Abstract
Celiac disease (CD) is manifested by a variety of clinical signs and symptoms that may begin either in childhood or adult life. Neurological symptoms without signs of malabsorption have been observed for a long time in CD. In this report, an 8-year-old girl with CD presented with rarely seen dilated cardiomyopathy and stroke. The girl was admitted with left side weakness. Her medical history indicated abdominal distention, chronic diarrhea, failure to thrive, and geophagia. On physical examination, short stature, pale skin and a grade 2 of 6 systolic murmur were detected. Muscle strength was 0/5 on the left side, and 5/5 on the right side. Coagulation examinations were normal. Tests for collagen tissue diseases were negative. Factor V Leiden and prothrombin GA20210 mutations were negative. Tandem mass spectrophotometry and blood carnitine profiles were normal. Brain magnetic resonance imaging and cerebral angiography showed an infarction area at the basal ganglia level. Examinations of serologic markers and intestinal biopsy revealed CD. We emphasize that in differential diagnosis of ischemic stroke, CD should be kept in mind.
Keywords: Celiac disease, Stroke, Cardiomyopathy, Children
INTRODUCTION
Celiac disease (CD) is a disease of the small intestine caused by an immune response to ingested gluten. This response results in characteristic damage to the villi, leading to malabsorption[1]. CD is manifested by a variety of clinical signs and symptoms that may begin in either childhood or adult life. Neurological symptoms without malabsorption signs have been observed for a long time in CD. Epilepsy, bilateral occipital calcification, cerebellar ataxia, degenerative central nervous system disease, peripheric neuropathy, myopathy and, rarely, stroke were defined as neurologic manifestations[2]. Tissue transglutaminase enzyme is an auto-antigen, which is related to gluten-associated immune events[3]. In this case report, an 8-year-old girl with CD presented with rarely seen stroke.
CASE REPORT
An 8-year-old girl was admitted to our emergency department with weakness of the left lower and upper extremities. It was learnt that the patient had geophagia, abdominal distention and chronic diarrhea for 2 years. On physical examination, her body weight and height were 16 kg (-2 SD) and 110 cm (-2.6 SD), respectively. Blood pressure was 110/70 mmHg. She had pale skin and mucosa, and a grade 2 of 6 systolic murmur at the inferior left side of the sternum. Muscle strength was determined as 0/5 on the left upper and lower extremities and 5/5 on the right side. The laboratory analysis, hemogram, serum electrolytes, glucose, cholesterol, triglyceride levels, liver and renal function tests were within normal limits. Thyroid hormone values were also found to be within the normal range [free T4: 1.4 ng/dL (normal range: 0.8-2.2 ng/dL), total T4: 7.4 mg/mL (normal range: 5.5-12.8 mg/L), thyroid stimulating hormone: 1.2 mU/mL (normal range: 0.6-5.5 mU/mL), total T3: 170 ng/dL (normal range: 119-218 ng/dL), free T3: 3.2 pg/mL (normal range: 2.0-4.0 pg/mL)]. C-reactive protein was negative and erythrocyte sedimentation rate was 19 mm/h. Vitamin B12 and folate levels were normal. Prothrombin time and activated partial thromboplastin time were 13.6 s (normal range: 11-14 s) and 29 s (normal range: 31-40 s), respectively. Fibrinogen, protein C, protein S, factors V, VII, VIII, XI, XII and antithrombin III levels were within normal limits. Serologic tests for human immunodeficiency virus, lupus anticoagulants, antinuclear antibody, anti-double-stranded DNA, anticardiolipin antibody immunoglobulin (Ig) G and M serologies were negative. Factor V Leiden and prothrombin GA20210 mutations were not detected. The tandem mass metabolic disease screening panel and detailed blood carnitine profile were normal.
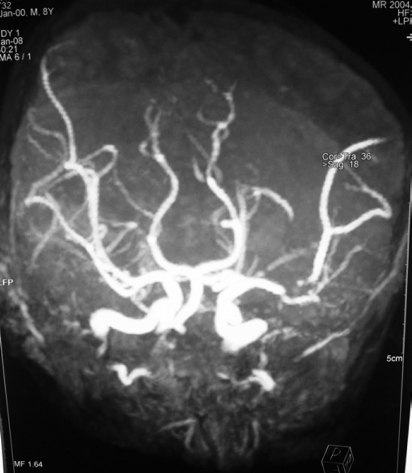
On brain magnetic resonance imaging, an infarction measuring 31 mm × 14 mm at the right basal ganglia level was seen (Figure 1). On cerebral angiography examination, a 1 cm segment occlusion was detected at the M2 branch of the right middle cerebral artery (Figure 2). Although dilated cardiomyopathy was identified on transesophagial and transthoracic echocardiographic examination, thrombus or vegetation was not seen. Serologic markers of CD were examined because of chronic diarrhea, abdominal distention, short stature, cerebral infarction and dilated cardiomyopathy, and anti-tissue transglutaminase IgA and IgG, and anti-endomysium IgA were found at a highly positive rate. Additionally, the examination of intestinal biopsy revealed CD. Duodenal biopsy showed villous atrophy with hyperplasia of the crypts and an increased intraepithelial lymphocyte count (above 40%).
