Abstract
While it has been shown that microchip electrophoresis with electrochemical detection can be used to separate and detect electroactive species, there is a need to increase the separation performance of these devices so that complex mixtures can be routinely analyzed. Previous work in microchip electrophoresis has demonstrated that increasing the separation channel length leads to an increase in resolution between closely eluting analytes. This paper details the use of lengthened serpentine microchannels for microchip electrophoresis and electrochemical detection where a palladium decoupler is used to ground the separation voltage so that the working electrodes remain in the fluidic network. In this work, palladium electrodepositions were used to increase the decoupler surface area and more efficiently dissipate hydrogen produced at the decoupler. Dopamine and norepinephrine, which only differ in structure by a hydroxyl group, were used as model analytes. It was found that increasing the separation channel length led to improvements in both resolution and the number of theoretical plates for these analytes. The use of a bi-layer valving device, where PDMS-based valves are utilized for the injection process, along with serpentine microchannels and amperometric detection resulted in a multi-analyte separation and an average of 28,700 theoretical plates. It was also shown that the increased channel length is beneficial when separating and detecting analytes from a high ionic strength matrix. This was demonstrated by monitoring the stimulated release of neuro-transmitters from a confluent layer of PC 12 cells.
1 Introduction
It has been shown that electrochemical (EC) detection is a power method of monitoring separations in microchip electrophoresis (ME). In particular, ME with amperometric detection is attractive for neurochemical measurements, as most neurotransmitters are electrochemically active and do not require derivatization for detection [1]. However, previous studies with ME and amperometric detection have used separation channels that are 2–5 cm in length [2–6], which limits the peak capacity of the separation. With electrophoresis, previous work has demonstrated that increasing the field strength improves the separation efficiency with an upper voltage limit that is based upon Joule heating [7, 8]. It has also been shown that resolution in free zone electrophoresis is directly proportional to the square root of separation length [8]. In ME, this has led to the development of serpentine channels that vary from 10 to 30 cm in length to increase the resolving power while maintaining high field strengths [9–12]. A serpentine fluidic design maintains a compact footprint by incorporating turns to increase the separation length. The turns have been shown to increase analyte dispersion but with the proper optimization band broadening can be minimized [9–12]. All of the previous studies with serpentine separation channels have utilized laser induced fluorescence (LIF) for detection and there have been a lack of reports where lengthened serpentine channels are used with electrochemical detection.
Electrochemical detection is ideal for ME devices for a variety of reasons. With the use of photolithographic techniques, microelectrodes can be fabricated directly onto the microchip device making the system relatively simple to construct. As opposed to LIF detection, electrochemistry can be used to detect many analytes without the need for derivatization [1]. Amperometric detection has been one of the most widely used types of electrochemical detection for ME since its initial description in 1998 [13]. To successfully implement amperometric detection with ME, it is necessary to isolate the detection electrodes from the electrophoresis separation voltage. The most common methods to accomplish this are end-channel [13, 14] and off-channel [4, 15] approaches. Although end-channel alignment schemes are often used in ME, major disadvantages of this approach are the resulting band broadening that arise from diffusion of the separation bands as they enter the relatively large detection reservoir and travel towards the detection electrodes [16]. The band broadening associated with end-channel detection can be circumvented using off-channel methods, where the detection electrodes remain in the fluidic network. Crucial to the operation of off-channel methods is the use of a decoupler to ground the separation voltage upstream from the detection electrodes and dissipate hydrogen formed at the cathode. Our group has previously shown that a sputtered and microfabricated palladium thin-film electrode can be used as a decoupler [4] and coupled with micromolded carbon ink electrodes to achieve amperometric detection for ME [3, 5, 17]. Henry’s group at Colorado State University also developed a palladium decoupler where a mircowire is manually placed into an electrode channel at the end of the separation channel [6]. However, to date these decouplers have been used with relatively simple buffer systems and short channel lengths.
In this paper, we detail the coupling of lengthened serpentine electrophoresis channels with a palladium decoupler and amperometric detection for the separation and detection of neurotransmitters. Palladium electrodepositions were used to increase the decoupler surface area and more efficiently dissipate hydrogen produced at the decoupler. Several different serpentine microchip designs for ME/amperometric detection were evaluated. The incorporation of a serpentine bi-layer microchip device, which uses pneumatic valves for injection, with the decoupler and detection electrodes was shown to result in a baseline separation of a neurotransmitter mixture. We demonstrate the applicability of this device by monitoring the stimulated release of dopamine and norepinephrine from PC 12 cells. To the best of our knowledge, this is the first report that integrates serpentine microchannels for microchip electrophoresis with a palladium decoupler and electrochemical detection.
2 Materials and Methods
2.1 Microchip Fabrication
Masters used in the production of poly(dimethylsiloxane) (PDMS) microchannels for pinched injection were fabricated based on previously published soft-lithography methods [14, 18] using a positive photoresist. Structure heights were measured using a surface profiler (Dektak II, Vecco Instruments, Woodbury, NY) and the resulting flow channel was 40 μm wide and either 3 or 8-cm in length depending on the design. The channel depths for the 3 and 8-cm pinched channels were 22.2 and 20.8 μm, respectively. Bi-layer valving devices were assembled as previously described [17, 19] but without a decoupler hole (discussed in results and discussion) [17]. The electrophoresis channel width was 40 μm and the channel depths varied for each study but ranged between 17.3 and 22.2 μm.
