Abstract
Ultraviolet radiation (UVR) is hazardous to patients with photosensitive skin disorders, such as lupus erythematosus, xeroderma pigmentosum and skin cancer. As such, these patients are advised to minimize their exposure to UVR. Classically, this is accomplished through careful avoidance of sun exposure and artificial tanning booths. Indoor light bulbs, however, are generally not considered to pose significant UVR hazard. We sought to test this notion by measuring the UV emissions of 19 different compact fluorescent light bulbs. The ability to induce skin damage was assessed with the CIE erythema action spectrum, ANSI S(λ) generalized UV hazard spectrum and the CIE photocarcinogenesis action spectrum. The results indicate that there is a great deal of variation amongst different bulbs, even within the same class. Although the irradiance of any given bulb is low, the possible daily exposure time is rather lengthy. This results in potential daily UVR doses ranging from 0.1 to 625 mJ cm−2, including a daily UVB (290–320 nm) dose of 0.01 to 15 mJ cm−2. Because patients are exposed continually over long time frames, this could lead to significant cumulative damage. It would therefore be prudent for patients to use bulbs with the lowest UV irradiance.
INTRODUCTION
It has long been known that ultraviolet radiation (UVR), particularly UVB (290–320 nm) and UVA2 (320–340 nm), can induce or exacerbate skin disease in a number of conditions, including systemic lupus erythematosus, xeroderma pigmentosum (XP) and skin cancer (1–5). The most significant source of UVR is the sun, and for years physicians have warned these patients to avoid direct sun exposure. It is now common practice for photosensitive patients to avoid being outdoors at peak sunlight hours and to wear sunscreen and sun-protective clothing for added protection.
Despite these efforts, patients may still be exposed to unwanted UVR from unexpected sources. In 1990, Diffey detailed the most common sources of UVR, listing sunlight and cosmetic tanning units first and fluorescent lamps last (6). It is becoming increasingly clear, however, that the effects of fluorescent lights are more substantial than was once assumed. In 1985, Cole et al. established that commercially available fluorescent lamps emit UVB and UVC (7). These findings proved to be clinically relevant in 1992, when Rihner and McGrath established that lupus patients reported worsening systemic and cutaneous symptoms after exposure to fluorescent light (8). A more recent survey of fluorescent bulbs performed by some of our group confirmed that both fluorescent tubes and energy-saving compact fluorescent light bulbs (CFL) emit appreciable levels of UVA, UVB and even UVC (9). This finding was confirmed by Khazova and O’Hagan, who found that several uncovered CFLs exceeded the UV exposure limits defined by the International Commission on Non-Ionizing Radiation Protection (ICNIRP) (10). However, they concluded that enveloped bulbs were safe (11).
Recently, Chignell et al. (12) evaluated the photosensitizing potential of CFLs measured from 300–750 nm and also tabulated UV emissions. Because their instrumentation differed substantially from ours (9), we sought to retest some of the bulbs using a highly sensitive spectroradiometer. We also tested an assortment of commercially available CFLs with various protective envelopes in an effort to identify sources with minimal UV emission. We quantified total spectral irradiance and photobiological hazards for each unique bulb in order to determine which brands and models are safest for patients with UV photosensitive conditions.
METHODS
Lamps
Lamps were purchased from local retail suppliers including Wal-Mart, Kroger, Snucks, Lowes, Home Depot and a Memphis lighting distributor (Table 1). We specifically selected bulbs with various protective envelopes in an effort to identify sources with minimal UV emission; the only unshielded bulbs included were the n:vision brand bulbs that were tested by Chignell’s group (12). With the exception of two lamps from a previous study, that are no longer available, all lamps were new and unseasoned.
Table 1.
Compact fluorescent sources examined listed by brand. Product family, base code, wattage and relative output as incandescent wattage are listed as indicated on packaging or lamp base. Configurations are indicated as incandescent (I), bare fluorescent (BF) and shielded fluorescent (SF) with covering material described.
