Abstract
Rationale
Hallucinogenic serotonin 2A (5-HT2A) receptor partial agonists, such as (±)-1-(2,5-dimethoxy-4-iodo-phenyl)-2-aminopropane hydrochloride (DOI), induce a frontal cortex-dependent head-twitch response (HTR) in rodents, a behavioral proxy of a hallucinogenic response that is blocked by 5-HT2A receptor antagonists. In addition to 5-HT2A receptors, DOI and most other serotonin-like hallucinogens have high affinity and potency as partial agonists at 5-HT2C receptors.
Objectives
We tested for involvement of 5-HT2C receptors in the HTR induced by DOI.
Results
Comparison of 5-HT2C receptor knockout and wild-type littermates revealed an approximately 50% reduction in DOI-induced HTR in knockout mice. Also, pretreatment with either the 5-HT2C receptor antagonist SB206553 or SB242084 eradicated a twofold difference in DOI-induced HTR between the standard inbred mouse strains C57BL/6J and DBA/2J, and decreased the DOI-induced HTR by at least 50% in both strains. None of several measures of 5-HT2A receptors in frontal cortex explained the strain difference, including 5-HT2A receptor density, Gαq or Gαi/o protein levels, phospholipase C activity, or DOI-induced expression of Egr1 and Egr2. 5-HT2C receptor density in the brains of C57BL/6J and DBA/2J was also equivalent, suggesting that 5-HT2C receptor-mediated intracellular signaling or other physiological modulators of the HTR may explain the strain difference in response to DOI.
Conclusions
We conclude that the HTR to DOI in mice is strongly modulated by 5-HT2C receptor activity. This novel finding invites reassessment of hallucinogenic mechanisms involving 5-HT2 receptors.
Keywords: Serotonin 2A receptor (5-HT2A), Serotonin 2C receptor (5-HT2C), Hallucinogens, Head-twitch response (HTR), Phospholipase C (PLC), Phospholipase A (PLA)
Introduction
Serotonin-based hallucinogens, such as the phenethylamine 2,5-dimethoxy-4-iodoamphetamine (DOI), the indoleamine psilocin, and ergoline d-lysergic acid diethylamide (LSD) profoundly alter sensory perception, mood, cognition, and behavior in humans. These effects inspired long ago the idea that hallucinogens could be models for understanding mental illnesses, such as schizophrenia (Cohen 1953; Fischer et al. 1951; Hollister 1964). A common denominator of serotonin-based hallucinogens is their high affinity for serotonin type 2 (5-HT2) receptors (Nichols 2004), including 5-HT2A and 5-HT2C receptors that are expressed densely in regions of the brain known to regulate emotion and memory, such as the frontal cortex and limbic structures (Lopez-Gimenez et al. 1997, 2001, 2002). 5-HT2A and 5-HT2C receptors are altered in psychiatric conditions, ranging from obsessive-compulsive disorder and depression (Benedetti et al. 2008; Millan 2005) to schizophrenia (Herrick-Davis et al. 2000; Kang et al. 2009; Quednow et al. 2009). In addition, atypical antipsychotics as well as novel antidepressants and anxiolytic medications target 5-HT2A and/or 5-HT2C receptors.
Although it is well appreciated that both 5-HT2A and 5-HT2C receptors are altered in psychiatric conditions, whether 5-HT2A or both 5-HT2A and 5-HT2C receptors mediate the effects of hallucinogens remains to be determined. Several lines of evidence suggest that the behavioral and cognitive effects of hallucinogens are driven primarily through activation of 5-HT2A receptors in the frontal cortex (Gonzalez-Maeso et al. 2007; Vollenweider et al. 1997; Willins and Meltzer 1997). The hallucinogenic indoleamine psilocybin causes significant increases in cerebral metabolic rate in the frontal cortex and anterior cingulate cortex (Vollenweider et al. 1997), and pretreatment with the 5-HT2A antagonist ketanserin dose-dependently prevents hallucinations caused by psilocybin in humans (Vollenweider et al. 1998). Also, the 5-HT2A antagonists MDL100907 and ketanserin block a frontal cortex-mediated hallucinogenic behavioral proxy in rodents, the head-twitch response (HTR; Vickers et al. 2001; Willins and Meltzer 1997), as well as the increase in synaptic activity in frontal cortex neurons caused by application of the 5-HT2A/2C agonists α-methyl-5-HT and DOI (Beique et al. 2007; Marek and Aghajanian 1996). Furthermore, mice devoid of the 5-HT2A receptor do not exhibit an HTR to DOI (Gonzalez-Maeso et al. 2007), and 5-HT2A antagonists attenuate the discriminative properties of several hallucinogens in rodents (Benneyworth et al. 2005; Fiorella et al. 1995b; Schreiber et al. 1994).
Although all known serotonin hallucinogens bind with high affinity and activate with high potency the 5-HT2C receptor, this receptor has largely been dismissed as a potential modulator of the hallucinogenic response for a number of reasons. Despite similar coupling to G-proteins and second messenger systems as 5-HT2A receptors, there is evidence that 5-HT2C receptor activity may oppose activity of the 5-HT2A receptor. Neurochemically, 5-HT2C receptors tonically inhibit dopamine release in the frontal cortex, while 5-HT2A receptor stimulation increases dopamine release in the frontal cortex (Gobert and Millan 1999; Gobert et al. 2000; Millan et al. 1998). However, a recent report shows that injections of the 5-HT2C agonist RO 60-0175 into the medial prefrontal cortex of rats enhance dopamine outflow in nucleus accumbens caused by cocaine administration (Leggio et al. 2009).
Behaviorally, 5-HT2A receptor agonism increases, whereas 5-HT2C agonism decreases locomotor activity, inferred from experiments testing 5-HT2A/2C receptor agonists in 5-HT2A knockout mice (Halberstadt et al. 2009) and selective 5-HT2C receptor agonists in 5-HT2C knockout mice (Fletcher et al. 2009). Also, systemic injections of selective 5-HT2C receptor agonists with low efficacy at 5-HT2A receptors, such as mCPP and MK212, do not cause an HTR in rats. However, when rats are pretreated with the 5-HT2C receptor antagonist, SB242084, both mCPP and MK212 induce an HTR (Vickers et al. 2001). Further suggesting that 5-HT2C receptors are not involved in the behavioral effects of hallucinogens are the observations that the HTR caused by direct injections of DOI into the frontal cortex of rats is blocked by systemic injections of MDL100907, yet not the 5-HT2C/2B antagonist, SDZ-SER 082 (Willins and Meltzer 1997). Finally, systemic injections of MK212 and mCPP have been reported to inhibit a DOI-induced HTR in rats (Berendsen and Broekkamp 1990). However, these latter findings may be confounded by the observations that 5-HT2C agonists dose-dependently decrease locomotor activity, perhaps disguising a role for 5-HT2C receptors in the DOI-induced HTR.
Other findings support the possibility of 5-HT2C receptor involvement in the hallucinogenic response. For example, direct injections of mCPP and MK212 into the frontal cortex of rats induce an HTR, an effect that is attenuated by systemic pretreatment with the 5-HT2C antagonist SDZ-SER 082 (Willins and Meltzer 1997). In addition, enhanced sensitivity to LSD drug discrimination in rats following p-chlorophenylalanine depletion of serotonin is associated with enhanced 5-HT2C, yet not 5-HT2A receptor-mediated phospholipase C (PLC) activity (Fiorella et al. 1995a).
