Abstract
Permeabilization of the outer mitochondrial membrane is the point of no return in most programmed cell deaths. This critical step is mainly regulated by the various protein-protein and protein-membrane interactions of the Bcl-2 family proteins. The two main models for regulation of mitochondrial outer membrane permeabilization, direct activation and displacement do not account for all of the experimental data and both largely neglect the importance of the membrane. We propose the embedding together model to emphasize the critical importance of Bcl-2 family protein interactions with and within membranes. The embedding together model proposes that both pro- and anti-apoptotic Bcl-2 family proteins engage in similar dynamic interactions that are governed by membrane dependent conformational changes and culminate in either aborted or productive membrane permeabilization depending on the final oligomeric state of pro-apoptotic Bax and/or Bak.
Keywords: Bcl-2, Bax, MOMP, Mitochondrial permeabilization, Membrane proteins
1. Introduction
There is a growing consensus that most programmed cell deaths (including apoptosis) are comprised of two distinct phases, initiation and execution. Bcl-2 family proteins function primarily during initiation of apoptosis. These proteins carry the moniker of Bcl-2 family proteins to indicate that they share sequence similarity with Bcl-2, the prototype of the family. Bcl-2 and its closest relatives, Bcl-XL, Mcl-1, Bcl-w share 4 regions of similarity denoted BH (Bcl-2 homology) regions. For most cells the commitment step that separates initiation from execution appears to be permeabilization of the outer mitochondrial membrane. It is at the commitment step that many intracellular signals must be integrated to determine the fate of the cell. Therefore, it is not surprising that mitochondrial outer membrane permeabilization (MOMP) is a point of convergence for a large variety of signaling pathways. Many of these signals are mediated by proteins of the Bcl-2 family called BH3 only proteins because the only sequence similarity is a BH3 sequence. It is likely that the rest of the protein sequence of BH3 only proteins is divergent because these proteins have other functions in healthy cells. Unlike BH3 proteins, the major apoptosis function of multi-BH region Bcl-2 family proteins is directly regulating the commitment step. In part convergence is driven by the fact that permeabilization is mediated by a small number of largely functionally redundant proteins, typically just Bax and Bak (and in some cells perhaps Bok). These pro-apoptotic proteins that contain BH regions 1-3 integrate signals from the BH3 only and other proteins generally via direct binding interactions.
MOMP, by releasing a number of proteins from the intermembrane space that participate in cellular execution, drives divergence of the process. Execution spreads throughout the cell when the proteins released from the mitochondrial intermembrane space each interact with a variety of other proteins that carry out cellular execution. These interactions activate both the proteases, including the caspases, as well as the nucleases that physically demolish the cell.
Cell free models using purified proteins have demonstrated unequivocally that once activated, either Bax or Bak is sufficient to permeabilize a variety of membranes including liposomes. Nevertheless, it is likely that in cells these proteins rarely if ever act alone. Instead they not only integrate cellular signals via direct binding interactions but also probably interact with other mitochondrial outer membrane (MOM) constituents (proteins and lipids) to carry out MOMP. In this review we will examine what is known about the molecular mechanism(s) by which Bax and Bak permeabilize MOM. We describe and critique two of the current models for the process (Direct Activation and Displacement) and use this information as well as other data to synthesize a new model (Embedding Together) to explain different aspects of the molecular mechanism. We describe some of the shortcomings of each of these models as well as prescribe experimental approaches for testing the salient features of each.