| Brand | Family name | Code | Wattage/incandescent comparison | Configuration |
|---|---|---|---|---|
| GE | POST LIGHT | FLE14/2/TC16/SWCD | 14/60 | SF (plastic) |
| Bug Light (yellow) | FLE14/2/TC16/BUG | 14/60 | SF (plastic) | |
| Ceiling Fan | FLE11/2/A17XL/CD | 11/40 | SF (glass) | |
| Incandescent Soft White | Incandescent | 60/60 | I | |
| Lights of America | Mini Globe | 2507 N, G-16 | 7/40 | SF (glass) |
| Mini Décor | 2107 N | 7/40 | SF (glass) | |
| Soft White | 2009A N | 9/40 | SF (glass) | |
| Reflector Flood | 2814-R20 | 14/60 | SF (glass) | |
| n:vision | Daylight | EDXO-14 5500K | 14/60 | BF |
| Soft White | EDXO-14 2700K | 14/60 | BF | |
| Bright White | EDXO-14 3500K | 14/60 | BF | |
| Mini Spot Reflector, Soft White | BR20, EDXR-20-14, 2700K | 14/50 | SF (glass) | |
| Philips | Energy Saver (ES) Soft White | EL/SWP, A19 | 14/60 | SF (glass) |
| ES Outdoor Postlight | EL/O | 14/60 | SF (glass) | |
| ES Outdoor Postlight | EL/O1 | 14/60 | SF (glass) | |
| ES BUG-A-WAY (yellow) | EL/SWP, A19 | 14/60 | SF (glass) | |
| MARATHON BUG-A-WAY (yellow) | EL/O | 15/75 | SF (plastic) | |
| MARATHON OUTDOOR* | BC-EL/O 18 LLG | 18/75 | SF (plastic) | |
| DAYLIGHT ES* | EL/O 15 DL50 | 15/50 | SF (plastic) | |
| Sylvania | Soft White 60 | CF14EL/A19 | 14/60 | SF (glass) |
Two lamps are out of production, although limited distributor inventory is available.
Spectral measurement methods
Following a 10 min warm up period, the emission spectrum of each lamp was measured using an Optronic Laboratories (Orlando, FL) model OL-756 spectroradiometer with 0.125, 0.5, 0.125 mm slits and a 4 inch integrating sphere with a 32 mm entrance aperture. As configured this spectroradiometer, with stray light <10−8 and single scan dynamic range of ~6 orders of magnitude of irradiance, satisfies the instrument requirements of CIE S 009/E:2002 Annex B.1 (13). The spectroradiometer was calibrated using a NIST traceable quartz-halogen calibration standard. Before each day’s measurements the wavelength offset and calibration response precision were checked using procedures designed for OL-756. Two spectra were averaged in 1 nm increments from 250 to 800 nm. As the specific needs of photosensitive patients falls under the ANSI guidelines for photobiological risk assessment of “specialty” lighting, the sources were measured at a distance of 20 cm accordingly (14–16).
In addition, each bulb was measured with a Solarmeter Digital Ultraviolet Meter, model 5.7 UVA + B Sensitive Microwatt Version meter (Solartech, Inc., Harrison Township, MI), at 20 cm. This small meter measures UV within the range of 1–1999 μW cm−2. It is recommended by the XP Society to provide a means for patients and physicians to check UV emissions from sources in their immediate environment.
Spectroradiometric measurement uncertainty
To evaluate measurement uncertainty, one must factor in all sources of uncertainty including contribution from instrument performance and traceability of calibration standards.
Rapid Precision Testing Laboratories has compiled a general measurement uncertainty budget based on a combination of the uncertainties provided by Optronic Laboratories as well as our own laboratory results, field experience and best estimates (Table 2).
Table 2.
Lamp measurement uncertainty budget.
| Uncertainty contributions | (ui) | (ui)2 | |
|---|---|---|---|
| 1. a. Uncertainty of NIST 1000W FEL standard (Max) | 1.56 (%) | 2.43 | |
| 1. b. Uncertainty of transfer to OL752-10 standard (Max) | 1.5 | 2.25 | |
| 2. Uncertainty of calibration transfer to spectroradiometer | 3 | 9 | |
| 3. Stray/scattered radiation in test environment | 0.1 | 0.01 | |
| 4. Nonlinearity of response throughout scan range | 1 | 1 | |
| 5. Wavelength uncertainty | 0.2 | 0.04 | |
| 6. Wavelength repeatability | 1 | 1 | |
| 7. Noise in measurements | 0.1 | 0.01 | |
| 8. Reproducibility of measurements | 1 | 1 | |
| 9. Source positioning and power uncertainty | 10 | 100 | |
| Combined squared uncertainty contributions | 116.74 | ||
| Expanded measurement uncertainty | 10.8% | ||
|
| |||
The major source of uncertainty is the 10% uncertainty in positioning the spectroradiometer relative to the sample source and the accuracy or stability of the sample source’s power supply. For this study, evaluating easily positioned bulbs operating on line current adjusted to 120 ± 0.2 V, we estimate source positioning and powering uncertainty to be less than 5% reducing expanded uncertainty to 6.5%. If source positioning and powering uncertainty is taken to be zero, then minimum uncertainty is 4.1%. Therefore the uncertainty of measurements reported in this study is estimated to be between 4.1% and 6.5%.