Stanford University’s Nanofabrication Facility was responsible for sputtering 200 Å titanium and 2000 Å palladium on high quality borosilicate glass. The decoupler design was patterned using positive resist and wet etching as previously reported [5]. Gold and platinum decoupler plates were also fabricated using this method and will be discussed in the results/discussion. The length of the decoupler in these studies was 1500 μm, with the width being fixed by the separation channel. PDMS micromolding channels (10 μm × 1 μm) were used to define structures for micromolding carbon ink electrodes as previously described [3, 5, 20].
2.2 Palladium Depositions
Palladium was electrodeposited from a 1011 mg/L palladium (II) chloride solution that was 5% in HCl (Sigma Aldrich, St. Louis, MO). Amperometric scans of either −100 mV or −300 mV were employed for electrodepositions [21] using a CH Instrument potientiostat (812B, Austin, TX, USA). When a deposition was carried out, the palladium decoupler served at the working electrode, while a Ag/AgCl reference and a platinum wire reference electrode were placed in a PDMS reservoir filled with solution over the decoupler. Cycles of 90s each were utilized depending on the desired height (see results/discussion).
2.3 Chip Operation
A LabSmith HVS448 3000V High Voltage Sequencer with eight independent high-voltage channels (LabSmith, Livermore, CA) was used as the electrophoresis voltage source. Pinched injections require the use of 3 voltage sources [22] and employ two steps, a load step and a separation step. During the load step of a pinched injection for both straight channel and serpentine designs, the buffer waste reservoir (BW) was held at ground, 0 V was applied to the sample waste reservoir (SW), and +400V to the sample (S) and buffer reservoirs (B), respectively (Figure 1). To initiate the separation using a straight channel, +1000 V was applied to B, +600 V to S and SW, while BW was constantly held at ground. In all cases, calculation of the junction voltage and the resulting field strength was based upon Kirchhoff’s rules as described by the Harrison group [23]. The field strength was found to be 226 V/cm with the straight channel design. To maintain the same field strength in an 8-cm serpentine microchip, +2400 V was applied to B, +1600 V applied to S and SW, while BW was held at ground.
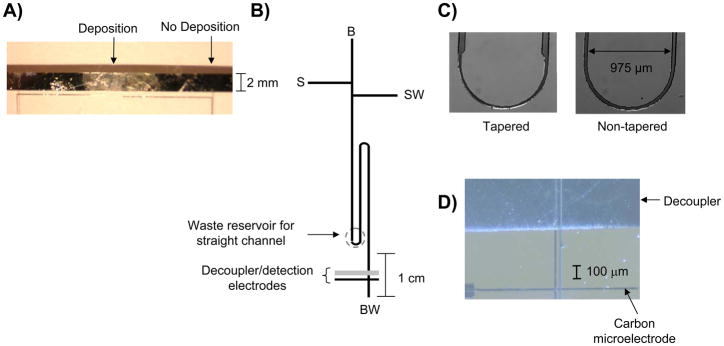
Figure 1.
A) Palladium electrodeposition on a palladium decoupler using −100 mV (vs Ag/AgCl, 3 depositions, 90 sec. each in duration); B) serpentine microchip used for the pinched injection scheme. The labels are as follows: B, buffer reservoir; BW, buffer waste; S, sample; and SW, sample waste. The dotted circle indicates the BW reservoir for a straight channel microchip; C) micrographs of the asymmetrically tapered turn and non-tapered turn; D) Micrograph showing palladium decoupler and carbon ink electrode reversibly sealed over the electrophoresis channel.
To operate the serpentine bi-layer microchip (Figure 2), PDMS valves were dead end filled with water via Tygon tubing by pressurizing a water-filled vial with nitrogen gas [17, 19]. MAC valves (MAC Fluid Power Engineering, St. Louis, MO) were used to trigger these PDMS valves by means of a timer-based control unit. In these studies, the PDMS valves are used as the interface between the hydrodynamic and electrophoresis flow regimes, with valve #1 being normally open and valve #2 being normally closed [19]. Under this condition, hydrodynamic flow is directed to sample waste (SW) and electrophoresis occurs on the opposite side of valve #2 (Figure 2). In all studies, the hydrodynamic flow rate was set by a syringe pump (Model 11 Plus, Harvard Apparatus, Holliston, MA) to be 0.2 μL/min. An injection is made by actuating the valves for hundreds of milliseconds (this momentarily closes valve #1 and opens valve #2) and the duration of the actuation is used to control the injection plug size [19]. During the actuation sequence, the separation voltage was temporarily shutoff while valve #2 was open [17]. Also, a pushback channel was used to eliminate stagnant sample at the valve interface and keep un-injected sample from leaking into the electrophoresis channel [18]. Two voltage sources are required to operate the bi-layer valving microchip. For the straight channel valving design, the high voltage (HV) applied was +1000 V and the pushback (PB) voltage was +700 V while the serpentine valving microchip used a HV of +2400 V and a PB voltage of +1200 V.