Also, although having greater affinity for 5-HT2A receptors than 5-HT2C receptors, MDL100907, ketanserin, and other 5-HT2A “selective” antagonists that block the effects of hallucinogens have appreciable affinity for 5-HT2C receptors (Knight et al. 2004; Roth et al. 1992), cautioning interpretation of observations with these drugs as evidence for specific 5-HT2A receptor dependence. Furthermore, caution should be present when interpreting data using “selective” 5-HT2C antagonists, such as SB242084, as recent studies show that it has inverse agonist properties at the 5-HT2C→phospholipase A2→arachidonic acid pathway, yet agonist properties at the 5-HT2C→PLC→inositol phosphate pathway in Chinese hamster ovary (CHO) cells (De Deurwaerdere et al. 2004). The second messengers activated by serotonin hallucinogens that are necessary for the hallucinogenic response are still unknown, although it has been shown that DOI-induced head-twitch responses are only partially prevented in Gq knockout mice, suggesting multiple second messenger pathways may be involved (Garcia et al. 2007). Finally, at least part of the electrophysiological effects of hallucinogens in the frontal cortex is mediated by the 5-HT2C receptor (Beique et al. 2007).
In the present study, we use pharmacological evidence, evidence from genetically modified mice lacking the 5-HT2C receptor, and evidence from a robust and reproducible mouse strain difference in HTR to DOI to show convincingly that 5-HT2C receptors potently modulate hallucinogen-induced head-twitch. These converging results suggest the possibility that coordinated activity of both 5-HT2A and 5-HT2C receptors underlie the hallucinogenic behavioral response in mice.
Methods
Mice
All experimental procedures were performed in accordance with (1) the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication 86–23) and (2) Institutional Animal Care and Use Committee. Eight-week-old male C57BL/6J and DBA/2J mice were purchased from Jackson Laboratories and allowed 2 weeks acclimation time in the vivarium prior to testing. Twenty-week-old 5-HT2C receptor null mice (Tecott et al. 1995) and wild-type littermates on a mixed 129 and C57BL/6 background, as well as 12–16-week-old 129SJ mice, were from established colonies in our laboratory. Mice were housed three to five per cage in standard rodent cages and had unlimited access to rodent chow and fresh water. All experiments involving live mice were performed between 1000 and 1600 hours.
Drugs
The 5-HT2A/2C receptor agonist, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), and the irreversible alkylating agent, N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) were purchased from Sigma-Aldrich (USA). The 5-HT2A receptor antagonist, MDL100907, was a gift from Merrill-Dow Corp (USA). The 5-HT2C receptor agonist, MK212, the 5-HT2C receptor inverse agonist/antagonist, SB206553, and the 5-HT2C receptor antagonist, SB242084, were each purchased from Tocris (USA). The mixed 5-HT1/2 receptor antagonist, methysergide, was also purchase from Tocris. [3H]-ketanserin and [125I]-DOI were purchased from Perkin-Elmer (USA). [3H]-mesulergine was purchased from G.E. Healthcare, Inc.
Head-twitch response
5-HT2A receptor hallucinogens induce a quantifiable HTR in rodents that is not induced by the non-hallucinogenic 5-HT2A receptor agonist lisuride (Gonzalez-Maeso et al. 2007). In addition, the HTR is not observed in 5-HT2A knockout mice administered 5-HT2A hallucinogens, and in wild-type animals, it is blocked by MDL100907 (Gonzalez-Maeso et al. 2007; Vickers et al. 2001). Also, 5-HT2A antagonists block the hallucinogenic response in humans. These data support the HTR as a valid behavioral proxy of hallucinogen action in mice. The role of 5-HT2A and 5-HT2C receptors in DOI-induced HTR was evaluated using both experimental genetic and pharmacologic manipulations. First, 5-HT2C receptor null mice (N=7) and littermate control mice (N=5) were treated with the hallucinogenic 5-HT2 receptor agonist DOI (1 mg/kg). Second, C57BL/6J and DBA/2J mice (N=6 per treatment group) were treated with DOI (1 mg/kg) alone or after pretreatment with 5-HT2A (MDL100987, 0.25 mg/kg) or 5-HT2C (SB206553, 0.3 and 3.0 mg/kg; SB242084, 3.0 mg/kg) receptor antagonists 10 min prior to the DOI treatment. To evaluate possible pharmacokinetic effects, C57BL/6J and DBA/2J mice (N=3 per treatment group) were also evaluated for dose–response to DOI at 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8 mg/kg without any pretreatment. DOI was injected intraperitoneally (IP: inbred strain dose–response and MDL100907 experiments) or subcutaneously (SC: 5-HT2C null mice and SB206553 and SB242084 experiments) at a volume of 10 ml/kg.
An HTR was defined as a clear, rapid, left to right or right to left tic movement of the head of the mouse following DOI treatment. Ten minutes after DOI injection, mice were placed inside a clean 3-L transparent glass beaker with a thin layer of fresh rodent bedding at the bottom. At least two observers counted HTRs in a 10-min period—observer's scores were averaged before analysis. Analyses of the data were by t test or analysis of variance (ANOVA; Stata, v.11).
EEDQ blockade of 5-HT2A receptor binding sites
To test whether SB206553 blocks 5-HT2A receptors in addition to 5-HT2C receptors in vivo, SB206553 was examined for its ability to prevent EEDQ binding to 5-HT2A receptors. EEDQ is an alkylating agent that binds irreversibly to 5-HT2A receptor binding sites (Kettle et al. 1999), blocking binding of [3H]-ketanserin to 5-HT2A receptors in membrane preparations. Mice were injected SC with saline (N=6), SB206553 (3 mg/kg, 10 ml/kg, N= 4), or MDL100907 (0.25 mg/kg, 10 ml/kg, N=4) 30 min prior to EEDQ injections (10 mg/kg, 10 ml/kg). Twenty-four hours later, mice were killed, and frontal cortex brain tissue was processed for [3H]-ketanserin radioligand binding (as described above). Ten-micromolar SB206553 was added to the radioligand assay buffer to block [3H]-ketanserin binding to 5-HT2C receptor sites. A single concentration of [3H]-ketanserin (5 nM) was used, and nonspecific binding was determined with the addition of 100 μM methysergide. All experiments were carried out in triplicate. Data were transformed to assess 5-HT2A receptor specific binding in drug-treated animals as percent of 5-HT2A receptor specific binding in saline-treated animals. A one-way ANOVA was performed to compare groups.
Saturation binding
5-HT2A receptor binding site density (Bmax) and antagonist affinity (Kd) were determined with [3H]-ketanserin, comparing membranes from frontal cortex of C57BL/6J and DBA/2J mice. Adult male C57BL/6J and DBA/2J mice were killed by brief isoflurane anesthesia and decapitation. Brains were quickly dissected free of the head, and olfactory bulbs were removed. The brain was placed into a cold, clean brain matrice, and frontal cortex, defined as cortex 2–3 mm caudal to the anterior pole of the brain, was cut free coronally using a clean razor blade. The tissue was homogenized in ice-cold 50-mM Tris assay buffer (50 mM Tris–HCl, 10 mM MgCl2, 0.1 mM EDTA (pH 7.3)). The homogenate was centrifuged at 20,000 g for 20 min at 4°C. The supernatant was discarded, and the pellet was resuspended in assay buffer. The centrifugation step was repeated two more times. Finally, the supernatant was discarded, the pellet was resuspended in assay buffer, briefly homogenized, and protein concentration was measured by Bradford assay (Bio-Rad).
