22.4)ab | 13.7 (10.6-17.9)a | 13.2 (8.7-17.1)b | < 0.001 |
| Sugar (mg/dL)1 | 91 (87-101)b | 98 (88-122)c | 114 (94-172)bc | < 0.001 |
| Insulin (μU/mL)1 | 10.3 (7.7-14.4)b | 11.7 (6.9-15.9)c | 14.8 (9.9-21.3)bc | 0.003 |
| HOMA-IR1 | 3.5 (2.6-4.7)b | 4.1 (2.6-5.9)c | 4.4 (2.9-6.6)bc | 0.018 |
| Adiponectin (μg/mL)1 | 5.0 (3.4-8.4) | 5.8 (4.1-9.7) | 7.9 (5.2-11.0) | 0.222 |
Median (interquartile range);
P value by one-way ANOVA test or χ2 test;
P < 0.05 between chronic hepatitis and advanced fibrosis;
P < 0.05 between chronic hepatitis and hepatocellular carcinoma;
P < 0.05 between advanced fibrosis and hepatocellular carcinoma with LSD post-hoc correction.
Figure 1.
Comparison of high homeostasis model assessment of insulin resistance (HOMA-IR) (> 4) among different stages of chronic hepatitis C virus (HCV) infection (P = 0.036).
Factors associated with serum adiponectin level in patients with chronic hepatitis C
Table 3 shows the factors associated with serum adiponectin level in 188 patients with chronic HCV infection. By univariate analysis, age (r = 0.388, P < 0.001), male sex (P < 0.001), BMI (r = -0.281, P < 0.001), AST level (r = 0.159, P = 0.030), platelet count (r = -0.198, P = 0.009), insulin level (r = -0.179, P = 0.014) and HOMA-IR (r = -0.290, P < 0.001) were significant factors associated with serum adiponectin level. Multiple linear regression analysis showed that age (regression coefficient = 0.140, P < 0.001), male sex (regression coefficient = -2.925, P < 0.001) and BMI (regression coefficient = -0.495, P < 0.001) were independent variables.
Table 3.
Univariate and multivariate analysis of factors associated serum adiponectin level in 188 patients with chronic HCV infection
|
Univariate |
Multivariate |
||||
| Coefficient | P value1 | Regression coefficient | SE | P value3 | |
| Age | 0.388 | < 0.001 | 0.140 | 0.028 | < 0.001 |
| Male sex2 | NA | < 0.001 | -2.925 | 0.744 | < 0.001 |
| BMI | -0.281 | < 0.001 | -0.495 | 0.101 | < 0.001 |
| HCC2 | NA | 0.136 | - | - | - |
| DM2 | NA | 0.629 | - | - | - |
| Child-Pugh classification | 0.107 | 0.145 | - | - | - |
| AST (U/L) | 0.159 | 0.030 | - | - | - |
| ALT (U/L) | -0.096 | 0.195 | - | - | - |
| Platelet (104/μL) | -0.198 | 0.009 | - | - | - |
| Insulin (μU/mL) | -0.179 | 0.014 | - | - | - |
| HOMA-IR | -0.290 | < 0.001 | - | - | - |
P value by Spearman’s test or
P value by Mann-Whitney U test;
P value by stepwise linear regression analysis. NA: Not applicable.
Stepwise logistic regression analysis for factors associated with development of HCC
Based on stepwise logistic regression analysis, significant factors associated with development of HCC in patients with chronic HCV infection were age (OR: 1.124, 95% CI: 1.067-1.183, P < 0.001), serum insulin level (OR: 1.585, 95% CI: 1.269-1.980, P < 0.001), HOMA-IR (OR: 0.495, 95% CI: 0.330-0.743, P = 0.001), DM (OR: 11.601, 95% CI: 2.50-53.8, P = 0.002) and male sex (OR: 3.877, 95% CI: 1.282-11.729, P = 0.016) (Table 4).
Table 4.
Stepwise logistic regression analysis of factors associated with HCC
| Comparison | OR | 95% CI | P value | |
| All patients | ||||
| Age | Per 1 year increase | 1.124 | 1.067-1.183 | < 0.001 |
| Insulin | Per 1 μU/mL increase | 1.585 | 1.269-1.980 | < 0.001 |
| HOMA-IR | Per 1 increase | 0.495 | 0.330-0.743 | 0.001 |
| DM | Yes vs no | 11.601 | 2.500-53.800 | 0.002 |
| Sex | Male vs female | 3.877 | 1.282-11.729 | 0.016 |
| Non-DM patients | ||||
| Age | Per 1 year increase | 1.170 | 1.075-1.272 | < 0.001 |
| Insulin | Per 1 μU/mL increase | 2.434 | 1.555-3.811 | < 0.001 |
| HOMA-IR | Per 1 increase | 0.158 | 0.055-0.452 | 0.001 |
| Sex | Male vs female | 6.111 | 1.310-28.499 | 0.021 |
When excluding DM cases, factors independently associated with HCC development in 142 non-DM patients were age (OR: 1.170, 95% CI: 1.075-1.272, P < 0.001), serum insulin level (OR: 2.434, 95% CI: 1.555-3.811, P < 0.001), HOMA-IR (OR: 0.158, 95% CI: 0.055-0.452, P = 0.001) and male sex (OR: 6.111, 95% CI: 1.310-28.49, P = 0.021).