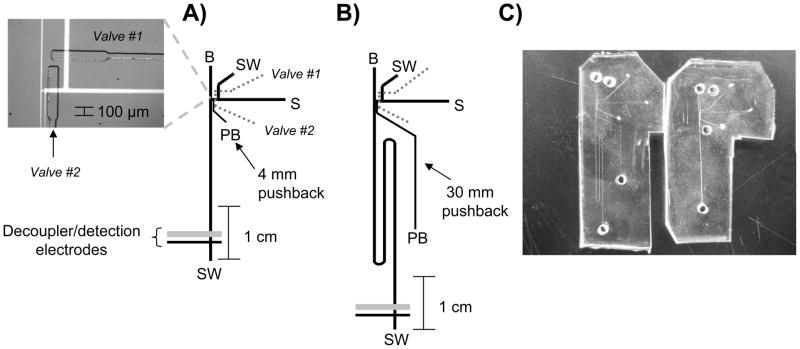
Figure 2.
A) Straight channel bilayer pneumatic valve microchip. The micrograph inset shows the continuous flow/electrophoresis interface with the channels filled with fluorescein. All labels same as Fig. 1 with addition of a PB, pushback reservoir; B) serpentine bilayer pneumatic valve microchip; C) picture of both microchips to show the small increase in the footprint when using serpentine channels.
Flow profiles as well as injection plug sizes were imaged using fluorescein and a fluorescence microscope (IX71, Olympus America) equipped with a 100 W Hg arc lamp, fluorescein filters, and a cooled 12-bit monochrome Qicam Fast digital CCD camera (QImaging, Montreal, Canada). Images were captured with Streampix Digital Video Recording software (Media Cybernetics, Silver Spring, MD, USA) and Q Capture Pro software (QImaging, Montreal, Canada) was used for determining sample plug length. This measurement, along with the cross-sectional area of separation channel, was used to calculate the volume of the peaks in nL.
Amperometric detection for these studies was achieved using micromolded carbon ink electrodes (made with Ercon E-978 carbon ink, Wareham, MA, USA) as the working electrode [3, 5, 20], while the palladium decoupler was used to ground the separation voltage [4] (see Figure 1D for micrograph). A two electrode format was used with a CH Instrument potentiostat (812B, Austin, TX, USA) to apply +0.9V to the carbon ink working electrode relative to a platinum counter electrode. The buffers used consisted of 10 mM boric acid with 25 mM SDS (pH 9.5) or 10 mM TES with 25 mM SDS (pH 8.3). For studies to determine the effects of injecting samples from a high ionic strength matrix, a cell-compatible buffer (pH 7.4) was made up of the following: 1.4 mM KCl, 72.5 mM NaCl, 0.5 mM MgCl2, 0.6 mM CaCl2, and 0.25 mM NaH2PO4 [17].
2.4 Cell Experiments
Off-chip cell stimulation experiments were carried out in Petri dishes (35 mm, Fisher Scientific, Springfield, NJ) there were coated with polygen (a mixture of collagen and poly-L-lysine), as described previously [17, 24]. After coating, the dishes were then rinsed with 10× phosphate-buffered saline solution (Fisher Scientific, Springfield, NJ) and replaced with 2 mL of F-12K medium (Kaighn’s modification of Ham’s F-12 medium; ATCC, Manassas, Virginia) that was supplemented with 10% penicillin-streptomycin solution, 5% fetal bovine serum (ATCC), and 10% horse serum (ATCC). PC 12 cells (ATCC) were then transferred into the Petri dishes and placed into the incubator at 37°C and 7% CO2. PC 12 cells were allowed to grow until ~90% confluency prior to analysis. The cells were pre-loaded before use by adding 150 μL of both dopamine and norepinephrine (1 mM each) to a confluent layer of PC 12 cells containing 1.2 mL of media for 20 min [25]. After this period of time, the solution was removed from the dish and the cells were rinsed four times with the cell-compatible buffer with the final rinse being saved as the blank. The cells were then exposed to 580 μL of a K+/Ca2+ stimulant solution (pH = 7.4, comprised of 80 mM KCl, 150 mM NaCl, 0.7 mM MgCl2, 2 mM CaCl2, and 1 mM NaH2PO4, and 10 mM HEPES) [24, 26, 27] and allowed to sit for 2 min. This solution, along with 20 μL of a 1 mM fluorescein solution, was then drawn into a syringe and hydrodynamically pumped into the bi-layer valving chip through the sample inlet at 0.2 μL/min. Cells were counted after stimulation with a hemocytometer.