Spectral analysis procedures
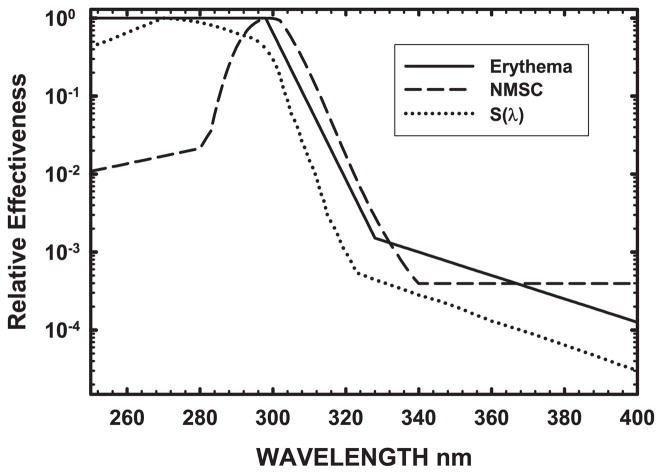
Our primary emphasis was identifying commercially available bulbs with minimal potential to exacerbate skin disease in photosensitive patients. However spectral hazard functions for such conditions are not well characterized. Some markers of UV-induced damage in normal individuals include erythema and skin cancer, therefore we analyzed the data using the CIE Erythema Reference Action Spectrum (17), the S(λ) Generalized UV Hazard Spectrum from ANSI/IESNA RP-27 (16) and the recently adopted CIE Photocarcinogenesis Action Spectrum (18). These response spectra (Fig. 1) extend through 4 orders of magnitude of effectiveness, ranging from 10−4 to 100. To appropriately compute source hazard using these action spectra, one needs to use spectral data measured with a precision double monochromator system capable of measuring at least 4–5 orders of magnitude or more of power as is explained in ANSI/IESNA RP-27.1 (14) and CIE S 009/E:2002 Annex B.1 (13).
Figure 1.
UV action spectra. Relative spectral effectiveness plots of the wavelengths capable of inducing erythema, generalized UV hazard, and nonmelanoma skin cancer (NMSC) as given by the CIE Erythema Reference Action Spectrum (17), ANSI/IESNA UV Hazard Function S(λ) (16) and the CIE Action Spectrum for Photocarcinogenesis (NMSC) (18). These UV hazard weighting functions, which peak in the UVB around ~300 nm and have minimum relative effectiveness at 400 nm as they reach visible light, are used to calculate spectral hazards from UV sources.
Each measured source spectrum was integrated with the aforementioned action spectra to produce a weighted spectral effectiveness, or hazard, for each source. This number reflects each lamp’s ability to elicit a particular biological response, based on the overlap between the lamp’s unique irradiance spectrum and the known action spectra. The effective irradiance of each source was calculated by weighting the absolute spectral irradiance at each wavelength by the action spectrum and summing over the specified wavelength range as shown in Eq. (1):
| (1) |
where Eas is effective irradiance, Eλ is the absolute spectral irradiance in W cm−2 nm−1 at wavelength λ in nm and Δλ is the wavelength interval used in the summation, in this case 1 nm, and σas(λ) is the relative effectiveness of wavelength λ in nm given in the respective action spectrum. For unweighted UV emission, the measured irradiance is simply summed from 250 to 400 nm and presented as μW cm−2.
The standard erythema dose (SED), an internationally recognized unit of UV exposure defined as equivalent to an erythemal effective radiant exposure of 0.01 J cm−2 (17), was calculated as the time required to accumulate 0.01 J cm−2 erythemal effective exposure as shown in Eq. (2):
| (2) |
where SED in seconds is equal to effective irradiance, Eas in effective W cm−2 from Eq. (1) using the CIE erythemal action spectrum as σas(λ), divided by Φ as 0.01 erythemal effective J cm−2.
RESULTS
Figure 1 shows the three UV action spectra used to evaluate the CFLs; these curves represent the relative effectiveness of UV wavelengths for inducing erythema (17) and nonmelanoma skin cancers (NMSC) (18), as well as the S(λ) generalized UV hazard (16). These action spectra were then used to calculate the spectral hazard of exposure to a specific bulb for each of these biological endpoints (Table 3).
Table 3.
