Abstract
Duodenal perforation during endoscopic retrograde cholangiopancreatography (ERCP) is a rare complication, but it has a relatively high mortality risk. Early diagnosis and prompt management are key factors for the successful treatment of ERCP-related perforation. The management of perforation can initially be conservative in cases resulting from sphincterotomy or guide wire trauma. However, the current standard treatment for duodenal free wall perforation is surgical repair. Recently, several case reports of endoscopic closure techniques using endoclips, endoloops, or fully covered metal stents have been described. We describe four cases of iatrogenic duodenal bulb or lateral wall perforation caused by the scope tip that occurred during ERCP in tertiary referral centers. All the cases were simply managed by endoclips under transparent cap-assisted endoscopy. Based on the available evidence and our experience, endoscopic closure was a safe and feasible method even for duodenoscope-induced perforations. Our results suggest that endoscopists may be more willing to use this treatment.
Keywords: Duodenal perforation, Endoscopic retrograde cholangiopancreatography, Endoscopic therapy, Endoclip
INTRODUCTION
Although MR cholangiopancreatography has almost completely replaced endoscopic retrograde cholangiopancreatography (ERCP) in the diagnosis of pancreato-biliary diseases, the risk of ERCP complications has increased as therapeutic endoscopists have taken on increasingly more complex cases, particularly at tertiary referral centers[1]. The frequency of duodenal perforation is 0.5%-2% of patients[2]. However, because of a relatively high mortality rate of 16%-18%, all duodenal perforations should be treated immediately after diagnosis[2-5].
Traditionally, the standard treatment for traumatic or iatrogenic duodenal perforation is surgical closure. Recently, endoscopic trials of perforation management have increased and successful primary repair of duodenal perforation using the endoscope itself has been reported. However, there is no clear consensus for primary repair due to the limited number of cases seen[6-8].
We report four cases in which ERCP done at tertiary referral centers induced a large duodenal perforation, which was then successfully managed with endoscopic approximation suture using multiple endoclips under transparent cap-assisted endoscopy.
CASE REPORT
Case 1
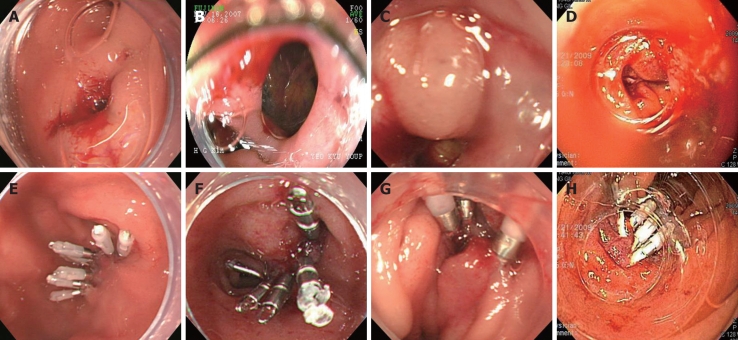
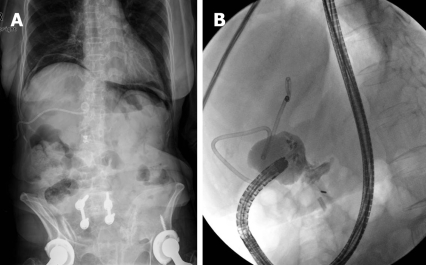
An 80-year-old woman with Klatskin tumor underwent ERCP. Three days later after endoscopic biliary drainage and biopsy, ERCP was attempted for the placement of the metallic stent. During the procedure, the duodenoscope slipped into the duodenum and caused an approximately 13 mm-sized perforation in the duodenal bulb. After early recognition of perforation, an immediate attempt to seal the perforation with 8 endoclips (Endoclip HX-600-090L; Olympus Optical Co., Ltd., Tokyo, Japan) was made successfully under transparent cap-assisted endoscopy (Figure 1A and E). Following the endotherapy, placement of percutaneous transhepatic biliary drainage (PTBD), peripheral parenteral nutrition, intravenous high-dose proton pump inhibitor (PPI), and broad-spectrum antibiotics (3rd generation cephalosporin + metronidazole) was initiated. A nasoduodenal tube was not placed. An abdominal X-ray showed a pneumoperitoneum in the subphrenic area (Figure 2A). However, she remained symptom-free without additional complications. Six days later, a contrast passage via endoscope showed no evidence of leakiness and the pneumoperitoneum was completely resolved (Figure 2B). She resumed a scheduled diet 6 d after the clipping and was discharged on day 12 (Tables 1 and 2).