3 Results and Discussion
3.1 Palladium deposition
When using off-channel detection, a method for grounding the electrophoresis voltage must be employed to isolate the working electrode and create a field-free zone for the detection of analytes. At an electrophoretic ground (cathode), the hydrolysis of H2O leads to the formation of hydrogen gas by the following equation [4, 28]:
| (1) |
Palladium is unique in its ability to absorb hydrogen in a Pd/H ratio of 0.6 [28]. To date, all palladium decouplers with ME have been used with relatively short electrophoresis channels, 2–5 cm in length [2–6]. Higher efficiency separations are needed for complex mixtures and, since it has been shown that resolution in free zone electrophoresis is directly proportional to the square root of separation length [8], the purpose of this study was to explore the use of longer separation channels with ME and EC detection. When lengthening the separation channel, a serpentine design is often utilized in order keep the microchip footprint compact [10–12]. Serpentine microchips utilize longer channels that require an increased separation voltage to maintain a constant field strength. Thin-layer palladium decouplers used in previous studies with simple boric acid buffer systems and shorter separation channels were ~2200 Å in height [3, 5]. These palladium plates are produced by sputtering 200 Å titanium and 2000 Å palladium onto glass rounds resulting in smooth, thin-layer electrodes that have a rectangular shape. Initial experiments using a non-tapered 8-cm serpentine channel (Fig 1B) sealed over a 2200 Å sputtered palladium decoupler (1500 μm in length, width defined by the separation channel) with a TES/SDS buffer at a field strength of 226 V/cm resulted in immediate hydrogen bubble formation at the decoupler. To increase the palladium through sputtering would be expensive and time-consuming (it takes approximately 3 hours to deposit 200 Å Ti and 2000 Å Pd at typical deposition rates). The use of palladium electrodepositions onto the palladium decoupler was explored to increase the decoupler surface area and more efficiently dissipate hydrogen produced at the decoupler.
Palladium was electrodeposited onto the palladium decoupler by applying a constant potential of −100 mV or −300 mV for 90s to the decoupler (vs. a Ag/AgCl reference electrode) while it is immersed in a 1011 mg/L palladium (II) chloride solution. As was seen in previous work where palladium was deposited onto glassy carbon [21], the more negative potential increases the amount of palladium deposited onto the surface. The −100 mV (vs. Ag/AgCl reference) deposition resulted in a smooth and shiny surface (Fig. 1A). The −300 mV deposition, however, yielded a darker and much rougher surface. These trends were also seen when depositing palladium onto glassy carbon [21]. Three depositions (90 seconds each in duration) at −100 mV effectively added an average of 1,480 Å (n = 4) of palladium to a majority of the 2200 Å decoupler. The edges of the decoupler had significantly more palladium deposited, with an average edge height of 1.3 μm. This can be explained by the increased surface area at the decoupler edges (as compared to the relatively smooth regions in between the edges) and the “edge effect”, where enhanced rates of mass transport are seen at the edge of a microelectrode [29]. Two 90 second depositions at −300 mV added an average of 3,060 Å (n = 4) of palladium to the 2200 Å decoupler. While the −300 mV depositions added a substantial amount of palladium, the resulting height and large degree of surface roughness led to periodic issues with reversibly sealing a PDMS flow channel over the decoupler. Therefore, all subsequent separation studies (see following sections) used the −100 mV conditions (3 depositions, 90 seconds each in duration).
In addition to increasing the surface area of a palladium decoupler, the deposition can be used to modify other thin-layer metal electrodes with palladium. In this study, palladium was also electrodeposited onto thin-layer sputtered gold (200 Å titanium and 2000 Å gold) or platinum (200 Å titanium and 2000 Å platinum) electrodes. The −100 mV deposition involved 4 cycles that were 90 seconds each in duration while the −300 mV deposition involved 2 cycles, 90 seconds each in duration. To test the effectiveness of the electrodes when used as a decoupler, a straight 2-cm flow channel (40 μm in width × 21 μm in depth) was sealed over each electrode (similar to Figure 1D) and a high voltage was applied to one reservoir while the electrode was held at ground. Both electrodes were 1500μm in length, with the width of the electrode being defined by the separation channel. The high voltage was increased over time to determine the field strength where hydrogen bubble formation ceased the electrophoresis. In previous work, a platinum decoupler has been shown to work effectively as a decoupler but only at low field strengths (<90 V/cm), as it is not able to absorb hydrogen to the extent of palladium [15]. When using platinum as a decoupler with no added palladium, we found that bubbles formed after one minute at 150 V/cm. A −100 mV (vs. a Ag/AgCl reference electrode) palladium deposition onto the platinum electrode effectively added 2300 Å palladium and allowed the microchip to run at 450 V/cm for ~15 min while the −300 mV deposition added 2500 Å of palladium and enabled field strengths of 450 V/cm to be applied for greater than 30 min. When using the thin-layer gold electrodes with no deposition it was found that a field strength of 100 V/cm led to bubble formation in less than 1 minute. The use of a −100 mV Pd deposition onto the Au decoupler added 2100 Å of palladium and allowed the decoupler to run at 300 V/cm for 20 min. A −300 mV Pd deposition added 3900 Å of palladium and, due to the absolute amount added to the decoupler, the microchannel did not reversibly seal over the decoupler. These studies show that electrodepositions can be used to coat a variety of electrode materials with palladium and the resulting electrode is an effective decoupler. This could be useful in situations where dissimilar decoupler and working electrode metals are desired, thus avoiding multiple sputtering and patterning steps when lithography is used to define the electrodes. One such example is the use of a palladium decoupler and gold working electrodes for pulsed amperometric detection [6].