The effective irradiance of each source lamp, in terms of eliciting erythema, generalized UV hazard and NMSC sorted according to erythemal effectiveness.
| Lamp | SED (h) | Erythemal effective irradiance (nW cm−2) | Total S(λ) weighted UV (nW cm−2) | NMSC effective irradiance (nW cm−2) |
|---|---|---|---|---|
| Sunlight AM 1.0 | 0.09 | 31691 | 8429 | 69491 |
| Sunlight AM 1.5 | 0.25 | 10971 | 2510 | 24001 |
| Sunlight AM 2.0 | 0.55 | 5022 | 1041 | 10495 |
| NVSoftWhite | 61 | 46 | 14 | 82 |
| GE incandescent | 111 | 25 | 10 | 36 |
| GE POST | 126 | 22 | 5.5 | 45 |
| GEBug (yellow) | 127 | 22 | 5.4 | 44 |
| NVBrightWhite | 151 | 18 | 6.2 | 29 |
| NVMiniSpotReflector | 239 | 12 | 3.4 | 12 |
| LAReflector | 358 | 7.8 | 2.5 | 6.7 |
| PhilipsES | 368 | 7.5 | 2.3 | 10.3 |
| Lights of America Mini Decor | 391 | 7.1 | 1.9 | 13.4 |
| NVDaylight | 473 | 5.9 | 2.4 | 7.0 |
| SylvaniaSW | 503 | 5.5 | 1.8 | 6.4 |
| PhilipsOPost1 | 505 | 5.5 | 1.9 | 4.9 |
| PhilipsOPost | 593 | 4.7 | 1.6 | 4.9 |
| Soft White | 863 | 3.2 | 1.5 | 2.2 |
| Mini Globe | 1022 | 2.7 | 1.1 | 2.0 |
| GE Ceiling | 1285 | 2.2 | 0.99 | 1.7 |
| Philips * MARATHON OUTDOOR | 2346 | 1.2 | 0.83 | 0.30 |
| Philips * DAYLIGHT ES | 2782 | 1.0 | 0.64 | 0.38 |
| Philips BUG-A-WAY (yellow) | 2934 | 0.95 | 0.60 | 0.30 |
| Philips MARATHON BUG-A-WAY (yellow) | 3007 | 0.92 | 0.66 | 0.26 |
Two lamps are out of production, although limited distributor inventory is available.
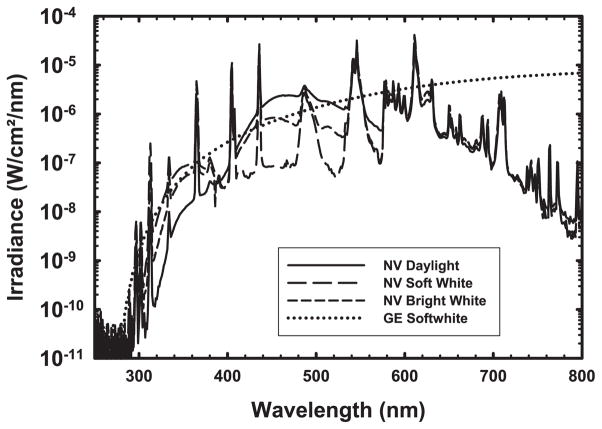
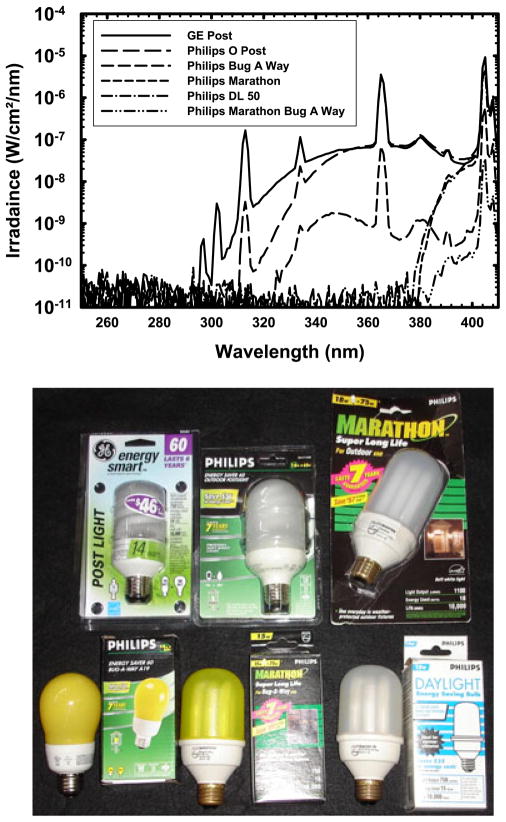
There is a great deal of variability in the emission spectra of CFLs. Figure 2 shows the emission spectra of the n:vision CFLs, some of which were also examined by Chignell et al. (12), compared to an incandescent bulb. Using our more sensitive spectroradiometer, UV irradiance is discernible starting at ~300 nm. All of these bulbs emit UV in the UVA and UVB range. Figure 3 compares the emission spectra of selected covered or shielded CFLs, which span a wide range of UV hazard; several lamps have virtually no UV emission while others show mercury emission lines in the UVB, including those at 294, 297, 302 and 313 nm. Despite their differences, they have very similar names and packaging (Fig. 3, panel 2).
Figure 2.