2. Direct activation model
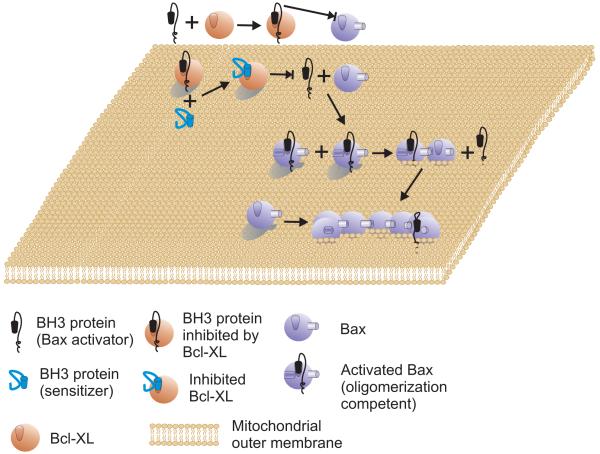
In the current formulation (1-3) the main features of the direct activation model (Figure 1) are as follows: BH3-only proteins are classified as activator or sensitizer (derepressor) proteins based on their multi-BH motif containing binding partners. Activators (Figure 1, Black) are the BH3-only proteins that bind both pro- and anti-apoptotic proteins such as Bax and Bcl-XL, respectively. In the direct activation model, Bax activation requires direct interaction with at least one of the BH3-only activators. Bcl-XL sequesters the BH3-only activators, and thereby prevents the activation of Bax. In contrast, sensitizer BH3-only proteins (Figure 1, Cyan) bind anti-apoptotic proteins releasing the activator BH3-only proteins from Bcl-XL. In the original version of the direct activation model Bcl-XL was also proposed to sequester activated Bax or Bak however, this activity is now usually excluded and often relegated to the status of a detergent induced artifact. At the very least it is secondary to the sequestration of BH3-only activators in the current version. Indeed, this has become a distinguishing feature of current direct activation models.
Figure 1.
The Direct Activation Model
An activator protein (black) such as tBid that contains a BH3 domain can bind to and activate Bax (powder blue). Activation of Bax leads to integration in the MOM and oligomerization. Oligomerization of Bax may displace the activator. Alternatively, one activated Bax may recruit additional Bax and therefore, there need be only one activator for each oligomer. Bak is predicted to function similarly but begins bound to the surface of MOM. Membrane permeabilization is inhibited by anti-apoptosis proteins (such as Bcl-XL shown) binding to the activator protein. Once bound to Bcl-XL the activator protein can no longer bind to or activate Bax. Sensitizer proteins compete with activator proteins for binding to Bcl-XL. Therefore, if the affinity for Bcl-XL or abundance of the sensitizer is greater than the activator it will displace it from Bcl-XL whereupon the activator can again bind to Bax.
The direct activation models do not explicitly distinguish between which multi-BH domain pro-apoptotic proteins are being activated, either Bax or Bak. In most, but not all, model systems Bax and Bak function in a redundant, parallel fashion (4). However, elucidating the molecular mechanisms entailed by a direct activation model will require separate investigations of both proteins, as activating Bax requires membrane localization and possibly integration into the MOM before oligomerization, whereas activating Bak requires only the last step. Thus, unless the migration to membranes, integration and oligomerization of Bax occur in one concerted step resulting from a single conformational change (and there is evidence from both endogenous and viral regulators of Bax activation that these are separate steps-see Section 3.1), Bax and Bak activation require different mechanisms.
BH3-only proteins Bid and Bim are the primary activators proposed in the direct activation model, although PUMA is sometimes considered an activator (5). Consistent with this view, a peptide corresponding to the BH3 motif of either Bid or Bim induced soluble monomeric Bax protein to insert into liposomal membranes with a composition of lipids often used as a mimic of mitochondrial outer membranes (MOM-like liposomes). Once activated, Bax forms oligomers in these MOM-like liposomes and causes membrane permeabilization (2). Bid and Bim peptides also induced Bax insertion into the MOM, Bax and Bak oligomerization and cytochrome c release from mitochondria isolated from various tissues or cell lines (1-3,5,6). Bax or Bak was required for either of these peptides to release cytochrome c from mitochondra (1,6). Moreover, when loaded into various cells, each peptide was sufficient to trigger cytochrome c release and apoptosis (1,2,6).
Consistent with these data, recombinant tBid protein activated full length, recombinant Bax to permeabilize MOM liposomes and isolated mitochondria (7-11). As part of this process, tBid causes Bax to insert into membranes and form large molecular weight oligomers (2,5,7,10-12). Full length, uncleaved Bid activates endogenous Bax in isolated mitochondria, but is less potent compared to tBid (13,14). Bid and tBid also induce cytochrome c release from mitochondria by activating and oligomerizing endogenous Bak (13,15,16).