DISCUSSION
There is increasing evidence linking chronic HCV infection and type 2 DM. Large community-based studies have shown that the prevalence of DM in HCV-infected patients is much higher than that observed in the general population, and in patients with other chronic liver diseases such as HBV and alcoholic liver disease[28,29]. In this study, we found that among HCC patients, type 2 DM was more prevalent in those infected with HCV compared to those with HBV or non-HBV, non-HCV infection. This observation in accordance with previous studies suggests a strong synergistic effect of metabolic factors and viral hepatitis in HCC development among HCV-infected patients[12,13]. Although there was no significant difference in BMI among HCC patients with different etiology, this could be explained by the low prevalence of obesity in our study population.
Chronic HCV infection is associated with the development of hepatic steatosis and unique, virus-specific alterations in host metabolism leading to the development of IR[19,30,31]. In this present study, we provide the first evidence that IR could potentially increase the risk of developing HCC in patients with chronic HCV infection. In a cross-sectional, hospital-based setting, we prospectively assessed the HOMA-IR value in different clinical stages of chronic HCV infection. Our data showed that patients with HCC had a higher ratio of HOMA-IR > 4 than those with chronic hepatitis and advanced fibrosis. Furthermore, after adjusting for age and sex, HOMA-IR was an independent factor associated with the development of HCC. A novel finding of our work, not specifically evaluated in other studies, was the association of IR, regardless of diabetes, with the development of HCC. An alternative explanation for the observed association between HOMA-IR and HCC is that advanced hepatic fibrosis and disease severity results in IR and impairs insulin clearance. This possibility could be excluded by the similar prevalence of DM and platelet count that has been considered as a fibrosis marker in chronic HCV infection between patients with HCC and those with advanced fibrosis. Also, HOMA-IR did not correlate with Child-Pugh classification, which suggests that disease severity was not associated with IR in patients with HCC or advanced fibrosis.
Although our work was not designed to clarify the pathogenic interaction between IR and the development of HCC, a few hypotheses can be put forward. IR is defined as an increased requirement for insulin to maintain normal metabolic function, which results in the compensatory development of hyperinsulinemia[32]. Recent evidence has suggested that hyperinsulinemia can promote the synthesis and biological activity of insulin-like growth factor 1 (IGF-1), which is a peptide hormone that regulates energy-dependent growth processes[33]. IGF-I stimulates cell proliferation and inhibits apoptosis and has been shown to have strong mitogenic effects on a wide variety of cancer cell lines. Changes in the expression pattern of IGF-system components have been observed in patients with HCC, in human HCC cell lines and in their conditioned culture medium, as well as in rodent models of hepatocarcinogenesis[34].
To study the role of adiponectin in HCC may be more complex because of its underlying chronic hepatitis infection[19,35,36]. Previous studies have demonstrated that circulating adiponectin levels are inversely associated with the risk of malignancies associated with IR, including endometrial, breast, colon and gastric cancer[37-40]. Moreover, serum adiponectin level has been reported to be significantly elevated in chronic liver disease, and correlated with stage of liver cirrhosis, liver cell injury, e.g. aminotransferase activity, and inflammatory markers[35,36]. Thus, serum adiponectin level is modified according to the two opposing factors, IR and underlying liver condition. In this study, we found no difference in serum adiponectin level among different clinical stages of chronic HCV infection. Although HOMA-IR score was inversely associated with serum adiponectin level by univariate analysis, multiple linear regression analysis did not support this correlation.
There are some limitations to our study. First, the analysis was carried out in a cross-sectional setting with a relatively small number of HCC patients, and it would be interesting to determine whether this association holds true in longitudinal follow-up studies of larger groups of patients. Second, the cohort of patients, at low prevalence of obesity, was enrolled in a tertiary referral center for liver disease, which limits the broader application of the results. A further methodological issue resides in the inability to dissect the temporal relation between IR and HCC. Another limitation lies in the fact that there is some concern on the use of HOMA-IR in the presence of long-standing DM. However, a diagnosis of DM is per se expression of IR, and HOMA-IR is a less invasive, inexpensive, and less labor-intensive method to measure IR as compared with the glucose clamp test.