Membrane preparations (200 μg/tube) were incubated with a range of concentrations of [3H]-ketanserin (0.25–8 nM) for 60 min at 37°C to determine total binding. Nonspecific binding was determined with 100 μM methy-sergide. All experiments were performed in duplicate. Following incubation, free radioligand was separated from bound by vacuum filtration through Whatman GF/C glass filters (Brandel, Gaithersburg, MD, USA). Filters were placed in vials with scintillation cocktail and counted 12 h later in a liquid scintillation counter.
Whole brain minus cerebellum was used for analysis of 5-HT2C receptor binding site density. Brain tissue was homogenized in cold assay buffer and centrifuged at 20,000 g for 20 min at 4°C. Supernatant was decanted, pellet was re-homogenized in fresh, cold assay buffer, then spun again at 20,000 g; this step was repeated, then samples were incubated for 30 min at 37°C to remove endogenous ligand. A final spin was performed, and the pellet was re-homogenized in ice-cold assay buffer. Protein concentration was determined using the BCA method. Samples were incubated at 37°C for 90 min with increasing concentrations of [3H]-mesulergine for 5-HT2C receptor binding (0.077–22.1 nM). One hundred nanomolar spiperone was added to block 5-HT2A receptor sites, and nonspecific binding was determined with the addition of 100 μM methysergide. All experiments were performed in duplicate. The reaction was terminated by adding ice-cold assay buffer, and membranes were collected, using a Brandel harvester, on Whatman GF/B glass filters presoaked with 0.3% polyethyleneimine. Filters were washed three times with cold 50 mM Tris–HCl buffer. Filters containing membrane-bound radioligand were placed in 5 mL scintillation fluid (Aquasol-2, Perkin-Elmer, USA), vortexed vigorously, and allowed to set overnight in the dark at room temperature. Bound radioactivity was determined by liquid scintillation spectrometry.
Nonlinear regression analysis (Stata, v.11, and Graphpad 5.02, USA) was used to determine Bmax and Kd estimates by simultaneously fitting the total and nonspecific curve data for six independent experiments per strain. The Bmax and Kd estimates for the C57BL/6J and DBA/2J samples were then compared by t test.
5-HT2A and 5-HT2C receptor autoradiography
Mice were anesthetized with isoflurane anesthesia then decapitated. Brains were removed and frozen quickly in isopentane on dry ice, then dabbed dry with a Kim Wipe and stored at −80°C until sectioning. Brains were thawed to −20°C inside a Cryostat chamber. Twenty-micrometer sections were thaw-mounted onto Superfrost Plus slides, then stored again at −80°C. At the start of the autoradiography experiments, slides were brought to room temperature then washed twice, 10 min each at room temperature in assay buffer containing 100 nM SB269770 to block 5-HT7 receptors. Slides were transferred to slide boxes containing assay buffer with 0.14 nM [125I]-DOI. Additional slides were incubated in radioligand plus 100 nM spiperone to define 5-HT2C receptors or 30 μM methysergide to define nonspecific binding. Slides were incubated for 60 min at room temperature then washed thrice, 10 min in ice-cold assay buffer, and briefly washed in cold deionized water, prior to drying with a stream of dehumidified air. Slides were exposed to Biomax MR film for 24, 48, or 72 h prior to developing. Autoradiograms were digitized using a CanoScan 4400F scanner (Canon, USA). Regional analysis of 5-HT2A or 5-HT2C receptor binding was quantified using NIH ImageJ version 1.42q (Abramoff et al. 2004). Brain areas, including anterior cingulate cortex, prefrontal cortex, somatosensory cortex, anterior striatum, and posterior striatum, were outlined based on a mouse brain atlas (Paxinos and Watson 2004). Average pixel density was determined and converted to microcuries per gram protein using 14C standards (ARC, Inc., St. Louis, MO, USA). Data from six C57BL/6J male mice were compared with data from six DBA/2J male mice using two-tailed unpaired t tests.
Western blots of Gαq/11 and Gαi/o subunits
To examine whether levels of G-proteins that couple to 5-HT2 receptors relate to the strain differences in the DOI-induced HTR, Western blots for Gαq/11 and Gαi/o in the frontal cortex were performed. C57BL/6J and DBA/2J mice (N=12, eight for each strain for Gαq/11 and Gαi/o, respectively) were anesthetized with isoflurane and decapitated. Brains were removed quickly and sectioned in a chilled brain block. Cortex tissue was placed in ice-cold cell lysis buffer, containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton-X, 1 mM freshly prepared PMSF, and Roche Complete® protease inhibitors and phosphatase inhibitors. Tissue was homogenized using a Kontes pellet pestle motor and pestle then centrifuged at 13,000 rpm for 20 min. Supernatant was aliquoted and stored at −80°C until Western blot procedures. Protein concentrations were determined using the BCA method. Twenty micrograms of protein was run on 10% acrylamide gels and transferred to PVDF membranes overnight. Membranes were washed in Tris-buffered saline (TBS), incubated in 5% non-fat dry milk with 0.1% Tween-20 in TBS, then incubated with Gαq/11 (rabbit, 1 to 500 dilution, Santa Cruz Biotechnology, USA) or Gαi/o/t/z/gust (rabbit, 1 to 500 dilution, Santa Cruz Biotechnology, USA), and α-tubulin (mouse, 1 to 7500, Sigma-Aldrich, USA) primary antibodies for 90 min. Membranes were then incubated in 4% non-fat dry milk with 0.1% Tween-20 in TBS, then with horseradish peroxidase labeled donkey anti-rabbit secondary antibody (1 to 100,000 dilution, G.E. Healthcare, USA) and sheep anti-mouse (1 to 100,000 dilution, G.E. Healthcare, USA) for 90 min, followed by several washes in TBS with 0.1% Tween-20. Protein was visualized using chemiluminescence and exposure of membranes to film. Film was scanned onto a PC, and densitometry analyses were performed using ImageJ software (Abramoff et al. 2004).
Serotonin-induced PLC activity
A comparison of basal and 5-HT-stimulated PLC activity was made in ex vivo cortex membrane preparations from six young adult male C57BL/6J mice and six young adult male DBA/2J mice. Frontal cortex was homogenized in 20 volumes of 25 mM HEPES-Tris, pH 7.4, containing 1 mM EGTA (“homogenization buffer”). Homogenates were centrifuged at 20,000 g for 10 min at 4°C. Buffer was decanted. Membranes were resuspended in 20 volumes of homogenization buffer and centrifuged at 20,000 g for 10 min at 4°C, four additional times. Membranes were then resuspended in 25 mM HEPES-Tris, pH 7.4, containing 3 mM EGTA, 10 mM LiCl, and centrifuged at 20,000 g for 10 min at 4°C. The supernatant was discarded, and the membrane pellet(s) were stored at −80°C. On the day of the assay, pellets were thawed, and protein concentration was measured by Bradford assay.