Figure 1.
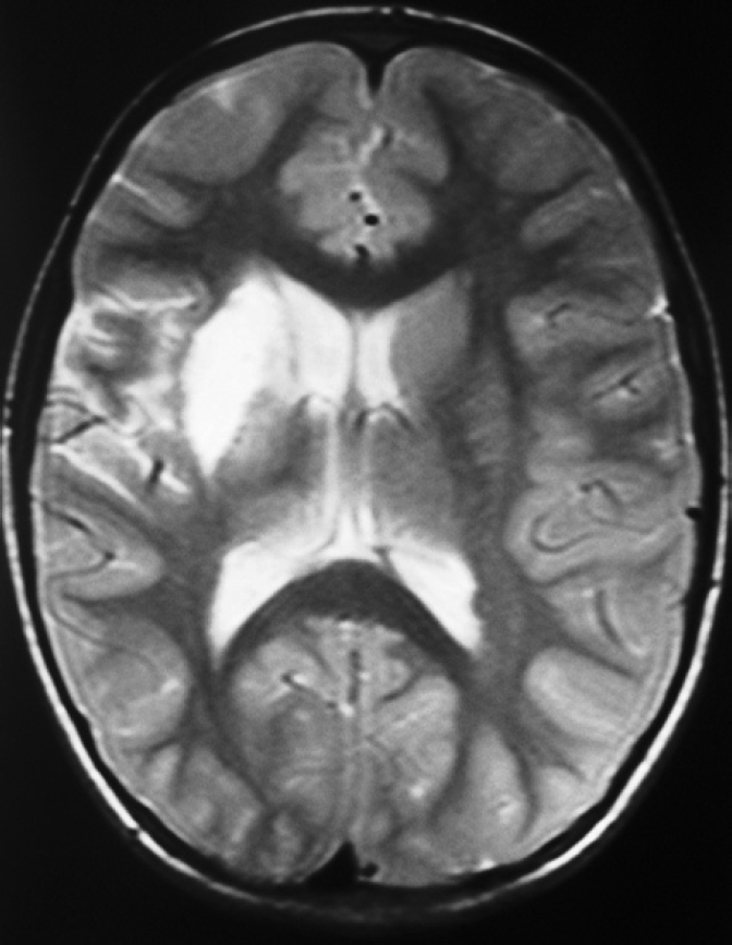
Brain magnetic resonance imaging shows an infarction area measuring 31 mm × 14 mm at the right basal ganglia level.
Figure 2.
Cerebral angiography examination shows a 1 cm segment occlusion at the M2 branch of the right middle cerebral artery.
A gluten-free diet and nadroparin calcium treatment were initiated and physiotherapy was performed. At the 18th day of hospitalization, the patient, whose symptoms had regressed, was discharged with a gluten-free diet, nadroparin calcium, co-enzyme Q and salicylate treatment. Symptoms resolved by the following 7th wk. Muscle strength at the left upper and lower extremities was 5/5, and other neurologic examinations were normal.
DISCUSSION
Classical findings of CD usually begin at 1-3 years of life. Toddlers and young children classically present with chronic diarrhea, vomiting, poor appetite, abdominal distension, abdominal pain, irritability, and failure to thrive some time after the introduction of gluten in the diet[1]. In adults, a variety of neuropsychiatric conditions, such as depression and anxiety, have been reported in individuals with CD[1]. In our case, abdominal distention, chronic diarrhea, geophagia, and short stature were observed. In addition, infarction and dilated cardiomyopathy were present.
The diagnosis of CD is established by positive results of serological testing and evidence of characteristic histopathology on intestinal biopsy[4]. If results of serologic testing are negative but clinical suspicion is high, intestinal biopsy should be performed. Characteristic histologic features of CD include varying degrees of villous atrophy, with hyperplasia of the crypts and an increased intraepithelial lymphocyte count[5]. Consistent with the literature, our case was positive for anti-tissue transglutaminase IgA and IgG, and anti-endomysium IgA, and duodenal biopsy examination showed villous atrophy with hyperplasia of the crypts and increased intraepithelial lymphocyte count (above 40%).