3.2 Initial characterization
A pinched injection scheme was initially utilized to introduce sample into the separation channel [30]. A schematic of the pinched channel design is shown in Figure 1B. When performing EC detection with ME, the buffer waste reservoir must be held at constant ground so that the detection electrodes are shielded from the electrophoresis voltages. It has been previously shown that this requirement of electrochemical detection results in sample plugs with pinched injections that are not confined within T intersection, as the sample migrates to both the sample and buffer waste reservoirs [22]. Turns are utilized in lengthened microchannels to maintain a compact microchip footprint. These turns have been shown to induce a “racetrack” effect where molecules on the inside of the wall will travel faster and shorter distances than those on the outside, leading to band broadening [10–12]. Subsequent studies demonstrated that asymmetrically tapered turns can be used to reduce this source of band broadening [9]. As shown in Figure 1C, this study initially used channels with tapered turns measuring 75 μm in width that tapered to 40 μm throughout the turns. When using moderate field strengths (226 V/cm) and separation channel lengths of 8-cm, the wider 75 μm channels utilized for the taper increased the hydrogen production at the decoupler such that consistent hydrogen bubble formation, which ceases electrophoresis, was seen even with palladium added to the decoupler through electrodeposition. The electrodeposited decoupler was able to effectively operate without hydrogen bubble formation in similar experiments with un-tapered turns that measure 40 μm in width throughout the channel network (Fig 1C). Culbertson et al. developed a relationship that shows the “racetrack” effect is a function of the analyte diffusion coefficient such that diffusion can help to counteract this phenomenon [7]; therefore the racetrack effect becomes less significant when small molecules like neurotransmitters are analyzed, as compared to larger molecules like proteins or DNA. Given the increased hydrogen production that results from use of the asymmetric turn design and since the analytes of interest for these studies are small molecules, un-tapered turns were used for the remainder of the experiments.
The neurotransmitters norepinephrine (NE) and dopamine (DA) only differ by one hydroxyl group and it is common for them to not be fully resolved in ME separations. The use of different buffer systems was explored to monitor the separation of these neurotransmitters in the lengthened channels. Sodium dodecyl sulfate (SDS) was used in all buffers as Roman et al. showed that its use in PDMS devices helps to increase and stabilize the electroosmotic flow (EOF) [31]. The buffers were 10 mM boric acid with 25 mM SDS (pH = 9.2) and 10 mM TES with 25 mM SDS (pH = 8.3). Using the boric acid buffer, the limit of detection (LOD, S/N =3) for DA on the 8-cm serpentine device was found to be slightly higher (6.7μM) than the 3-cm straight channel device (1.1 μM), probably due to diffusion-based band broadening throughout the longer channels. The straight channel LOD is similar to our previous work with these carbon ink microelectrodes [5, 17]. Use of the boric acid buffer and a field strength of 226 V/cm with the 3-cm straight channel design led to a resolution between NE and DA of 0.9 while the same experiment using the TES buffer led to a resolution between NE and DA of 1.8. A similar trend was seen when comparing resolution on the lengthened serpentine channels. Use of the boric acid buffer and a field strength of 226 V/cm with the 2-turn, 8-cm microchip resulted in a resolution between NE and DA of 2.3 while the same experiment with the TES buffer led to a resolution between NE and DA of 2.8. Since the TES buffer offered the greatest resolution for the separation of NE and DA on both the straight and serpentine designs, this buffer was used throughout the rest of these studies.
3.3 Use of valving microchip
Although pinched injections were good for initially characterizing the serpentine microchannels, more complex injection systems are necessary for studying in vitro or in vivo biological systems. Our group has shown that bi-layer microchips, where PDMS-based pneumatic valves enable the discrete injection of sample from a continuous hydrodynamic flow stream into an electrophoresis channel, can be used for integrating cell culture with electrophoretic analysis on-chip [24] or to continuously analyze species sampled by microdialysis [17]. As opposed to a pinched injection scheme, the injections are pressure based and the injection plug size can be accurately controlled/varied by changing the valve actuation time [18, 19]. Since our group has shown that these bi-layer devices can be used to analyze neurotransmitters released from cells [17, 24], the effect of increasing the separation channel length in these microchips was investigated. Figure 2 shows the schematic for the straight and serpentine channel bi-layer devices that were used in these studies. As compared to previous studies [17, 24], two chip fabrication/operation findings arose from lengthening the separation channel and using decouplers of increased palladium surface area. When integrating ME with the palladium decoupler, we previously showed that a hole must be punched through the thick (~3.5 mm) valving layer during the fabrication procedure so that this hole (2 mm in diameter) overlays the decoupler [17]. This ensured that hydrogen produced at the decoupler only had to diffuse through the thin flow layer (about 40 μm). With the use of palladium depositions, however, the hydrogen is efficiently dissipated without having to punch this extra hole and this simplifies the fabrication procedure. In addition, when using these bi-layer devices a pushback channel is necessary to remove stagnant sample from the interface and to ensure no leakage occurs into the separation channel [18, 19]. To keep the pushback channel length proportional to the separation channel length, the 8-cm serpentine device utilized a longer pushback channel than the 3-cm straight channel (see Fig. 2C).