The assay protocol was based on methods previously described (Damjanoska et al. 2004; Wolf and Schutz 1997). One hundred micrograms of membrane protein was diluted on ice into 100 μl of total volume with a buffer containing 25 mM HEPES-Tris pH 7.4, 3 mM EGTA, 10 mM LiCl, 12 mM MgCl2, 1.44 mM sodium deoxycholate, 200 nM Ca2+, 1 μM GTPγS, 3 μM 5-HT, and 1 mM unlabeled phosphatidyl inositol. 5-HT was not included for basal measures. After addition of 100 μM [3H]-phosphatidyl inositol, the reaction tubes were incubated in a 37°C water bath for 20 min. The reactions were terminated by the addition of 0.9 ml 1:2 chloroform:methanol mixture, followed by 0.3 ml chloroform and 0.3 ml 0.25 M HCl, and 2 min of vigorous shaking. Tubes were then centrifuged at 8,000 g for 2 min. Of the upper aqueous phase, 0.3 ml was mixed with 6 ml scintillation cocktail and counted 1 h later on a liquid scintillation counter. PLC activity for each biological sample (mouse) was measured in triplicate under basal and 5-HT-stimulated conditions. Triplicates were averaged by mouse before analysis.
The comparison of basal and 5-HT-stimulated PLC activity was made between strains by use of an ANOVA model that included one within mouse factor (basal versus stimulated), a factor to account for correlated measures by mouse (mouse), one between mouse factor (C57BL/6J versus DBA/2J), and an interaction between stimulation and strain factors. Of interest in this ANOVA is the interaction that tests whether PLC activity differs between strains.
DOI-induced Egr1 and Egr2 gene expression
Early growth response 1 (Egr1) and 2 (Egr2), two gene markers induced by hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007), were measured to test for potential differences in DOI-induced cellular signaling in frontal cortex of DBA/2J and C57BL/6J. Egr1 and Egr2 RNA transcript relative abundance was compared by realtime polymerase chain reaction (PCR) between saline (0.9%)- and DOI (3 mg/kg, IP route)-treated young adult male C57BL/6J and DBA/2J mice (N=5 mice per condition). Tissue was collected 45 min after injection.
Frontal cortex was placed immediately into RNAlater and stored until RNA isolation. The tissue was homogenized in TRI Reagent (Sigma), chloroform was mixed with the lysate, and the mixture was separated into three phases by centrifugation. The RNA was then precipitated from the aqueous phase with isopropanol. DNA-free DNase treatment and removal reagents (Ambion) were used to remove contaminating genomic DNA. RNA quality control and assessment was checked by measuring the 260/280 absorption ratio in 10 mM Tris using a Nanodrop ND-1000 spectrophotometer. In addition, an RNA integrity number (RIN) using an Agilent 2100 Bioanalyzer was determined. All 20 samples used for real-time PCR were high quality, intact RNA, without significant protein or DNA contamination.
FAM-labeled Taqman probes for relative quantification of Egr1 (assay ID Mm00656724 m1) and Egr2 (assay ID Mm00456650 m1) were purchased from Applied Biosystems, along with two control gene probes (mouse ACTB and mouse GAPD). The real-time PCR assay was run by the Vanderbilt Microarray Shared Resource core facility (http://array.mc.vanderbilt.edu/services/realtime.vmsr) using the ABI 7900HT Fast real-time PCR system. All samples were run in quadruplicate, and cycle times were calculated using ABI SDS software.
Cycle times and sample identifier data for target and control genes were exported from ABI SDS software to a tab-delimited text file. Data were imported to Stata (v.11) for statistical analyses. Because there is no justification for paired comparisons between individual C57BL/6J mice and DBA/2J mice, the 2ˆ(−ΔΔ Ct) method cannot be used (Schmittgen and Livak 2008). Instead, statistical comparisons are made on 2ˆ(−Δ Ct) data, for example, on 2ˆ(−(CtEgr1−CtGapdh)) (Schmittgen and Livak 2008). Analysis of the target genes was by comparison to Gapdh, because it did not appear to be regulated by DOI as did Actb. Target genes were tested jointly by multivariate analysis of variance (MANOVA) for an interaction between strain and DOI treatment, because both Egr1 and Egr2 are reportedly downstream readouts of DOI-induced 5-HT2A receptor activation (Gonzalez-Maeso et al. 2007).
Results
DOI induces a 5-HT2A receptor-dependent head-twitch response that is modulated by the 5-HT2C receptor
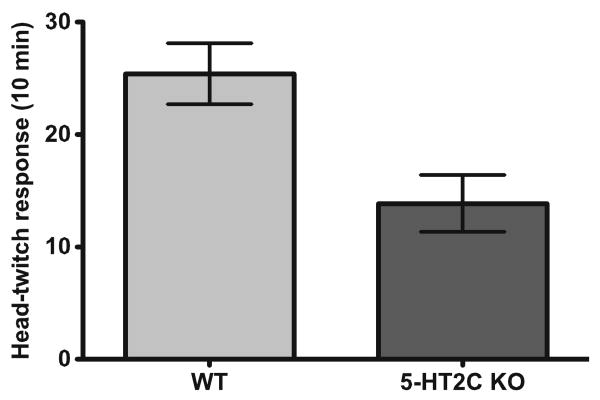
As shown in Fig. 1, 5-HT2C knockout mice exhibited a significantly lower number of HTR in 10 min relative to their wild-type littermates (t test, p=0.01; mean ± SEM number of HTR: WT (N=5), 25±3; 5-HT2C knockout (N=7), 14±3).
Fig. 1.
5-HT2C receptor knockout male mice (N=7) exhibit significantly fewer head-twitch responses compared to littermate control mice (N=5) after 1 mg/kg DOI treatment
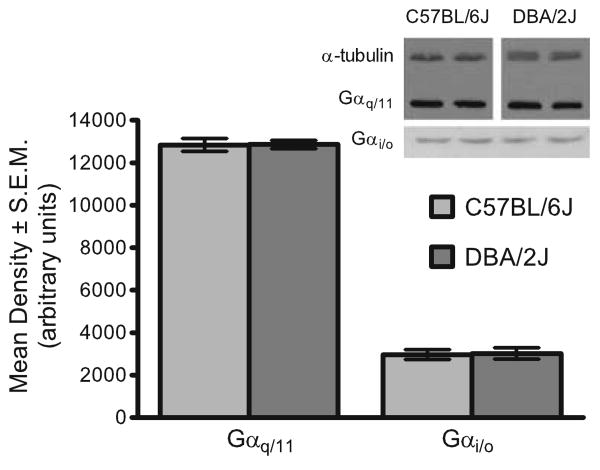
Fig. 5.
There were no detectable differences in densities of Gαq/11 and Gαi/o proteins in cortex of C57BL/6J and DBA/2J. Inset shows a representative photomicrograph of Western blots of frontal cortex from C57BL/6J and DBA/2J mice showing equivalent levels of Gαq/11 and Gαi/o proteins
To assess head-twitch dose–response to DOI, HTRs were counted in three C57BL/6J and three DBA/2J mice for each of eight DOI dose levels (48 total mice): 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8 mg/kg (Fig. 2a). Group means (with ±1 SEM error bars) are shown for each strain in Fig. 2a. A 2×8 factorial ANOVA produced a significant interaction between strain and dose level (F(7,32)=4.93, p<0.01). Bonferroni corrected simple main effects (SME) analysis resolved the interaction into strain differences at DOI doses of 0.4 mg/kg and above (*p<0.05 as indicated in Fig. 2a), but not at either 0.1 or 0.2 mg/kg. Both strains appear to reach maxima in HTR between 0.8 and 1.6 mg/kg DOI dose and maintain this response at higher doses. A small decrement in DBA/2J response was noticed at the higher doses and was accompanied by apparent reduction of overall activity during the observation period. When these data are fit by a four-parameter logistic (dose–response) equation, including parameters for top, bottom, ED50, and Hill slope, only the strain difference in the maximum was significant (t=7.57, p<0.001, C57BL/6J top=38 head-twitches, DBA/2J top=64 head-twitches, model fit not shown). The dose–response profiles suggest a robust strain difference in DOI-induced HTR that is not readily explained by pharmacokinetic or metabolic factors presenting as a left–right shift in the ED50 but similar maxima.