Development of autoimmune diseases is one of the complications of the CD. Tissue transglutaminase enzyme is an auto-antigen that is related to gluten-associated immune events. Evidence of a central nervous system vasculitis was reported by Ozge et al[6] in a patient with recurrent stroke and CD. Pratesi et al[7] found that sera from patients with active CD contain IgA antibodies that reacted with human brain vessel structures, giving intense fluorescence. These antibodies were not present in sera from celiac patients on a gluten-free diet or non-celiac controls. They emphasized that this finding might be involved in the abnormal nervous system manifestations frequently described in association with CD. Tissue transglutaminase is the major auto-antigen in CD and is thought to maintain vascular endothelial integrity. Anti-endomysial IgA antibodies, demonstrated to be the same autoantibody as anti-transglutaminase, react with the cerebral vasculature, suggesting an autoimmune mechanism for CD-associated vasculopathy. Because CD is a potentially treatable cause of cerebral vasculopathy, serology (specifically for anti-tissue transglutaminase antibodies) should be included in the evaluation of cryptogenic stroke in childhood, even in the absence of typical gut symptoms.
Dilated cardiomyopathy is the most commonly seen type of cardiomyopathy. Regarding its etiology, genetic causes, endocrine disorders, collagen vascular diseases, drugs, congenital metabolism diseases, muscular dystrophies, structural heart diseases, acute and chronic myocarditis and toxins can be present. However, 50% of cases remain idiopathic. An increased incidence of CD in patients with idiopathic dilated cardiomyopathy as well as in patients with secondary cardiomyopathy has been reported recently[8]. In our case, dilated cardiomyopathy was diagnosed with echocardiography. However, dilated cardiomyopathy was not thought to be the primary factor causing the stroke, because there was no thrombus or vegetation at echocardiography, and regression of symptoms occurred with a gluten-free diet. In addition, in patients with a cryptogenic stroke, the presence of a patent foramen ovale should be evaluated. Transthoracic 2-dimensional echocardiography can generally show the atrial septum and the flap of the foramen ovale in infants and small children. Color Doppler flow across the atrial septum proves the presence of the foramen ovale. In older children and adults, transthoracic echocardiography does not visualize the atrial septum as well. Transesophageal echocardiography is preferred in patients where the atrial septum is inadequately visualized by transthoracic echocardiography. Older children and adults fall into this category. In addition to a patent foramen ovale, redundancy of the septum primum can also be seen. When the redundancy of the septum moves more than 1 cm, it is called an atrial septal aneurysm. In the presence of a patent foramen ovale in patients who have had a prior stroke, an atrial septal aneurysm confers an increased risk for a subsequent neurologic event[9,10]. In our patient, a patent foremen ovale was not found in both transthorasic and transesophageal echocardiographic examination. Therefore, a patent foremen ovale was not thought to be a cause of the stroke in our patient.
In conclusion, the cause of ischemic stroke in our case is thought to be multifactorial. We suggest that CD was a primary factor in its etiology, secondary to a contribution from dilated cardiomyopathy. In conclusion, we emphasize that in the differential diagnosis of ischemic stroke, CD should be kept in mind.
Footnotes
Peer reviewer: Weekitt Kittisupamongkol, MD, Hua Chiew Hospital, 665 Bumrungmuang Road, Bangkok 10100, Thailand
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 2.Vaknin A, Eliakim R, Ackerman Z, Steiner I. Neurological abnormalities associated with celiac disease. J Neurol. 2004;251:1393–1397. doi: 10.1007/s00415-004-0550-9. [DOI] [PubMed] [Google Scholar]
- 3.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 4.Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 6.Ozge A, Karakelle A, Kaleağasi H. Celiac disease associated with recurrent stroke: a coincidence or cerebral vasculitis? Eur J Neurol. 2001;8:373–374. doi: 10.1046/j.1468-1331.2001.00233.x. [DOI] [PubMed] [Google Scholar]
- 7.Pratesi R, Gandolfi L, Friedman H, Farage L, de Castro CA, Catassi C. Serum IgA antibodies from patients with coeliac disease react strongly with human brain blood-vessel structures. Scand J Gastroenterol. 1998;33:817–821. doi: 10.1080/00365529850171468. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, Maseri A, Gasbarrini G. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–2618. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]
- 9.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 10.Telman G, Yalonetsky S, Kouperberg E, Sprecher E, Lorber A, Yarnitsky D. Size of PFO and amount of microembolic signals in patients with ischaemic stroke or TIA. Eur J Neurol. 2008;15:969–972. doi: 10.1111/j.1468-1331.2008.02232.x. [DOI] [PubMed] [Google Scholar]