Figure 1.
Endoscopic images of four cases demonstrating a large perforation on bulb and lateral wall of the second portion of duodenum, and successful primary endoscopic closure using multiple endoclips.
Figure 2.
A simple abdomen X-ray shows both subphrenic pneumoperitoneum (A) and 6 d later, the follow-up upper gastrointestinal investigation (UGI) reveals no contrast leaks (B).
Table 1.
Patient characteristics
| Case No. | Age (yr)/sex | ERCP indication | Perforation site | Perforation size (mm) | Diagnosis | Experience of ERCPs (yr) |
| Case 1 | 80/F | Klatskin tumor | Bulb | > 10 | During ERCP | < 2 |
| Case 2 | 72/F | IHD stricture | 2nd portion, lateral wall | > 10 | During ERCP | < 2 |
| Case 3 | 69/F | Jaundice | 2nd portion, lateral wall | > 10 | During ERCP | < 1 |
| Case 4 | 63/M | Choledocholithiasis | 2nd portion, lateral wall | > 10 | During ERCP | < 1 |
ERCP: Endoscopic retrograde cholangiopancreatography; IHD: Intrahepatic duct.
Table 2.
Therapeutic outcomes
| Case No. | No. of endoclip | Antibiotics (d) | PPI | Gastric drainage | Biliary drainage | Start diet/hospital stay (d) | Mortality or Cx. |
| Case 1 | 8 | 3rd generation cephalosporin/metronidazole (7/5) | Pantoprazole 40 mg IV | - | PTBD | 6/12 | - |
| Case 2 | 5 | 3rd generation cephalosporin/metronidazole (7/3) | Pantoprazole 40 mg IV | - | PTBD, PTCS | 7/10 | - |
| Case 3 | 4 | 3rd generation cephalosporin/metronidazole (8/5) | Pantoprazole 40 mg IV | - | PTBD | 8/10 | - |
| Case 4 | 5 | 3rd generation cephalosporin/metronidazole (14/7) | Pantoprazole 40 mg IV | - | PTBD | 4/27 | - |
PPI: Proton pump inhibitor; PTBD: Percutaneous transhepatic biliary drainage; PTCS: Percutaneous transhepatic cholangioscopy.
Case 2
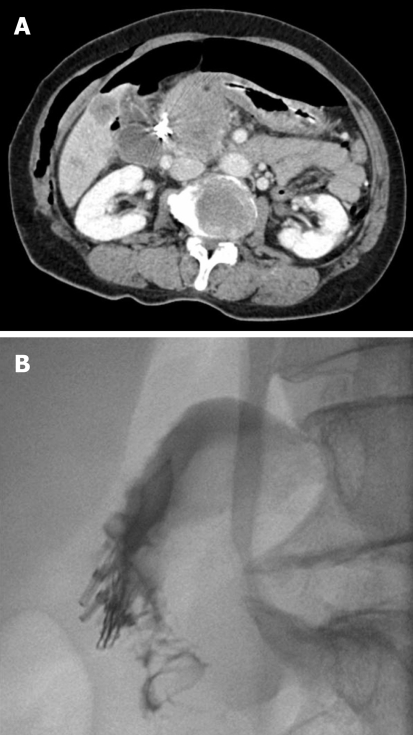
A 72-year-old woman with a history of left hepatic lobectomy and cholecystectomy 11 years prior to the procedure underwent ERCP for the segmental stricture of right intrahepatic duct and stones after PTBD. During the insertion of the duodenoscope into the stomach, severe rigidity was felt and the duodenoscope suddenly slipped into the duodenum. This caused an approximately 13 mm-sized perforation in the lateral wall of the second portion of the duodenum. Subsequently, an attempt to seal the perforation was successfully made with 5 endoclips under transparent cap-assisted endoscopy (Figure 1B and F). Peripheral parenteral nutrition, intravenous high-dose PPI, and broad-spectrum antibiotics were initiated. An abdominal computed tomography (CT) showed a pneumoretroperitoneum (Figure 3A). She remained symptom-free 2 d later, and did not develop any complications. A repeat abdominal CT performed 6 d later showed markedly decreased pneumoretroperitoneum and fluid collection, and there was no contrast leak on upper gastrointestinal investigations (UGIs) (Figure 3B). She resumed a normal scheduled diet 7 d after clipping and was discharged on day 10.