Fig. 2.
a C57BL/6J and DBA/2J male mice exhibit a robust difference in head-twitch response to DOI (N=3 mice per dose level per strain). Dose–response profiles show a significant difference in maxima between strains. DBA/2J has approximately 1.5- to twofold greater head-twitch response than C57BL/6J. A 2×8 factorial analysis of variance reveals a significant strain by dose interaction that is resolved into differences between strains at dose levels of 0.4 mg/kg or higher, as indicated by stars. b DOI-induced head-twitch in C57BL/6J and DBA/2J mice is reduced but not eliminated by 5-HT2C antagonist pretreatment, and the strain difference in head-twitch is eradicated by 5-HT2C antagonist pretreatment (N=6 per group). The stars indicate both a significant reduction in head-twitch response after SB206553 or SB242084 pretreatment compared to saline pretreatment, and significant reduction in the difference between strains after 5-HT2C antagonist pretreatment compared to saline (see text for details)
The role of 5-HT2A receptors in the HTR to DOI was assessed using the antagonist MDL100907. When MDL100907 was given as a pretreatment (0.25 mg/kg) 10 min prior to 1 mg/kg DOI, the DOI-induced HTR was completely blocked in both strains (C57BL/6J=0(0) (mean (SEM)), N=6; DBA/2J=1.25(0.54), n=6; 10 min observation; data not shown). Neither C57BL/6J nor DBA/2J mice head-twitch following only saline injection (C57BL/6J=2.3 (0.44), N=6; DBA/2J=0.75(0.36), N=6; 10 min observation; data not shown).
The role of 5-HT2C receptors in the HTR to DOI was assessed using the inverse agonist/antagonist SB206553 and the antagonist SB242084. Figure 2b shows head-twitches after saline pretreatment, 3 mg/kg SB206553 pretreatment, or 3 mg/kg SB242084 pretreatment prior to a 1-mg/kg DOI treatment in C57BL/6J and DBA/2J mice (N=6 per group). SB206553 was also used at a pretreatment dose of 0.3 mg/kg, and it produced similar results to 3 mg/kg (data not shown). As expected from the non-parallel lines in Fig. 2b, a 2×3 factorial ANOVA produced a significant interaction between strain and pretreatment (F (2,30)=10.41, p<0.01). Bonferroni corrected SME analysis resolved the interaction into a significant strain difference after saline pretreatment (F(1,30)=48.34, p<0.01), but not after SB206553 pretreatment (F(1,30)=3.94, p=NS), nor after SB242084 pretreatment (F(1,30)=0.82, p=NS). Alternatively, Bonferroni corrected SME analysis describes differences within each strain between saline and either antagonist pretreatments as significantly different (p<0.05), but that head-twitch between antagonist pretreatments do not differ (p=NS). Finally, the difference in HTR between C57BL/6J and DBA/2J mice after saline pretreatment is significantly different than the difference between strains after either SB206553 pretreatment (F(1,30)=18.30, p<0.01) or SB242084 pretreatment (F(1,30)=12.34, p<0.01), indicated by the stars in Fig. 2b. We conclude that DOI-induced head-twitch in C57BL/6J and DBA/2J mice is reduced but not eliminated by 5-HT2C antagonist pretreatment. Furthermore, we conclude that the strain difference in head-twitch is eradicated by 5-HT2C antagonist pretreatment.
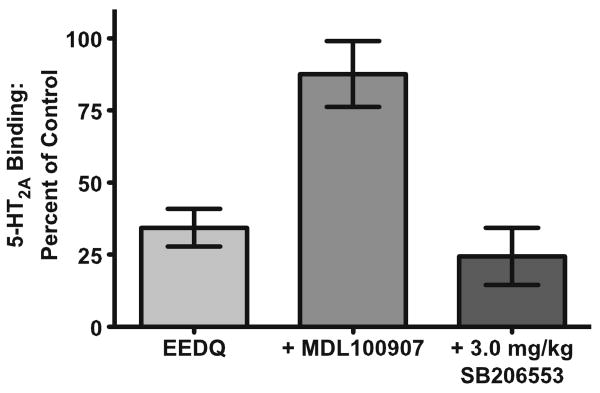
Reduction in DOI-induced head-twitch response by pretreatment with SB206553 is not mediated by 5-HT2A receptors
Figure 3 shows the results of an EEDQ inactivation study, a classic pharmacological demonstration of drug specificity. One-way ANOVA analysis revealed a significant main effect of drug treatment on 5-HT2A specific binding (F(2,13)=13.09, p<0.01). Pretreatment of mice (N=6) with EEDQ significantly reduced [3H]-ketanserin binding to 5-HT2A sites after 24 h, eliminating 65% of specific binding. Pretreatment with 0.25 mg/kg MDL100907 (N=4) 30 min prior to EEDQ significantly prevented EEDQ inactivation of 5-HT2A receptors (p<0.05), restoring 5-HT2A specific [3H]-ketanserin binding to 90% of controls. In contrast, pretreatment with 3.0 mg/kg SB206553 (N=4) 30 min prior to EEDQ treatment did not alter the ability of EEDQ to reduce 5-HT2A specific [3H]-ketanserin binding, suggesting that this dose of SB206553 does not appreciably bind 5-HT2A receptor binding sites in the mouse brain in vivo.
Fig. 3.
EEDQ treatment inactivates 5-HT2A receptor binding (N=6) in the mouse brain which is prevented by pretreatment with MDL100907 (0.25 mg/kg, N=4), but not SB206553 (3.0 mg/kg, N=4), suggesting that 3.0 mg/kg SB206553 does not bind to 5-HT2A receptor binding sites at this dose or lower doses
Candidate mechanisms explaining the C57BL/6J and DBA/2J difference in head-twitch
5-HT2A and 5-HT2C receptor saturation binding and autoradiography
Saturation binding was used to test for differences in 5-HT2A receptor density in the frontal cortex and 5-HT2C receptor density in whole brains, from C57BL/6J and DBA/2J mice. Nonlinear regression analysis was used to determine the Bmax and Kd estimates from total binding and nonspecific binding data for each sample (Table 1). Comparisons of the 5-HT2A Bmax values (femtomoles per milligram protein) between strains (t test, t=0.54, df=10, p=NS) and of the 5-HT2A Kd values (nanomolar) between strains (t test, t=0.06, df=10, p=NS) showed no significant differences. Comparisons of the 5-HT2C Bmax values between strains (t test, t=0.21, df=10, p=NS) and of the 5-HT2C Kd values between strains (t test, t=1.34, df=10, p=NS) also showed no significant differences.
Table 1.