In conclusion, we demonstrated the independent association between IR and HCC development in chronic HCV infection. These findings may have important prognostic and therapeutic implications in the management of chronic HCV-infected patients. Since IR is a potentially modifiable factor, therapeutic intervention aimed at decreasing IR may be warranted in these patients.
COMMENTS
Background
Recent studies have demonstrated that diabetes mellitus (DM) is associated with high risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis C. Insulin resistance (IR), which correlates inversely with circulating adiponectin concentration, is a consistent finding in patients with type 2 DM. Chronic hepatitis C virus (HCV) infection has been reported to be associated with increased IR. Recent studies have suggested that IR plays a crucial role in fibrosis progression, and has been demonstrated to have a negative impact on treatment responses to antiviral therapy in patients with chronic hepatitis C.
Research frontiers
IR has emerged as a risk factor for a wide variety of cancers. In a cross-sectional, hospital-based setting, the authors assessed the role of IR assessed by the homeostasis model (HOMA-IR) and serum adiponectin level in the development of HCC associated with chronic HCV infection.
Innovations and breakthroughs
The authors have provided the first evidence that IR can potentially increase the risk of developing HCC in patients with chronic HCV infection. A novel finding, not specifically evaluated in other studies, is the association of IR, regardless of diabetes, with the development of HCC.
Applications
These findings may have important prognostic and therapeutic implications in the management of chronic HCV-infected patients. Since IR is a potentially modifiable factor, therapeutic intervention aimed at decreasing IR may be warranted in these patients.
Peer review
The authors present a clinical investigation of the correlation between IR and HCC. The title accurately reflects the major contents of the article, and the abstract delineates briefly and concisely the research background, objectives, materials and methods, results and conclusions.
Footnotes
Supported by National Science Council (Republic of China, Taiwan), Grant No. NSC96-2314-B182A-088
Peer reviewer: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, Hollinger FB, Gitnick G, Knodell RG, Perrillo RP. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 3.Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 4.Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132–2137. [PubMed] [Google Scholar]
- 5.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Kew MC, Popper H. Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis. 1984;4:136–146. doi: 10.1055/s-2008-1040653. [DOI] [PubMed] [Google Scholar]
- 7.Schirmacher P, Rogler CE, Dienes HP. Current pathogenetic and molecular concepts in viral liver carcinogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:71–89. doi: 10.1007/BF02899246. [DOI] [PubMed] [Google Scholar]
- 8.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 9.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024–1029. doi: 10.1053/j.gastro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Hung CH, Chen CH, Lee CM, Wu CM, Hu TH, Wang JH, Yen YH, Lu SN. Association of amino acid variations in the NS5A and E2-PePHD region of hepatitis C virus 1b with hepatocellular carcinoma. J Viral Hepat. 2008;15:58–65. doi: 10.1111/j.1365-2893.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 12.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 14.Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 15.N'Kontchou G, Paries J, Htar MT, Ganne-Carrie N, Costentin L, Grando-Lemaire V, Trinchet JC, Beaugrand M. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062–1068. doi: 10.1016/j.cgh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Cahill GF Jr. Beta-cell deficiency, insulin resistance, or both? N Engl J Med. 1988;318:1268–1270. doi: 10.1056/NEJM198805123181909. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 18.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Hung CH, Lee CM, Chen CH, Hu TH, Jiang SR, Wang JH, Lu SN, Wang PW. Association of inflammatory and anti-inflammatory cytokines with insulin resistance in chronic hepatitis C. Liver Int. 2009;29:1086–1093. doi: 10.1111/j.1478-3231.2009.01991.x. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 23.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 24.Møller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 26.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 27.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 28.Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, Lee LP, Lin ZY, Chen SC, Hsieh MY, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237–1243. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 29.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 30.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 31.Hung CH, Lee CM, Kuo FY, Jiang SR, Hu TH, Chen CH, Wang JH, Lu SN, Eng HL, Changchien CS. Steatosis correlates with hepatic expression of death receptors and activation of nuclear factor-kappaB in chronic hepatitis C. Liver Int. 2008;28:339–346. doi: 10.1111/j.1478-3231.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 32.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 33.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 34.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003–1015. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Tacke F, Wüstefeld T, Horn R, Luedde T, Srinivas Rao A, Manns MP, Trautwein C, Brabant G. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42:666–673. doi: 10.1016/j.jhep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Kaser S, Moschen A, Kaser A, Ludwiczek O, Ebenbichler CF, Vogel W, Jaschke W, Patsch JR, Tilg H. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis. J Intern Med. 2005;258:274–280. doi: 10.1111/j.1365-2796.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- 37.Housa D, Housová J, Vernerová Z, Haluzík M. Adipocytokines and cancer. Physiol Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 38.Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, Chrousos G, Trichopoulos D. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 39.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]