Figure 3.
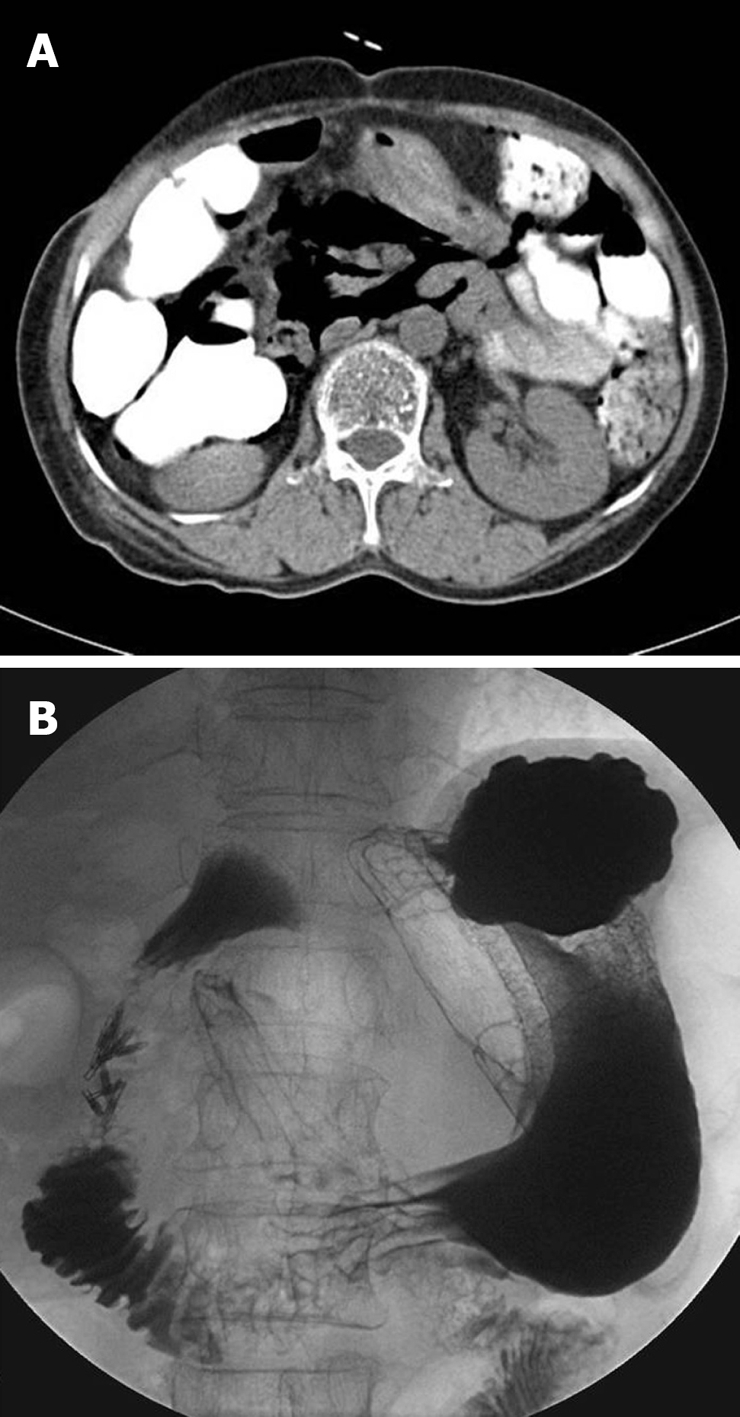
An abdominal computed tomography (CT) shows a severe pneumoretroperitoneum (A), and follow-up UGIs done 6 d later reveal no contrast leaks (B).