5HT2A and 5HT2C receptor saturation binding Bmax (femtomoles per milligram protein) and Kd (nanomolar)
| Strain | n | 5HT2A | 5HT2C | ||
|---|---|---|---|---|---|
| Bmax (SEM) | Kd (SEM) | Bmax (SEM) | Kd (SEM) | ||
| C57BL/6J | 6 | 410 (28.7) | 1.3 (0.11) | 40 (4.0) | 2.2 (0.40) |
| DBA/2J | 6 | 384 (39.5) | 1.3 (0.24) | 39 (4.3) | 3.0 (0.56) |
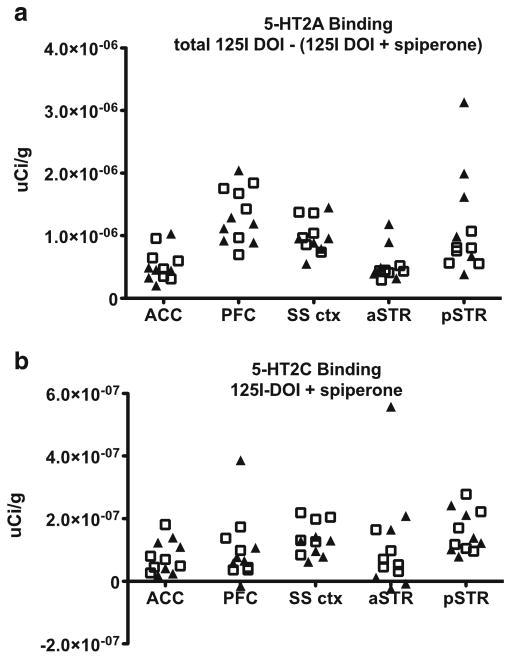
[125I]-DOI autoradiography for cortical and striatal brain areas in C57BL/6J and DBA/2J did not reveal obvious differences in either 5-HT2A or 5-HT2C receptor high-affinity binding (Fig. 4a, b, respectively). Kruskal–Wallis tests of prefrontal cortex and two other cortical regions, as well as two striatal regions, were not significant (p>0.05).
Fig. 4.
[125I]-DOI autoradiography data showing 5-HT2A and 5-HT2C receptor high-affinity binding in cortical and striatal brain regions comparing six C57BL/6J mice (squares, N=6) and six DBA/2J mice (triangles, N=6). The y-axis is microcuries per gram of tissue, and the x-axis defines the brain regions anterior cingulate cortex (ACC), prefrontal cortex (PFC), somatosensory cortex (SS ctx), anterior striatum (ASTR), and posterior striatum (PSTR). a [125I]-DOI 5-HT2A receptor binding. b [125I]-DOI 5-HT2C receptor binding
Western blot for Gαq/11 or Gαi/o
No evidence could be established to support a difference in levels of Gαq/11 or Gαi/o in frontal cortex between C57BL/6J and DBA/2J mice. Because α-tubulin appeared to be regulated between strains (data not shown; however, see Fig. 5 inset for an example), we compared raw density measures for Gαq/11 and Gαi/o between C57BL/6J and DBA/2J mice. Frontal cortex from 12 mice of each strain was used for Gαq/11 Western blots, and frontal cortex from eight mice of each strain was used for Gαi/o Western blots. Unpaired t tests comparing mean protein densities in C57BL/6J and DBA/2J mice showed no significant differences between groups (Gαq/11, p=0.94; Gαi/o, p=NS).
PLC activity
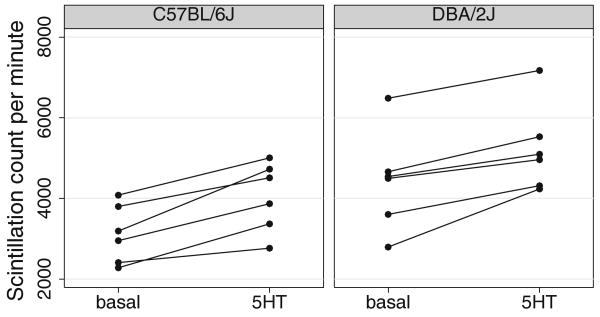
Serotonin-stimulated PLC activity was measured by the amount of [3H]-inositol phosphate produced by PLC in the membrane fractions of C57BL/6J (N=6 mice) and DBA/2J (N=6 mice) frontal cortex. Figure 6 shows PLC activity measures for basal and 5-HT-stimulated conditions by mouse and strain. A strain difference in PLC activity would be evident as a difference in the slope from basal to stimulated conditions. While ANOVA confirms significant 5-HT-stimulated PLC activity across strains (F(1,10)= 63.89, p<0.01), showing that 5-HT stimulated PLC activity, the interaction between strain and PLC activity was not significant (F(1,10)=0.40, p=NS). There is no evidence for differing PLC activity in frontal cortex between C57BL/6J and DBA/2J mice. PLC activity induced by 5-HT2A receptors does not likely underlie DOI-induced head-twitch differences between C57BL/6J and DBA/2J mice.
Fig. 6.
The figure plots the basal and stimulated counts connected by mouse for each strain. Despite induction of significant 5-HT-stimulated [3H]-inositol phosphate levels produced by phospholipase C in frontal cortex from C57BL/6J and DBA/2J mice (N=6 per strain), there were no differences between strains
DOI-induced Egr1 and Egr2 gene expression
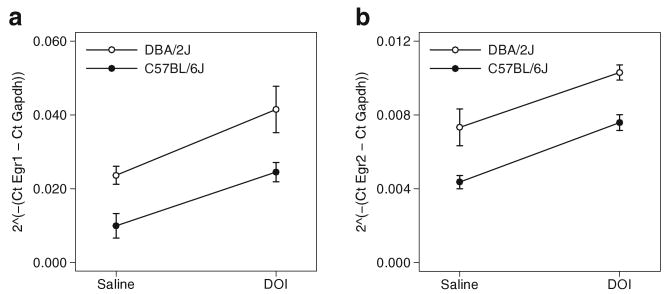
The relative RNA transcript abundance of Egr1 and Egr2 genes in the frontal cortex of C57BL/6J and DBA/2J mice after saline and DOI (3 mg/kg) injections was measured using real-time PCR and analyzed by the comparative CT method (n=5 mice per group). Figure 7 shows in panels (a) and (b) the delta cycle times for Egr1 and Egr2 relative to Gapdh. A MANOVA predicting Egr1 and Egr2 expression from strain and DOI treatment confirmed the strain (F(2,15)=17.39, p<0.01) and DOI (F(2,15)=20.18, p< 0.01) effects apparent in panels (a) and (b). The interaction between strain and DOI was not significant (F(2,15)=0.10, p=NS). Egr1 and Egr2 expression in the frontal cortex of C57BL/6J and DBA/2J mice are increased equivalently by DOI, independent of a basal strain difference in gene expression.
Fig. 7.