Representative plot of lamp spectra. Irradiance spectra for select bulbs, including three n:vision compact fluorescent lamps and a GE soft white incandescent bulb. All compact fluorescent bulbs exhibited numerous strong, primarily mercury, emission lines. In the UV, several compact fluorescent lamps also exhibited continuous emission from their phosphors detectable down to around 300 nm. In the visible, ~400–700 nm, the different visible phosphor types can be distinguished. The GE Soft White lamp is an incandescent tungsten filament lamp and has a continuous emission rising from about 300 nm and reaching a maximum at 800 nm in the near-infrared.
Figure 3.
UVR from six covered and shielded compact fluorescent lamps. This figure plots (upper panel) the UV spectrum of six covered or shielded compact fluorescent lamps with both glass and plastic covers. Several have almost identical names and packaging as evident in the photograph (lower panel). Several lamps have virtually no UV emission while others show many mercury emission lines including those at 294, 297, 302 and 313 nm in the UVB. The spectra of the two discontinued Philips lamps show that it is feasible to manufacture CFLs emitting white light, with good color rendering, that have nearly undetectable UV hazard.
Of all of the bulbs tested, the Philips BUG-A-WAY and MARATHON BUG-A-WAY appear to be the safest. They emit the lowest levels of UVA, UVB and UVC as determined using both the meter and the spectroradiometer (Table 4). They also have the lowest erythemal effective irradiance, total weighted UV and NMSC effective irradiance (Table 3). Assuming eight hours of exposure per day, 5 days a week, it would take about a year and a half to receive 1 SED, or 10 mJ cm−2, of erythemally effective UV exposure. However, both of these bulbs emit less esthetically pleasing yellow light, which will not provide accurate color rendering for optimal visual acuity. The next best bulbs are the Philips MARATHON OUTDOOR and the similar Philips DAYLIGHT ES. Both bulbs are enclosed by a white plastic cover similar to that found on the MARATHON BUG-A-WAY, and the light they emit is not yellow. Unfortunately, the manufacturing of these lamps has been discontinued, although distributor inventory remains available as of this writing. Nevertheless, the spectra of the two discontinued Philips lamps show that it is technically feasible to manufacture CFLs emitting white light, with good color rendering, that have nearly undetectable UV hazard.
Table 4.
Detailed emission spectra of various CFL, compared to incandescent. Data are sorted according to the UV emission measured by the Solattech Inc. Solar Meter listed in column 2, and columns 3–7 list the UV emissions in various subdivisions of the UV measured by the OL-756 spectroradiometer.
| Lamp | Meter (μW cm−2) | UVC 250–290 nm (μW cm−2) | UVB 290–320 nm (μW cm−2) | UVA2 320–340 nm (μW cm−2) | UVA1 340–400 nm (μW cm−2) | Total UV 250–400 nm (μW cm−2) |
|---|---|---|---|---|---|---|
| NVMiniSpotReflector | 27 | 7.19E–04 | 0.03 | 0.39 | 21.3 | 21.7 |
| LAReflector | 19 | 9.08E–04 | 4.33E–04 | 0.06 | 16.4 | 16.5 |
| NVSoftWhite | 19 | 1.20E–03 | 0.52 | 0.72 | 13.8 | 15.0 |
| GE POST | 15 | 9.06E–04 | 0.33 | 0.51 | 10.4 | 11.2 |
| NVBrightWhite | 14 | 1.08E–03 | 0.15 | 0.29 | 9.91 | 10.4 |
| GEBug | 13 | 1.02E–03 | 0.33 | 0.57 | 10.5 | 11.4 |
| PhilipsOPost1 | 10 | 8.97E–04 | 6.87E–03 | 0.12 | 9.91 | 10.0 |
| PhilipsES | 10 | 8.72E–04 | 0.06 | 0.41 | 7.6 | 8.1 |
| SylvaniaSW | 9 | 7.99E–04 | 0.03 | 0.26 | 6.95 | 7.2 |
| PhilipsOPost | 8 | 7.72E–04 | 0.02 | 0.15 | 7.14 | 7.3 |
| GE incandescent | 7 | 2.62E–03 | 1.22E–03 | 0.45 | 8.19 | 8.6 |
| Soft White | 5 | 1.23E–03 | 2.95E–03 | 0.06 | 3.9 | 4.0 |
| Lights of America Mini Decor | 5 | 8.59E–04 | 0.11 | 0.18 | 3.14 | 3.4 |
| NVDaylight | 4 | 8.26E–04 | 0.02 | 0.04 | 4.06 | 4.1 |
| Mini Globe | 4 | 9.17E–04 | 3.39E–03 | 0.07 | 3.53 | 3.6 |
| GECeiling | 3 | 8.08E–04 | 4.17E–03 | 0.01 | 2.55 | 2.6 |
| Philips * MARATHON OUTDOOR | 0 | 9.8.E–04 | 3.72E–04 | 2.05E–04 | 0.22 | 0.22 |
| Philips * DAYLIGHT ES | 0 | 7.2.E–04 | 4.97E–04 | 2.17E–04 | 0.25 | 0.25 |
| Philips BUG-A-WAY | 0 | 6.94E–04 | 3.94E–04 | 6.52E–03 | 0.19 | 0.20 |
| Philips MARATHON BUG-A-WAY | 0 | 7.48E–04 | 4.75E–04 | 1.84E–04 | 2.46E–03 | 0.004 |
Two lamps are out of production, although limited distributor inventory is available.
