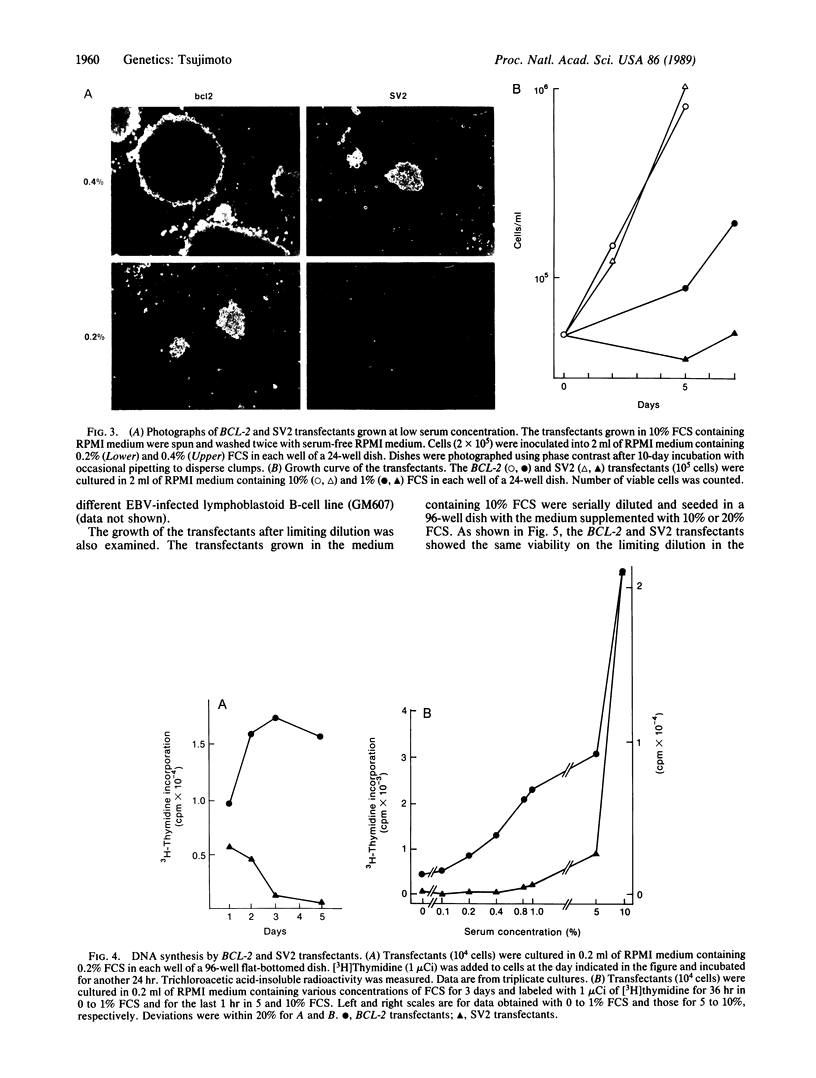
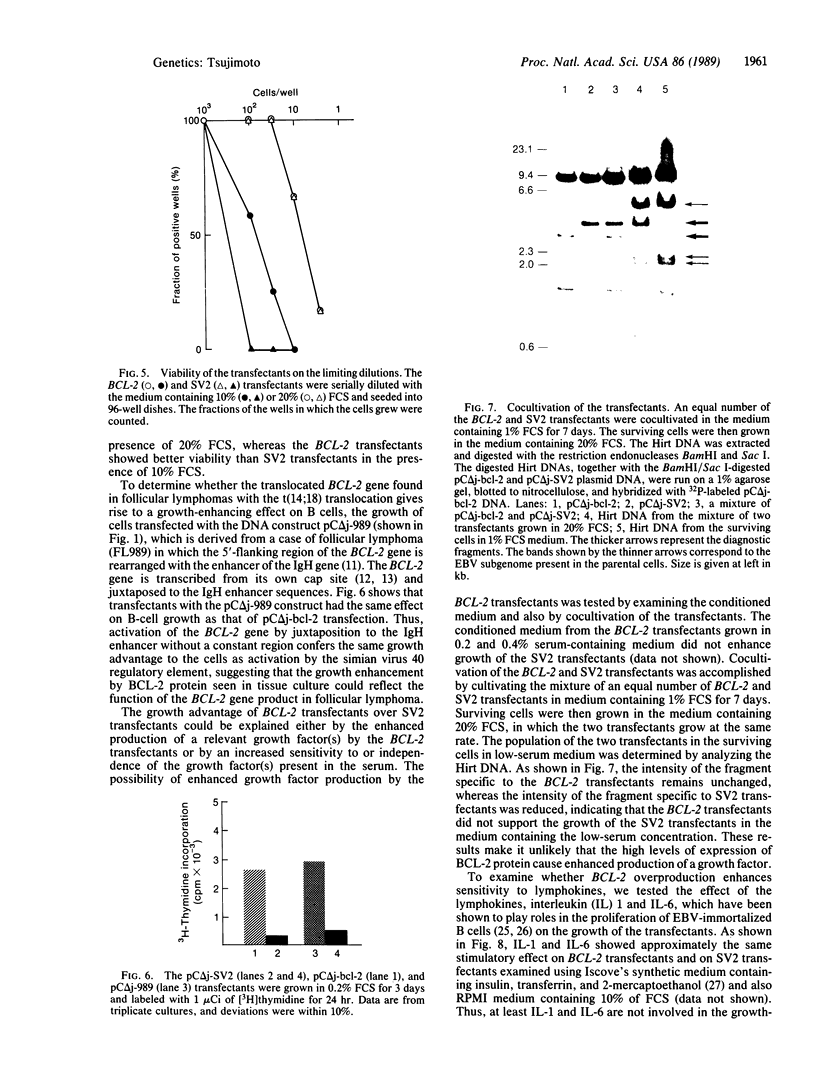
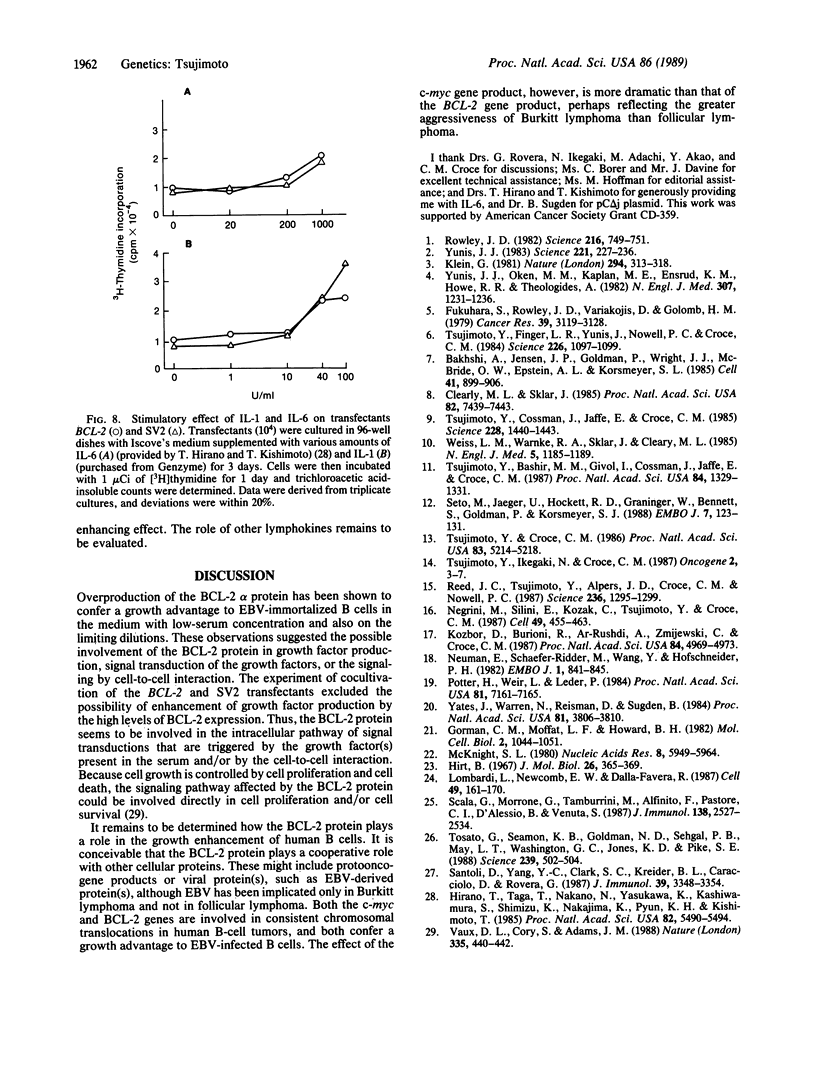
Abstract
The biological activity of the human BCL-2 gene product was analyzed in an Epstein-Barr virus (EBV)-infected human lymphoblastoid B-cell line transfected with BCL-2 sequences driven by the simian virus 40 promoter and enhancer. Overproduction of the BCL-2 protein conferred a selective growth advantage to the EBV-infected B cells as selective growth advantage to the EBV-infected B cells as compared with control transfectants in low-serum medium and also after seeding at limiting dilution but did not render the cells tumorigenic in athymic nude mice. This growth enhancement was also seen in cells transfected with the BCL-2 gene with its own promoter juxtaposed to the immunoglobulin heavy chain gene enhancer, which represents the translocated form of the BCL-2 gene observed in follicular lymphomas with the t(14;18) translocation. The growth advantage of EBV-infected B cells overproducing the BCL-2 protein is neither due to the enhanced growth factor production nor due to an enhanced sensitivity of the BCL-2 transfectants to interleukins 1 or 6, although both lymphokines are known to stimulate proliferation of EBV-infected B-cell lines. The growth advantage of EBV-infected B cells by overproduction of the BCL-2 protein suggests the direct involvement of the BCL-2 gene product in the pathogenesis of follicular lymphoma.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Rowley J. D., Variakojis D., Golomb H. M. Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res. 1979 Aug;39(8):3119–3128. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Burioni R., Ar-Rushdi A., Zmijewski C., Croce C. M. Expression of members of immunoglobulin gene family in somatic cell hybrids between human B and T cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4969–4973. doi: 10.1073/pnas.84.14.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Alpers J. D., Croce C. M., Nowell P. C. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. doi: 10.1126/science.3495884. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Santoli D., Yang Y. C., Clark S. C., Kreider B. L., Caracciolo D., Rovera G. Synergistic and antagonistic effects of recombinant human interleukin (IL) 3, IL-1 alpha, granulocyte and macrophage colony-stimulating factors (G-CSF and M-CSF) on the growth of GM-CSF-dependent leukemic cell lines. J Immunol. 1987 Nov 15;139(10):3348–3354. [PubMed] [Google Scholar]
- Scala G., Morrone G., Tamburrini M., Alfinito F., Pastore C. I., D'Alessio G., Venuta S. Autocrine growth function of human interleukin 1 molecules on ROHA-9, an EBV-transformed human B cell line. J Immunol. 1987 Apr 15;138(8):2527–2534. [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Bashir M. M., Givol I., Cossman J., Jaffe E., Croce C. M. DNA rearrangements in human follicular lymphoma can involve the 5' or the 3' region of the bcl-2 gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1329–1331. doi: 10.1073/pnas.84.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C. M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2(1):3–7. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]