Case 3
A 69-year-old woman was referred due to pancreatic cancer. An abdominal CT scan revealed about a 5 cm-sized pancreatic head cancer with multiple hepatic metastases. ERCP was considered for jaundice and evaluation of the biliary system. At first, a trainee who had less than 6 mo experience inserted the duodenoscope. He tried to shorten the scope loop in the duodenum several times, but failed to shorten the scope loop due to duodenal rigidity resulting from the duodenal invasion of pancreatic cancer. The trainee then notified his supervisor, who was observing the procedure. He found about a 10 mm-sized duodenal perforation, which might have been caused by excessive shortening of the scope in the restricted lumen. Successful closure was immediately performed using 4 endoclips under transparent cap-assisted endoscopy (Figure 1C and G). The next day, the patient complained of abdominal pain, but there was no interval change except mild leukocytosis. Fasting and hydration were ordered and abdominal CT scans were taken twice, 24 h apart, but the pneumoperitoneum was not found to be progressive (Figure 4A). On the 8th day after duodenal perforation, UGIs showed no contrast leakage (Figure 4B), and she was put on a diet. After discharge from the hospital 10 d later, the patient received gemcitabine chemotherapy.
Figure 4.
Initial abdominal CT following perforation shows pneumoperitoneum and subcutaneous emphysema (A), and follow up UGIs performed 8 d later reveal no contrast leaks (B).
Case 4
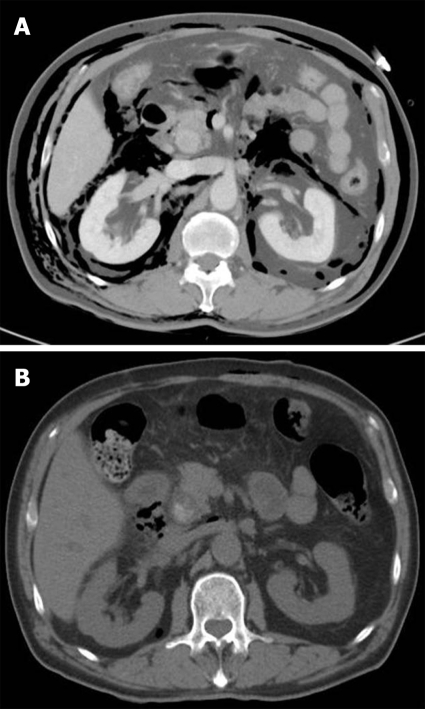
A 63-year-old man with a history of cholecystectomy 10 years before the procedure underwent ERCP for choledocholithiasis. During insertion of the duodenoscope, an approximately 12 mm-sized perforation occurred in second portion of the duodenum secondary to trauma from difficult passage of the duodenoscope due to bulb deformity caused by recurrent duodenal ulcers. A successful attempt to seal the perforation with 5 endoclips was made under long transparent cap-assisted endoscopy (Figure 1D and H), followed by PTBD. Conservative managements were initiated as mentioned above. Simple X-ray and abdominal CT showed a pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema. The patient remained symptom-free 3 d later and did not develop any complications. An abdominal CT repeated 4 d later showed markedly decreased pneumoretroperitoneum (Figure 5) and a scheduled diet was started. The remaining CBD stones were removed by ERCP 25 d later.
Figure 5.
Abdominal CT images showing a pneumoretroperitoneum, and subcutaneous emphysema following perforation (A) and a marked improvement 4 d after conservative management (B).
DISCUSSION
Duodenal perforation is an infrequent complication of ERCP. It extends beyond the intramural portion of the bile duct and is usually associated with sphincterotomy in about 1% of patients[9]. Retroperitoneal duodenal perforations represent the majority of cases and usually are due to papillotomy, whereas intraperitoneal perforations are much less common and caused by the endoscope itself[10]. Direct duodenoscope-induced perforation is much less common, accounting for 0.1% of patients who undergo ERCP, but tends to be large and further away from the ampulla[4,5,11,12]. Known risk factors of an ERCP-related perforation might include old age, suspected sphincter of Oddi dysfunction, dilated bile duct, papillary stenosis, Billroth-II reconstruction, pre-cut sphincterotomy, and long procedure duration[5,13-15].
Immediate surgery after diagnosis is the current standard treatment for duodenal perforations caused by an endoscope. Stapfer et al[4] and Howard et al[3] have proposed a classification scheme for duodenal injury by dividing it into four and three types, respectively, according to anatomical location, mechanism of injury, and severity. Type I (lateral or medial wall duodenal perforation; Stapfer et al) or Group III (duodenal perforation remote from the papilla; Howard et al) injuries are usually large and require immediate surgery for repair. In a study by Stapfer et al, surgery was recommended for patients with the following criteria: large contrast extravasation on ERCP/UGIs, contrast-enhanced CT scans showing intra- or retroperitoneal fluid collection, massive subcutaneous emphysema or suspected perforation in association with retained material (i.e. stones, ERCP wire/basket)[4]. In cases of perivaterian injuries, they suggested conservative management with serial radiographic examination. Howard et al[3] also suggested the use of endoscopic drainage to divert the bile, pancreatic, and/or duodenal fluids away from the perforation, and showed that the endoscopic approach reduced the rates of surgery, mortality, and length of hospital stay.