The next best currently manufactured bulb is the GE ceiling fan bulb, followed by the Lights of America mini globe (Tables 3 and 4). The CFLs with the greatest UV hazard were the unshielded n:vision soft white and the plastic-enclosed GE POST LIGHT, which had the highest levels of UV irradiance, erythemal effective irradiance, total S(λ) weighted UV and NMSC effective irradiance. With these bulbs, assuming the same exposure times listed above, it would take 1½ and 3 weeks, respectively, to receive 1 SED (Table 3).
As a point of reference, the incandescent lamp has intermediate levels of unweighted irradiance. Though not as high as the n:vision soft-white CFL, its erythemal effective irradiance, total S(λ) weighted UV and NMSC effective irradiance were higher than most of the fluorescents we examined. It would take about 3 weeks to receive 1 SED from the GE soft white incandescent (Table 3). However, its total UV, and UVB in particular, were lower than many of the CFLs (Table 4).
As expected, each bulb emits significantly less UVR than the sun. For example, with exposure to the midaltitude AM 1.5 solar spectrum, it would take 15 min to receive 1 SED (Table 3).
DISCUSSION
These results verify that CFLs may emit low levels of UVR. While our lamp sampling was intentionally biased toward covered or shielding lamps, our findings clearly indicate that among these types of CFLs, there is a great deal of variation in the actual level of biologically effective UVR emitted and the resulting exposure hazard. Assuming 8 h of exposure per day, the dose of total UV ranged from 0.1 to 625 mJ cm−2 day−1, while the UVB dose ranged from 0.01 to 15 mJ cm−2 day−1.
This variability is in part a result of differences in the composition of the outer coverings. We found bulbs with plastic outer coverings at both ends of our range of UV emission, with glass enclosed and bare compact fluorescent bulbs mixed in between. Common window glass blocks UVC, UVB and some UVA2, whereas very pure quartz-type glasses are essentially UV transparent. Likewise, clear plastics such as polycarbonate are effectively opaque to UV, while acrylics may be highly transparent to UV. Some types of coverings may be composed of materials that yellow with use. We did not attempt to evaluate such spectral degradation as a function of lamp ageing or potential lot or batch variation within any individual lamp product.
Our results did not clearly indicate that covered bulbs consistently pose lower UV hazards than uncovered bulbs, in contrast to Khazova and O’Hagan’s report (11). There were several covered bulbs among our sampling with higher irradiance and effective irradiance than the limited number of uncovered bulbs we examined, particularly the uncovered n:vision Daylight (Tables 3 and 4).
In general, however, the light bulbs emitted very low levels of UVR. This degree of UVR exposure is not expected to cause erythema, which is often used as a general index of UV hazard, in normal individuals with intact skin repair processes. However, these same repair mechanisms are deficient or impaired in photosensitive individuals. Similarly, accumulation of very low level photocarcinogenic exposure, as represented by our NMSC hazard weighting, is obviously of greater consequence in XP patients with known propensity to skin cancers. The question thus remains as to whether or not this level of UVR is clinically significant for photosensitive patients.
There are only a handful of studies that have evaluated the effects of UVR in doses as low as those measured here. In 2000, Runger et al. exposed Epstein–Barr virus-transformed lymphoblasts to varying doses of UVR. They found that a single dose of 3 mJ cm−2 of UVB (280–320 nm) could elicit DNA damage in normal donor cells and inhibit growth and stimulate apoptosis in XP donor cells (19). However, they irradiated monolayers of cells in culture, which lack the protection of skin and connective tissue that humans possess. These layers both attenuate and spectrally modify incoming radiation, which affords some protection to the underlying cells. Moreover, transformed lymphoblasts do not necessarily behave like normal cells in vitro, further complicating any direct comparison. Thus, while the data are suggestive of risk to photosensitive patients, it is difficult to extrapolate the extent of the danger.
