Abstract
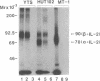
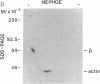
The human high-affinity receptor for interleukin 2 (IL-2) has been proposed as being a membrane complex composed of at least two distinct polypeptide chains: p55 (alpha chain), recognized by the anti-Tac monoclonal antibody (mAb), and p75 (beta chain), both of which are capable of binding IL-2. Whereas the alpha chain itself has been shown to be nonfunctional, the beta chain appears to be pivotal in the IL-2 signal transduction, although the beta chain is otherwise poorly characterized. Three beta chain-specific mAbs, designated Mik-beta 1, -beta 2, and -beta 3, were developed. Mik-beta 1 and -beta 2 completely inhibited the IL-2 binding to the beta chain, whereas Mik-beta 3 immunoprecipitated the beta chain crosslinked with 125I-labeled IL-2. The beta chain immunoprecipitated by these mAbs was revealed to have a Mr of 68,000-72,000. High-affinity IL-2 binding was completely abolished by Mik-beta 1. Although IL-2-dependent T-cell growth at high IL-2 concentrations was not inhibited by the anti-Tac, it was almost completely inhibited by Mik-beta 1 in the presence of the anti-Tac. These results clearly indicate that the beta chain is an indispensable component to the high-affinity IL-2 receptor and is responsible for the IL-2 signal transduction. The beta chain was found to be constitutively expressed without the alpha chain on the surface of peripheral blood Leu-19+ natural killer cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bich-Thuy L. T., Dukovich M., Peffer N. J., Fauci A. S., Kehrl J. H., Greene W. C. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987 Sep 1;139(5):1550–1556. [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Svetlik P. B., Rusk C. M., Depper J. M., Leonard W. J. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. doi: 10.1084/jem.162.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T., Diamantstein T. The human intermediate-affinity interleukin 2 receptor consists of two distinct, partially homologous glycoproteins. Eur J Immunol. 1988 Jul;18(7):1051–1057. doi: 10.1002/eji.1830180713. [DOI] [PubMed] [Google Scholar]
- Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987 Oct;70(4):1069–1072. [PubMed] [Google Scholar]
- Kondo S., Shimizu A., Saito Y., Kinoshita M., Honjo T. Molecular basis for two different affinity states of the interleukin 2 receptor: affinity conversion model. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9026–9029. doi: 10.1073/pnas.83.23.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Benike C. J., Phillips J. H., Engleman E. G. Recombinant interleukin 2 enhanced natural killer cell-mediated cytotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu 11 antigens. J Immunol. 1985 Feb;134(2):794–801. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Greene W. C. Contrasting interleukin 2 binding properties of the alpha (p55) and beta (p70) protein subunits of the human high-affinity interleukin 2 receptor. J Exp Med. 1987 Oct 1;166(4):1156–1161. doi: 10.1084/jem.166.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Nishi M., Ishida Y., Honjo T. Expression of functional interleukin-2 receptors in human light chain/Tac transgenic mice. Nature. 1988 Jan 21;331(6153):267–269. doi: 10.1038/331267a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. Direct identification of the murine IL-2 receptor p55-p75 heterodimer in the absence of IL-2. J Immunol. 1988 Jul 15;141(2):476–482. [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sharon M., Siegel J. P., Tosato G., Yodoi J., Gerrard T. L., Leonard W. J. The human interleukin 2 receptor beta chain (p70). Direct identification, partial purification, and patterns of expression on peripheral blood mononuclear cells. J Exp Med. 1988 Mar 1;167(3):1265–1270. doi: 10.1084/jem.167.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Goldman C. K., Bongiovanni K. F., Chan W. C., Winton E. F., Yagita M., Grimm E. A., Waldmann T. A. The p75 peptide is the receptor for interleukin 2 expressed on large granular lymphocytes and is responsible for the interleukin 2 activation of these cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5394–5398. doi: 10.1073/pnas.84.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]