However, unlike more common spontaneous perforation resulting from peptic ulcer disease, endoscopic therapy-related iatrogenic perforations have a relatively lower chance of bacterial contamination in a fasting state, and there is therefore sometimes an opportunity to manage these patients using nonsurgical means. A small amount of bacterial contamination may be controlled by the administration of antibiotics[16]. Recently, trials of endoscopic management have been performed. There have been sporadic reports about the use of an endoscopic clipping device for the closure of iatrogenic perforations during endoscopic mucosal resection (EMR) or sphincterotomy in the esophagus, stomach, and duodenum[17-19]. Though surgery remains the standard treatment for duodenal perforations caused by the endoscope itself, the outcomes from case reports support the beneficial role of endoclips in the closure of these defects[6-8,20]. In particular, some reports described that nonsurgical treatment is possible for the perforation of the upper gastrointestinal tract when peritonitis remains localized. The clinician’s familiarity with endoclips and their immediate availability and proper use may avoid surgery for a selected group of patients with a high surgical risk.
Kaneko et al[16] suggested some conditions for endoscopic repair using a clipping device in EMR-related perforation. Their suggestions included prior preparation of the patients, quick recognition of perforation, the diameter of perforation being less than the width of the clip’s nail, the shape of the opening must be smooth and suitable for drawing the edges together, and an excellent visual field. In endoscopic management, quick recognition and rapid endoscopic closure were the keys to success in limiting the degree of peritoneal contamination and pneumoperitoneum, as delayed diagnosis and surgery are associated with a high mortality rate[4,21]. However, nonsurgical suturing therapy using endoclips is not yet widely accepted as the primary management of ERCP-related duodenal perforation.
In the four cases presented here, the experience of endoscopist and patient old age may be risk factors. All the perforations were done by inexperienced endoscopists who only had one or two years of therapeutic ERCP experiences. However, routine surgery was not required in all patients. The endoscopists could detect the injury early, the visual field was relatively clear, and the endoscopic manipulation was performed in minimal time in all cases. These were the reasons why the primary closure was successful despite a large perforation of more than 10 mm. The perforation was detected very early during ERCP because it occurred in the course of duodenoscope insertion. Cap-assisted endoscopy method under direct vision through a transparent hood was also helpful in reducing the manipulation time of the procedure by allowing a good visual field and ensuring the safety margin during clipping. The cap can facilitate the displacement of any mucosal folds that obscure the lumen and is very useful for overcoming the sharp angulations[22].
Conservative treatment includes giving the patients nothing by mouth, broad-spectrum antibiotics, PPI, and diversion of the bile and pancreatic secretion, or nasogastric or nasoduodenal decompression. However, there were differences in the follow-up method and interval, duration of conservative management methods (fasting, PPI, and antibiotics), and the time when a normal diet was started. Following immediate closure, the use and duration of broad-spectrum antibiotics or PPI was not clear. In our cases, routine nasogastric or duodenal drainage was not used because of early successful closure and biliary diversion by PTBD. Therefore, we think that these procedures are not always required in such cases. Normal diet should be resumed after confirming the complete closure of the wound by UGIs. If patients don’t have any clinical symptoms and contrast leakage, earlier resumption of normal diet may be possible.
In summary, primary approximation closure using endoclips under cap-assisted endoscopy of iatrogenic duodenal perforation during ERCP was a safe and feasible technique for even a large free wall perforation. Although the surgical operation remains the standard treatment for duodenal perforation, these cases support the use of endoscopic closure of the perforation with conservative treatments for selected cases of the injury caused by the endoscope itself.