A more direct comparison was made by De Gruijl et al., who irradiated albino hairless mice with broad-spectrum UVR (~280–360) on a daily basis. A range of doses was tested, the lowest of which was 5.7 mJ cm−2 day−1. The primary endpoint was tumor formation, which approached 100% regardless of the dose of UVR administered. Of note, the amount of time needed to reach this prevalence increased as the dose decreased (20).
Kaidbey et al. performed similar experiments with humans, looking at the primary endpoint of erythema after 5 days of repeated exposure to UVR. He determined spectrum-specific threshold doses, at which little to no erythema developed; for UVA (320–410 nm), this dose was 3.8 J cm−2, for UVB (270–320) it was 4.7 mJ cm−2 and for UVC it was 6.5 mJ cm−2 (21). These results provide a rough estimate of the upper limit of acceptable UV exposure; however, they might underestimate the risk to photosensitive patients for a number of reasons. First, these experiments were performed on healthy subjects, and it is possible that lupus patients and certainly XP patients would have reacted more intensely. Second, the subjects were only followed for 5 days, whereas the average patient is exposed to bulbs for many years. It is conceivable that erythema would have developed at these doses if the exposure was sustained for longer. It is also difficult to make a direct comparison between these results and the irradiance measured in this study. The spectroradiometer used here is far more sensitive than the broadband radiometer used in the Kaidbey study. Moreover, the source they used to irradiate with UVB extended into the UVA range, which might have influenced the results.
It is also important to recognize that although the level of UV emitted from bulbs is much lower than that of the sun, patients spend considerably more time exposed to lamps than they do outdoors. This has the potential to lead to significant cumulative damage. Damage is able to accumulate when the skin does not have adequate time to recover from the initial insult, which usually takes 24–48 h (22). Thus, if someone is exposed to fluorescent lights on a daily basis, the damage may build and eventually result in clinically apparent changes. Several studies have substantiated this principle by demonstrating that even suberythemal doses of UVR can elicit erythema if applied to the skin chronically (21,23). Moreover, repeated exposure to suberythemal doses of UVR within short enough time frames sensitizes the skin, causing the minimal erythema dose to decrease in a time-dependent manner (21,23).
Collectively, these studies indicate that the risk of chronic exacerbation of photosensitivity from frequent exposure to CFLs is not negligible. Moreover, the action spectra used indicate significant variability in terms of the bulbs’ erythemal, NMSC and general S(λ) UV hazard. Until further studies are performed to determine what dose of UVR is safe in photosensitive patients, it would be prudent for these individuals to protect themselves by using bulbs with the lowest UV emission and effective irradiance. Unfortunately, given the variability of UV hazard from covered CFLs, and the lack of distinguishing package labeling (Fig. 3, lower) it is challenging for photosensitive patients to know which bulbs are safest. We would therefore encourage physicians to refer to Tables 1, 3 and 4 when making recommendations to patients.
We urge responsible lamp manufacturers to provide alternative UV-safe bulbs that are clearly marked with an internationally recognizable symbol, as proposed in Fig. 4, consisting of the letters “UV” set inside a circle bisected by a diagonal slash. Aside from providing much needed information to photosensitive patients, the use of such a symbol, coincident with the development of relevant consensus standards, has other applications to general lighting. These broader applications include protection of artwork in homes or museums, prevention of color fading of products and packaging in retail environments and in UV-sensitive manufacturing processes.
Figure 4.
Proposed symbol to mark UV shielded compact fluorescent lamps. The internationally recognized format of this symbol will permit the unambiguous selection of lamps that are encapsulated with effective UV blocking materials.
Until lamp manufacturers provide improved UV hazard label information, individuals that are particularly vulnerable may prefer to use appropriate personal UV meters, as recommended by the XP society, to actually implement this precaution.
Acknowledgments
This material is based upon work supported in part by a Merit Review Grant from the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and by the National Institutes of Health (NIH K24-AR 02207) to V.P.W. and NIH training grant (NIH T32-AR007465-25) to R.S.K.