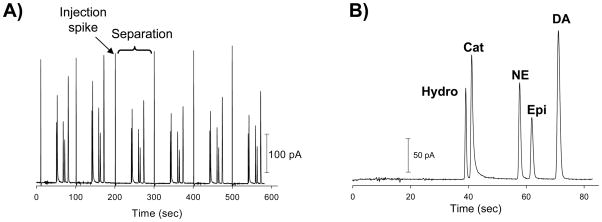
Use of an 8-cm separation channel with the increased pushback channel length, a field strength of 250 V/cm, and a 10 mM TES/25 mM SDS buffer (pH = 8.3) enabled the mutli-analyte separation shown in Figure 3. Impressively, this separation shows the ability to separate the neurotransmitters NE, epinephrine, and DA along with catechol and hydroquinone. The smallest resolution value was 1.6 (for the isomers catechol and hydroquinone) and all other analytes were baseline resolved, with resolution values of 2.7 or greater. The response was reproducible, as the average peak heights were found to be (n = 6): 138.50 ± 3.55 pA (2.6% RSD) for hydroquinone, 189.20 ± 2.32 pA (1.2% RSD) for catechol, 138.00 ± 3.04 pA (2.2% RSD) for NE, 94.73 ± 1.88 pA (2.0% RSD) for epinephrine, 212.88 ± 2.84 pA (1.3% RSD) for DA. The average number of theoretical plates for this separation was 28,700, with hydroquinone generating 36,280 plates. This type of separation performance is similar to what has been achieved with conventional CE/EC [32, 33] as well as ME studies that utilize LIF detection.
Figure 3.
Electropherograms of: A) 6 repetitive injections and amperometric detection of 500 μM hydroquinone (Hydro), catechol (Cat), norepinephrine (NE), epinephrine (Epi), and dopamine (DA) using 10 mM TES/25 mM SDS buffer (pH=8.3) and a field strength of 250 V/cm; and B) inset of one injection, separation, and detection sequence.
Most biological samples (either from cellular or in vivo systems) have matrices with a large number of ions so the ionic strength is relatively high. In order to utilize the bi-layer valving device for biological studies and to study the effect of increasing the separation channel length in ME with EC detection, a cell-compatible buffer (pH 7.4) of the following composition was used: 1.4 mM KCl, 72.5 mM NaCl, 0.5 mM MgCl2, 0.6 mM CaCl2, and 0.25 mM NaH2PO4 [17]. Analytes of interest were dissolved in this buffer and injected into the separation channel containing the 10 mM TES/25 mM SDS buffer (pH = 8.3). One drawback of injecting samples that have a high ionic strength relative to the separation buffer is possible band broadening from de-stacking due to differences in conductivity of the two buffer zones [8, 34]. The effect of increasing the separation channel length on the separation between NE and DA was studied with a 3-cm straight channel valving chip and a 2-turn, 8-cm serpentine valving microchip (see Figure 2 for chip designs). For these comparison studies, the injection volume was increased as the channel volume (length) increased. If a constant injection volume were used, it would not be possible to make comparisons of different channel lengths as each would have a different amount of band broadening contributed by the injection process. It has been established that in order to minimize band broadening in CE, extra column volumes from the injector and detector should ~1% of the separation column volume [35, 36]. Since the electrodes used here are 10 μm in width, the contribution from the detector is minimal.
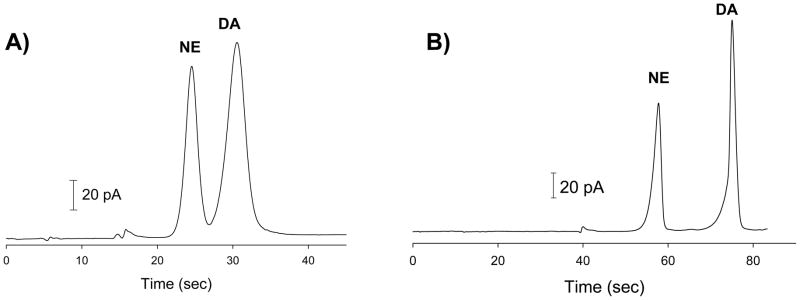
Figure 4 shows the comparison when the analytes are dissolved in the cell-compatible buffer and injected into the separation channel containing the TES/SDS buffer. The 3-cm device had a channel volume of 20.80 nL and an average injection volume of 0.28 nL. This resulted in the injection volume being 1.35% of the channel volume. Using a field strength of 250 V/cm led to a resolution between NE and DA of 1.9, with the number of theoretical plates being 960 for NE and 800 for DA. With the 8-cm, 2 turn device, the channel volume was 61.80 nL and the average injection volume was 0.62 nL leading to the injection volume being 1.0% of the channel volume. Using a field strength of 250 V/cm, the resolution between NE and DA on the 2-turn, 8-cm microchip was found to be 4.9 with the number of theoretical plates being 7,270 for NE and 3,080 for DA. This data shows that increasing the channel length significantly improves the separation performance of ME with EC detection in terms of both the number of theoretical plates and resolution. In addition, it is clear that increasing the channel length is beneficial with regard to analyzing samples from a high ionic strength biological matrix where de-stacking may be an issue.
Figure 4.