Footnotes
Peer reviewer: Yuk-Tong Lee, MD, Department of Medicine and Therapeutics, Prince of Wales Hospital, Shatin, New Territories, Hong Kong, China
S- Editor Tian L L- Editor O'Neill M E- Editor Lin YP
References
- 1.Fatima J, Baron TH, Topazian MD, Houghton SG, Iqbal CW, Ott BJ, Farley DR, Farnell MB, Sarr MG. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: diagnosis and management. Arch Surg. 2007;142:448–454; discussion 454-455. doi: 10.1001/archsurg.142.5.448. [DOI] [PubMed] [Google Scholar]
- 2.Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652–656. doi: 10.1067/mge.2002.129086. [DOI] [PubMed] [Google Scholar]
- 3.Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, Swack ML, Kopecky KK. Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999;126:658–663; discussion 664-665. [PubMed] [Google Scholar]
- 4.Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, Garry D. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191–198. doi: 10.1097/00000658-200008000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, Baillie J. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34:293–298. doi: 10.1055/s-2002-23650. [DOI] [PubMed] [Google Scholar]
- 6.Mutignani M, Iacopini F, Dokas S, Larghi A, Familiari P, Tringali A, Costamagna G. Successful endoscopic closure of a lateral duodenal perforation at ERCP with fibrin glue. Gastrointest Endosc. 2006;63:725–727. doi: 10.1016/j.gie.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Seibert DG. Use of an endoscopic clipping device to repair a duodenal perforation. Endoscopy. 2003;35:189. doi: 10.1055/s-2003-37004. [DOI] [PubMed] [Google Scholar]
- 8.Sebastian S, Byrne AT, Torreggiani WC, Buckley M. Endoscopic closure of iatrogenic duodenal perforation during endoscopic ultrasound. Endoscopy. 2004;36:245. doi: 10.1055/s-2004-814257. [DOI] [PubMed] [Google Scholar]
- 9.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin DF, Tweedle DE. Retroperitoneal perforation during ERCP and endoscopic sphincterotomy: causes, clinical features and management. Endoscopy. 1990;22:174–175. doi: 10.1055/s-2007-1012833. [DOI] [PubMed] [Google Scholar]
- 11.Mosca S. Is ERCP a safe procedure, but for experts only? Endoscopy. 2002;34:1021–1022; author reply 1023. doi: 10.1055/s-2002-35837. [DOI] [PubMed] [Google Scholar]
- 12.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ML. Complications of endoscopic biliary sphincterotomy: a review. Endoscopy. 1997;29:288–297. doi: 10.1055/s-2007-1004193. [DOI] [PubMed] [Google Scholar]
- 14.Krims PE, Cotton PB. Papillotomy and functional disorders of the sphincter of Oddi. Endoscopy. 1988;20 Suppl 1:203–206. doi: 10.1055/s-2007-1018176. [DOI] [PubMed] [Google Scholar]
- 15.Wu HM, Dixon E, May GR, Sutherland FR. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): a population-based review. HPB (Oxford) 2006;8:393–399. doi: 10.1080/13651820600700617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko T, Akamatsu T, Shimodaira K, Ueno T, Gotoh A, Mukawa K, Nakamura N, Kiyosawa K. Nonsurgical treatment of duodenal perforation by endoscopic repair using a clipping device. Gastrointest Endosc. 1999;50:410–413. doi: 10.1053/ge.1999.v50.97235. [DOI] [PubMed] [Google Scholar]
- 17.Katsinelos P, Paroutoglou G, Papaziogas B, Beltsis A, Dimiropoulos S, Atmatzidis K. Treatment of a duodenal perforation secondary to an endoscopic sphincterotomy with clips. World J Gastroenterol. 2005;11:6232–6234. doi: 10.3748/wjg.v11.i39.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Komatsu Y, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc. 2004;60:636–639. doi: 10.1016/s0016-5107(04)01960-1. [DOI] [PubMed] [Google Scholar]
- 19.Baron TH, Gostout CJ, Herman L. Hemoclip repair of a sphincterotomy-induced duodenal perforation. Gastrointest Endosc. 2000;52:566–568. [PubMed] [Google Scholar]
- 20.Raju GS, Gajula L. Endoclips for GI endoscopy. Gastrointest Endosc. 2004;59:267–279. doi: 10.1016/s0016-5107(03)02110-2. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary A, Aranya RC. Surgery in perforation after endoscopic sphincterotomy: sooner, later or not at all? Ann R Coll Surg Engl. 1996;78:206–208. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YT, Hui AJ, Wong VW, Hung LC, Sung JJ. Improved colonoscopy success rate with a distally attached mucosectomy cap. Endoscopy. 2006;38:739–742. doi: 10.1055/s-2006-925238. [DOI] [PubMed] [Google Scholar]