References
- 1.Epstein JH, Tuffanelli D, Dubois EL. Light sensitivity and lupus erythematosus. Arch Dermatol. 1965;91:483–485. doi: 10.1001/archderm.1965.01600110069013. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 3.Kuhn A, Beissert S. Photosensitivity in lupus erythematosus. Autoimmunity. 2005;38:519–529. doi: 10.1080/08916930500285626. [DOI] [PubMed] [Google Scholar]
- 4.Robbins JH, Kraemer KH, Lutzner MA, Festoff BW, Coon HG. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- 5.Wysenbeek AJ, Block DA, Fries JF. Prevalence and expression of photosensitivity in systemic lupus erythematosus. Ann Rheum Dis. 1989;48:461–463. doi: 10.1136/ard.48.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diffey BL. Human exposure to ultraviolet radiation. Semin Dermatol. 1990;9:2–10. [PubMed] [Google Scholar]
- 7.Cole C, Forbes PD, Davies RE, Urbach F. Effect of indoor lighting on normal skin. Ann N Y Acad Sci. 1985;453:305–316. doi: 10.1111/j.1749-6632.1985.tb11819.x. [DOI] [PubMed] [Google Scholar]
- 8.Rihner M, McGrath H., Jr Fluorescent light photosensitivity in patients with systemic lupus erythematosus. Arthritis Rheum. 1992;35:949–952. doi: 10.1002/art.1780350816. [DOI] [PubMed] [Google Scholar]
- 9.Sayre RM, Dowdy JC, Poh-Fitzpatrick M. Dermatological risk of indoor ultraviolet exposure from contemporary lighting sources. Photochem Photobiol. 2004;80:47–51. doi: 10.1562/2004-02-03-RA-074.1. [DOI] [PubMed] [Google Scholar]
- 10.ICNIRP. Guidelines on Limits of Exposure to Ultraviolet Radiation of Wavelengths between 180 nm and 400 nm (Incoherent Optical Radiation) Health Physics Society; Oberschleissheim, Germany: 2004. [DOI] [PubMed] [Google Scholar]
- 11.Khazova M, O’Hagan JB. Optical radiation emissions from compact fluorescent lamps. Radiat Prot Dosimetry. 2008;131:521–525. doi: 10.1093/rpd/ncn234. [DOI] [PubMed] [Google Scholar]
- 12.Chignell CF, Sik RH, Bilski PJ. The photosensitizing potential of compact fluorescent vs incandescent light bulbs. Photochem Photobiol. 2008;84:1291–1293. doi: 10.1111/j.1751-1097.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 13.6-47, C. T. C. CIE Standard 009/E:2002, Photobiological Safety of Lamps and Lamp Systems. Commission Internationale de l’Eclairage (CIE) Central Bureau; Vienna, Austria: 2002. [Google Scholar]
- 14.IESNA Photobiology Committee. ANSI/IESNA RP-27.1-05, Recommended Practice for Photobiological Safety for Lamps & Lamp Systems—General Requirements. Illuminating Engineering Society of North America; New York: 2005. [Google Scholar]
- 15.IESNA Photobiology Committee. ANSI/IESNA RP-27.2-00, Recommended Practice for Photobiological Safety for Lamps & Lamp Systems—Measurement Techniques. Illuminating Engineering Society of North America; New York: 2001. [Google Scholar]
- 16.IESNA Photobiology Committee. ANSI/IESNA RP-27.3-96, Recommended Practice for Photobiological Safety for Lamps & Lamp Systems—Risk Group Classification & Labeling. Illuminating Engineering Society of North America; New York: 1996. [Google Scholar]
- 17.International Commission on Illumination (CIE) International Standard ISO 17166:1999(E)—CIE 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. 1. International Organization for Standardization (ISO); Geneva, Switzerland: 1999. [Google Scholar]
- 18.CIE Technical Committee 6-32. CIE Standard S 019/E:2006, Photocarcinogenesis Action Spectrum (Non-Melanoma Skin Cancers) Commission Internationale de l’Eclairage (CIE) Central Bureau; Vienna, Austria: 2006. [Google Scholar]
- 19.Runger TM, Moller K, Jung T, Dekant B. DNA damage formation, DNA repair, and survival after exposure of DNA repair-proficient and nucleotide excision repair-deficient human lymphoblasts to UVA1 and UVB. Int J Radiat Biol. 2000;76:789–797. doi: 10.1080/09553000050028940. [DOI] [PubMed] [Google Scholar]
- 20.De Gruijl FR, Van Der Meer JB, Van Der Leun JC. Dose-time dependency of tumor formation by chronic UV exposure. Photochem Photobiol. 1983;37:53–62. doi: 10.1111/j.1751-1097.1983.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaidbey KH, Kligman AM. Cumulative effects from repeated exposures to ultraviolet radiation. J Invest Dermatol. 1981;76:352–355. doi: 10.1111/1523-1747.ep12520007. [DOI] [PubMed] [Google Scholar]
- 22.Arbabi L, Gange RW, Parrish JA. Recovery of skin from a single suberythemal dose of ultraviolet radiation. J Invest Dermatol. 1983;81:78–82. doi: 10.1111/1523-1747.ep12539063. [DOI] [PubMed] [Google Scholar]
- 23.Parrish JA, Zaynoun S, Anderson RR. Cumulative effects of repeated subthreshold doses of ultraviolet radiation. J Invest Dermatol. 1981;76:356–358. doi: 10.1111/1523-1747.ep12520019. [DOI] [PubMed] [Google Scholar]