Electropherogram of 500 μM dopamine (DA) and norepinephrine (NE) prepared in cell-compatible buffer (pH=7.4) and injected into electrophoresis channel containing 10 mM TES/25 mM SDS (pH=8.3) run buffer using the bilayer pneumatic schematic on A) a 3-cm straight channel microchip and B) an 8-cm serpentine channel microchip. Field strength for both = 250 V/cm
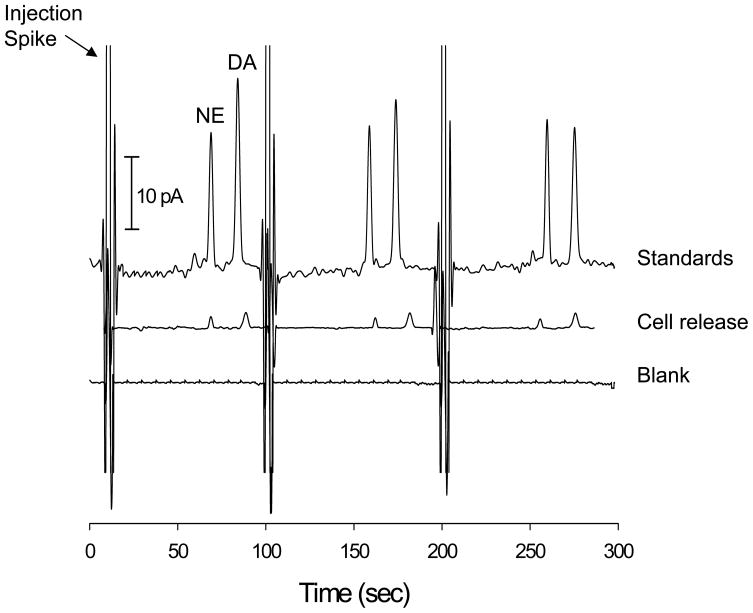
Off-chip stimulation of a confluent layer of PC 12 cells was used to demonstrate the applicability of the bi-layer serpentine device with amperometric detection to monitor an actual biological system. Prior to analysis, the cells were preloaded with NE and DA [25]. The cells were rinsed 4 times with cell-compatible buffer (last rinse was saved as a blank) and cells were stimulated with a K+/Ca2+ solution (pH = 7.4, comprised of 80 mM KCl, 150 mM NaCl, 0.7 mM MgCl2, 2 mM CaCl2, and 1 mM NaH2PO4, and 10 mM HEPES). After analysis, NE and DA standards (100 μM) were used to determine the concentration of the release seen from the PC 12 cells. The data shown in Figure 5 shows the cellular release along with the standards and blank. The average DA concentration was found to be 18.6 μM while the average NE concentration was found to be 15.2 μM. In addition, the resolution between NE and DA was found to be 3.9 in the cell release data. Measuring NE release was not possible in a previous study in our lab due to problems with detection limits [24]. This study shows that both neurotransmitters can be separated and detected from a high ionic strength biological matrix (total salt concentration of stimulant solution is ~230 mM) with more than baseline resolution.
Figure 5.
Three repetitive injections and amperometric detection of releasate from PC 12 cells upon stimulant with a K+/Ca2+ solution. Also shown are 3 repetitive injections and amperometric detection of standards and a blank. Electrophoresis buffer = 10 mM TES with 25 mM SDS (pH=8.3). Sequence: 1.2 s valve actuation with ~2 s high-voltage shutoff repeated every 100 s.
4 Conclusions
These studies have shown that serpentine microchannels can be used with electrochemical detection for efficiently separating complex mixtures of neurotransmitters. The use of palladium electrodepositions onto the sputtered palladium decoupler was required to increase the decoupler surface area. It was shown that increasing the channel length leads to a baseline separation of dopamine and norepinephrine as well as an increased number of theoretical plates. A bi-layer PDMS device with pneumatic valves and serpentine channels offered a multi-analyte separation that is comparable to separations seen in conventional CE/EC. It was also shown that the increased channel length is beneficial when separating and detecting analytes from a high ionic strength matrix. To the best of our knowledge, this is the first report that integrates serpentine microchannels for microchip electrophoresis with a palladium decoupler and electrochemical detection. Future work will detail studies that improve the limit of detection and integrate cell culture directly onto the bi-layer serpentine device.
Acknowledgments
This research was supported grants from Stanford University (CIS New User Grant) and the National Institutes of Health (9R15GM084470-02).