Interaction plots are shown for relative gene expression from (a) Egr1 and (b) Egr2 real-time polymerase chain reaction experiments. Means ± SEM for gene expression (y-axis) are shown against DOI condition (x-axis) and strain (white or black symbols, N=5 per group, 20 mice total). Egr1 and Egr2 expression in the frontal cortex of C57BL/6J and DBA/2J mice are equivalently increased by DOI, independent of a basal strain difference in gene expression; there is no indication of a strain difference
Discussion
5-HT type 2 receptors are central mechanistic components of the psychoactive response to serotonin-based hallucinogenic drugs, and it is accepted that the 5-HT2A receptor subtype is the most essential receptor mediating the hallucinatory effects (Nichols 2004; Winter 2009). In humans, antagonists of 5-HT2A receptors such as chlorpromazine dampen the psychoactive effects of hallucinogens (Freedman 1986), and pretreatment in humans with the 5-HT2A antagonist ketanserin dose-dependently prevents the hallucinogenic effects caused by psilocybin ingestion (Vollenweider et al. 1998). In addition, mice devoid of the 5-HT2A receptor do not exhibit a regularly observed behavioral response to hallucinogens, the HTR (Gonzalez-Maeso et al. 2007). Fairly selective 5-HT2A antagonists, such as MDL100907, also prevent this response, and block drug discrimination of DOI and the related hallucinogenic analogue, DOB (Benneyworth et al. 2005; Schreiber et al. 1994).
However, several findings confound the interpretation that the psychoactive response to serotonin-like hallucinogens is mediated solely by the 5-HT2A receptor. Results from radioligand binding studies show that the 5-HT2A receptor antagonists ketanserin, chlorpromazine, and MDL100907 have appreciable affinity for 5-HT2C receptors (PDSP, http://pdsp.med.unc.edu/). In addition, all known 5-HT2 receptor agonists that are hallucinogenic have equal or greater affinities and potencies at 5-HT2C receptors relative to 5-HT2A receptors. These data leave open the possibility that 5-HT2C receptors functionally participate in inducing the hallucinogenic response (Fiorella et al. 1995a; Winter 2009).
Despite speculation that activation of the 5-HT2C receptor could modulate the psychoactive effects caused by hallucinogens, the collective results of drug discrimination studies suggest that 5-HT2C receptors are not central to the subjective effects of serotonin-like hallucinogens (Winter 2009). 5-HT2C receptor antagonists do not block drug discrimination for DOI in mice (Schreiber et al. 1994; Smith et al. 2003). We have also observed previously that 3.0 mg/kg of the 5-HT2C inverse agonist/antagonist SB206553 does not reduce the ability of mice to discriminate LSD from vehicle (data not shown). Hence, we were surprised to observe a blunted DOI-induced HTR in 5-HT2C knockout mice, our initial hint that 5-HT2C receptors may be involved in this response elicited by hallucinogens. The reduced HTR to DOI in 5-HT2C knockout mice was likely not due to developmental alterations in 5-HT2A receptor levels, as it has been shown that 5-HT2C knockout mice have equivalent levels of 5-HT2A receptors as their wild-type littermates (Lopez-Gimenez et al. 2002). Similarly, 5-HT2A knockout mice do not have alterations in 5-HT2C levels (Weisstaub et al. 2006), suggesting that genetically manipulating either 5-HT2C or 5-HT2A receptors does not alter expression of the other.
In a separate set of experiments, we observed that DBA/2J and C57BL/6J mice have dramatically different HTRs to DOI with DBA/2J mice showing 75% more head-twitches. This difference was likely not due to pharmacokinetic or metabolic factors, as it was maintained across a wide range of doses. The HTR was completely eliminated by pretreat-ing either strain of mouse with MDL100907, suggesting it is 5-HT2A mediated. However, several measures aimed at discovering a difference between the strains in 5-HT2A receptor function in the frontal cortex, including receptor and G-protein density, Egr1 and Egr2 expression, and PLC activity, proved futile. Collectively, these data suggested that the 5-HT2A receptor was not mediating the strain differences in response to DOI.
We next tested whether the 5-HT2C receptor mediated the strain differences. The 5-HT2C selective inverse agonist and antagonist SB206553 completely eliminated the strain differences in response to DOI at two doses, 3.0 mg/kg and a tenfold lower dose, 0.3 mg/kg. This effect was not due to non-selective blockade of 5-HT2A receptors, as pretreatment of mice with 3.0 mg/kg SB206553 did not prevent EEDQ inactivation of 5-HT2A receptors. Recent reports show that SB206553 is a positive allosteric modulator of nicotinic α7 receptors (Dunlop et al. 2009), which called into question whether other targets of SB206553 could be mediating its effects on the DOI HTR. However, a different 5-HT2C antagonist, SB242084, also attenuated DOI HTR and eliminated the strain differences; these data conflict with a previous report using rats (Vickers et al. 2001), suggesting there may be species-related differences in mechanisms controlling the DOI-induced HTR in rodents. The observation that at high concentrations SB242084 activates 5-HT2C receptor→PLC signaling in CHO cells may also be pertinent to species differences in the effects of SB242084 (De Deurwaerdere et al. 2004). In summary, our data showing suppressive effects of 5-HT2C receptor antagonists on DOI-induced HTR in mice combined with the attenuated response to DOI in 5-HT2C knockout mice strongly support the conclusion that the 5-HT2C receptor potently modulates the behavioral response in mice induced by the hallucinogenic 5-HT2 agonist DOI.
We attempted to discover a difference in 5-HT2C density or cellular function in C57BL/6J and DBA/2J mice but were unsuccessful. [3H]-mesulergine saturation binding did not reveal a difference in total 5-HT2C density in brains of the two strains. Also, [125I]-DOI autoradiography which preferentially labels high-affinity agonist sites did not reveal a significant difference between the strains in 5-HT2C binding sites in regions thought to underlie the DOI-induced HTR. A forward genetic screen of DOI-induced HTR in experimental crosses of mice derived from C57BL/6J and DBA/2J may point to the genetic loci that underlie the strain difference. Speculative possibilities that may underlie the different HTR induced by DOI in C57BL/6J and DBA/2J mice include alterations in glutamatergic, dopaminergic, and/or 5-HT1A receptors or the serotonin transporter, which have been shown to modulate the stimulus properties and head-twitch-inducing properties of hallucinogens in rodents (Jennings et al. 2008; Krall et al. 2008; Winter 2009).
The role of the 5-HT2C receptor in modulating the behavioral response to DOI in mice is interesting in light of observations that the 5-HT2A receptor agonist, lisuride, does not have LSD-like hallucinatory effects in humans (Freedman and Boggan 1982; Herrmann et al. 1977) and does not cause an HTR in mice (Gonzalez-Maeso et al. 2007), an observation confirmed in our laboratory (data not shown). These findings have been interpreted to support the idea that 5-HT2A receptor agonists with hallucinogenic properties stabilize a unique conformation of the 5-HT2A receptor that is not induced by non-hallucinogenic 5-HT2A agonists, suggesting that serotonin-based hallucinogens are conformationally selective ligands, activating specific signaling pathways downstream of the 5-HT2A receptor (Gonzalez-Maeso and Sealfon 2009; Gonzalez-Maeso et al. 2007).