References
- 1.Vandaveer WR, IV, Passas-Farmer SA, Fischer DJ, Frankenfeld CN, Lunte SM. Electrophoresis. 2004;25:3528–3549. doi: 10.1002/elps.200406115. [DOI] [PubMed] [Google Scholar]
- 2.Chen DC, Hsu FL, Zhan DZ, Chen CH. Anal Chem. 2001;73:758–762. doi: 10.1021/ac000452u. [DOI] [PubMed] [Google Scholar]
- 3.Kovarik ML, Li MW, Martin RS. Electrophoresis. 2005;26:202–210. doi: 10.1002/elps.200406188. [DOI] [PubMed] [Google Scholar]
- 4.Lacher NA, Lunte SM, Martin RS. Anal Chem. 2004;76:2482–2491. doi: 10.1021/ac030327t. [DOI] [PubMed] [Google Scholar]
- 5.Mecker LC, Martin RS. Electrophoresis. 2006;27:5032–5042. doi: 10.1002/elps.200600401. [DOI] [PubMed] [Google Scholar]
- 6.Vickers JA, Henry CS. Electrophoresis. 2005;26:4641–4647. doi: 10.1002/elps.200500508. [DOI] [PubMed] [Google Scholar]
- 7.Culbertson CT, Jacobson SC, Ramsey JM. Anal Chem. 2000;72:5814–5819. doi: 10.1021/ac0006268. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger R. Practical Capillary Electrophoresis. Academic Press; San Diego, CA: 2000. [Google Scholar]
- 9.Ramsey JD, Jacobson CT, Culbertson CT, Ramsey JM. Anal Chem. 2003;75:3758–3764. doi: 10.1021/ac0264574. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths SK, Nilson RH. Anal Chem. 2001;73:272–278. doi: 10.1021/ac000936q. [DOI] [PubMed] [Google Scholar]
- 11.Culbertson CT, Jacobson SC, Ramsey JM. Anal Chem. 1998;70:3781–3789. [Google Scholar]
- 12.Paegel BM, Hut LD, Simpson PC, Mathies RA. Anal Chem. 2000;72:3030–3037. doi: 10.1021/ac000054r. [DOI] [PubMed] [Google Scholar]
- 13.Woolley AT, Lao K, Glazer AN, Mathies RA. Anal Chem. 1998;70:684–688. doi: 10.1021/ac971135z. [DOI] [PubMed] [Google Scholar]
- 14.Martin RS, Gawron AJ, Lunte SM. Anal Chem. 2000;72:3196–3202. doi: 10.1021/ac000160t. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Wu RG, Huang JG, Lin YC, Chang HC. Anal Chem. 2003;75:947–952. doi: 10.1021/ac025912t. [DOI] [PubMed] [Google Scholar]
- 16.Martin RS, Ratzlaff KL, Huynh BH, Lunte SM. Anal Chem. 2002;74:1136–1143. doi: 10.1021/ac011087p. [DOI] [PubMed] [Google Scholar]
- 17.Mecker LC, Martin RS. Anal Chem. 2008;80:9257–9264. doi: 10.1021/ac801614r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li MW, Huynh BH, Hulvey MK, Lunte SM, Martin RS. Anal Chem. 2006;78:1042–1051. doi: 10.1021/ac051592c. [DOI] [PubMed] [Google Scholar]
- 19.Li MW, Martin RS. Electrophoresis. 2007;28:2478–2488. doi: 10.1002/elps.200600713. [DOI] [PubMed] [Google Scholar]
- 20.Kovarik ML, Torrence NJ, Spence DM, Martin RS. Analyst. 2004;129:400–405. doi: 10.1039/b401380h. [DOI] [PubMed] [Google Scholar]
- 21.Soreta TR, Strutwolf J, O’Sullivan CK. Langmuir. 2007;23:10823–10830. doi: 10.1021/la7006777. [DOI] [PubMed] [Google Scholar]
- 22.Garcia CD, Liu Y, Anderson P, Henry CS. Lab Chip. 2003;3:324–328. doi: 10.1039/b309339e. [DOI] [PubMed] [Google Scholar]
- 23.Seiler K, Fan ZHH, Fluri K, Harrison DJ. Anal Chem. 1994;66:3485–3491. [Google Scholar]
- 24.Li MW, Martin RS. Analyst. 2008;133:1358–1366. doi: 10.1039/b807093h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore JM, Papke JB, Cahill AL, Harkins AB. Am J Physiol: Cell Physiol. 2006;291:270–281. doi: 10.1152/ajpcell.00539.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing AG. Anal Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- 27.Li MW, Spence DM, Martin RS. Electroanalysis. 2005;7:1171–1180. [Google Scholar]
- 28.Kok WT, Sahin Y. Anal Chem. 1993;65:2497–2501. [Google Scholar]
- 29.Wang J. Analytical Electrochemistry. Wiley-VCH; New York: 2000. [Google Scholar]
- 30.Jacobson SC, Hergenroder R, Moore AW, Ramsey JM. Anal Chem. 1994;66:1107–1113. [Google Scholar]
- 31.Roman GT, McDaniel K, Culbertson CT. Analyst. 2006;131:194–201. doi: 10.1039/b510765b. [DOI] [PubMed] [Google Scholar]
- 32.Wallingford RA, Ewing AG. Anal Chem. 1987;59:1762–1766. doi: 10.1021/ac00141a005. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Lunte SM, Lunte CE. Anal Chem. 1995;67:911–918. [Google Scholar]
- 34.Gillogly JA, Lunte CE. Electrophoresis. 2005;26:633–639. doi: 10.1002/elps.200410061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grossman PD, Colburn JC. Capillary Electrophoresis: Theory and Practive. Academic Press, Inc; San Diego: 1992. [Google Scholar]
- 36.Poole CF. The Essence of Chromatography. Elsevier; Amsterdam: 2003. [Google Scholar]