An alternative or additional possibility is that lisuride differentially interacts with 5-HT2A and 5-HT2C receptors. In cultured cells of the rat choroid plexus, lisuride behaves as an antagonist at 5-HT2C receptors, whereas the hallucinogens LSD, DOB, DOM, and 5-methoxy-DMT are potent 5-HT2C agonists (Burris et al. 1991; Sanders-Bush and Breeding 1991). This is in contrast to in vitro studies using 5-HT2C-VSV receptors stably expressed at high levels in CHO cells, where lisuride behaves as a weak partial agonist (Cussac et al. 2002) or agonist (Cussac et al. 2008). The differences in 5-HT2C receptor expression levels and edited isoforms between native cells from the choroid plexus and CHO cells transfected with 5-HT2C-VSV (Burns et al. 1997; Cussac et al. 2008; Sanders-Bush and Breeding 1991) could account for this discrepancy; alterations in 5-HT2C receptor levels and RNA editing of 5-HT2C receptor can alter ligand pharmacology at 5-HT2C receptors (Berg et al. 2008; Fitzgerald et al. 1999). Also, weak partial agonist activity in vitro may translate to antagonism in vivo. The inability of lisuride to induce LSD-like hallucinations may relate to its antagonism of 5-HT2C receptors expressed in vivo. Taken together, the converging evidence suggests that the behavioral response in mice elicited by the hallucinogen DOI is potently modulated by 5-HT2C receptors.
Acknowledgments
We thank research assistants Katherine Smith, Dongmei Li, and Jason Abramo for diligent and careful work in the laboratory. The authors report no conflicts of interest. Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # NO1MH32004 (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
Supported by grants P50 MH78028, DA005181, and T32 MH0652150.
Contributor Information
Clinton E. Canal, Email: clinton.canal@ufl.edu.
David C. Airey, Email: david.airey@vanderbilt.edu.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Bernasconi A, Fulgosi MC, Colombo C, Dallaspezia S, Gavinelli C, Marino E, Pirovano A, Radaelli D, Smeraldi E. Serotonin 5-HT2A receptor gene variants influence antidepressant response to repeated total sleep deprivation in bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1863–1866. doi: 10.1016/j.pnpbp.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology (Berl) 2005;179:854–862. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br J Pharmacol. 1990;101:667–673. doi: 10.1111/j.1476-5381.1990.tb14138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Cunningham KA, Spampinato U. Fine-tuning serotonin2c receptor function in the brain: molecular and functional implications. Neuropharmacology. 2008;55:969–976. doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–896. [PubMed] [Google Scholar]
- Cohen S. The toxic psychoses and allied states. Am J Med. 1953;15:813–828. doi: 10.1016/0002-9343(53)90173-9. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Duqueyroix D, Pasteau V, Millan MJ. Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: influence of receptor reserve and relationship to agonist-directed trafficking. Mol Pharmacol. 2002;62:578–589. doi: 10.1124/mol.62.3.578. [DOI] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C -VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Damjanoska KJ, Heidenreich BA, Kindel GH, D'Souza DN, Zhang Y, Garcia F, Battaglia G, Wolf WA, Van de Kar LD, Muma NA. Agonist-induced serotonin 2A receptor desensitization in the rat frontal cortex and hypothalamus. J Pharmacol Exp Ther. 2004;309:1043–1050. doi: 10.1124/jpet.103.062067. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Lock T, Jow B, Sitzia F, Grauer S, Jow F, Kramer A, Bowlby MR, Randall A, Kowal D, Gilbert A, Comery TA, Larocque J, Soloveva V, Brown J, Roncarati R. Old and new pharmacology: positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor by the 5-hydroxytryptamine(2B/C) receptor antagonist SB-206553 (3, 5-dihydro-5-methyl-N-3-pyridinylbenzo[1, 2-b:4, 5-b']di pyrrole-1(2H)-carboxamide) J Pharmacol Exp Ther. 2009;328:766–776. doi: 10.1124/jpet.108.146514. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Lorrain DS, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: the mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology (Berl) 1995a;121:364–372. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: reassessment of LSD false positives. Psychopharmacology (Berl) 1995b;121:357–363. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fischer R, Georgi F, Weber R. Psychophysical correlations. VIII. Experimental tests in schizophrenia: lysergic acid diethylamide and mescaline. Schweiz Med Wochenschr. 1951;81:817–819. contd. [PubMed] [Google Scholar]
- Fitzgerald LW, Conklin DS, Krause CM, Marshall AP, Patterson JP, Tran DP, Iyer G, Kostich WA, Largent BL, Hartig PR. High-affinity agonist binding correlates with efficacy (intrinsic activity) at the human serotonin 5-HT2A and 5-HT2C receptors: evidence favoring the ternary complex and two-state models of agonist action. J Neurochem. 1999;72:2127–2134. doi: 10.1046/j.1471-4159.1999.0722127.x. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009;57(3):259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Freedman DX. Hallucinogenic drug research–if so, who what?: Symposium summary and Commentary. Pharmacol Biochem Behav. 1986;24:407–415. doi: 10.1016/0091-3057(86)90371-0. [DOI] [PubMed] [Google Scholar]
- Freedman DX, Boggan WO. Biochemical pharmacology of psychotomimetics. Springer-Verlag; Berlin: 1982. [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Measo J, Sealfon SC. Agonist-trafficking and hallucinogens. Curr Med Chem. 2009;16(8):1017–1027. doi: 10.2174/092986709787581851. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT (2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295:226–232. [PubMed] [Google Scholar]
- Herrmann WM, Horowski R, Dannehl K, Kramer U, Lurati K. Clinical effectiveness of lisuride hydrogen maleate: a double-blind trial versus methysergide. Headache. 1977;17:54–60. doi: 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Chemical psychoses. Annu Rev Med. 1964;15:203–214. doi: 10.1146/annurev.me.15.020164.001223. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kang K, Huang XF, Wang Q, Deng C. Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia-a postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:867–871. doi: 10.1016/j.pnpbp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kettle CJ, Cheetham SC, Martin KF, Prow MR, Heal DJ. The effects of the peptide-coupling agent, EEDQ, on 5-HT2A receptor binding and function in rat frontal cortex. Neuropharmacology. 1999;38:1421–1430. doi: 10.1016/s0028-3908(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Krall CM, Richards JB, Rabin RA, Winter JC. Marked decrease of LSD-induced stimulus control in serotonin transporter knockout mice. Pharmacol Biochem Behav. 2008;88:349–357. doi: 10.1016/j.pbb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Moison D, Cunningham KA, Piazza PV, Spampinato U. Serotonin2C receptors in the medial prefrontal cortex facilitate cocaine-induced dopamine release in the rat nucleus accumbens. Neuropharmacology. 2009;56:507–513. doi: 10.1016/j.neuropharm.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100, 907. Naunyn Schmiede-bergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G. Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100, 907 autoradiography and in situ hybridization studies. J Comp Neurol. 2001;429:571–589. doi: 10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT. Serotonin 5- HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67:69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson KBJ. The mouse brain in stereotaxic coordinates. 2nd. Elsevier Academic Press; Amsterdam, Boston: 2004. [Google Scholar]
- Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, Wagner M, Kumari V. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-o-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66(6):614–620. doi: 10.1016/j.biopsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD, Meltzer HY. Binding of typical and atypical antipsychotic agents to transiently expressed 5-HT1C receptors. J Pharmacol Exp Ther. 1992;260:1361–1365. [PubMed] [Google Scholar]
- Sanders-Bush E, Breeding M. Choroid plexus epithelial cells in primary culture: a model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacology (Berl) 1991;105:340–346. doi: 10.1007/BF02244428. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Lockade of the discriminative stimulus effects of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/-)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluoro-deoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology (Berl) 2009;203:251–263. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- Wolf WA, Schutz LJ. The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J Neurochem. 1997;69:1449–1458. doi: 10.1046/j.1471-4159.1997.69041449.x. [DOI] [PubMed] [Google Scholar]