The role of Bim as a BH3-only activator has not been studied as extensively as tBid/Bid, and the results are complicated by the existence of multiple splice isoforms and post-translational modifications of Bim. Nevertheless, studies done to date do point to significant differences between Bid and Bim. Recombinant Bax can be activated by purified BimEL protein (but not BimL, (17) to release cytochrome c from mitochondria. However, compared to tBid, BimEL was less effective at releasing cytochrome c from mitochondria. Furthermore, while tBid releases 10-kDa molecules from liposomes when combined with Bax protein, BimEL with Bax generated smaller pores that released only 0.4-kDa molecules (9). Thus in cell free sytems, BimEL may be an incomplete activator of Bax. Consistent with incomplete activation, in cells transfected with BimS and BimAD, Bax changes conformation and induces apoptosis, but Bim S/AD does not bind to inactive Bax in cells solubilized with CHAPS, indicating that another activation step for Bax is required before it interacts with Bim (18). Similarly, BimEL was co-precipitated with Bax in mitochondria from hematopoeitic cells only after apoptosis induction by IL-3 withdrawal; phosphorylation of BimEL inhibited the interaction (19). Whether any Bim isoforms can activate Bak directly remain to be determined by reconstitution experiments similar to those performed for tBid.
The status of PUMA as a Bid-like activator of Bax is even more controversial, as a PUMA BH3 peptide and purified PUMA protein acted like an activator in one study(5) but like a sensitizer in two other studies (2, 3).
How Bax and Bak are activated by BH3-only activators is unclear. The NMR structure of soluble monomeric Bax indicates that the C-terminal hydrophobic helix (helix 9) is buried in the hydrophobic groove that corresponds to the hydrophobic groove of Bcl-XL demonstrated by crystallography to be a BH3 binding pocket (20, 21). Therefore, by analogy, the BH3 motif of tBid or Bim may bind to the similar hydrophobic groove in Bax displacing helix 9, which then inserts into the mitochondrial membrane. If this is true, Bak must be activated by a different mechanism since the C-terminal hydrophobic sequence of Bak is constituitively inserted into membranes and therefore cannot occupy the hydrophobic groove. However, the evidence that tBid binds to Bcl-XL and Bax similarly is controversial; data with Bax mutants indicates that the BH3 motif of tBid may interact with helix 1 of Bax rather than with the BH3 motif of Bax that is part of the hydrophobic groove (8).
The location and persistence of the binding of BH3-only activators to Bax and Bak is also debatable. Purified tBid pull-down Bax synthesized by in vitro translation in the absence of detergent suggests that the initial interaction can take place in cytoplasm (5). However, we have shown that tBid causes the conformational change associated with Bax activation only in the presence of membrane, suggesting that to be effective the interaction between Bax and tBid must take place at the membrane(12). Interestingly tBid targeted to mitochondrial membrane efficiently via multiple internal helices (and in some cases additionally by a myristoylated N-terminus) resulting in exposure of the tBid BH3 motif (22-26). Moreover, a recent report indicated that a membrane-tethered Bid BH3 peptide is much more potent in activating Bax than the corresponding soluble peptide 27). However, we did not detect any tBid bound to Bax oligomers after activation in mitochondria, suggesting most tBid dissociates from Bax after Bax inserts into the membrane (11). Moreover, in another study tBid-Bax hetero-adducts were not detected in the mitochondria when Bax homo-adducts were present (28). Since Bak is constitutively inserted into mitochondrial membranes, its interaction with BH3-only and other activators must occur at the membrane. Thus tBid was co-immunoprecipitated with Bak in the mitochondrial membrane (15), but tBid was not crosslinked to Bak that was crosslinked with other Bak proteins. These results are consistent with a model in which tBid is released when either Bax or Bak homo-oligomerizes in the membrane as shown in Figure 1. The precise sequence of binding and dissociation may differ between BH3-only activators, as co-immunoprecipitation of Bim with Bax in the heavy membrane fraction suggests that this complex persists in membrane (19). The details of the interaction between Bim and Bak have not yet been investigated.
The direct activation model proposes that anti-apoptotic Bcl-2 family proteins inhibit Bax activation mainly by sequestration of the activator BH3-only proteins. Consistent with this feature of the model, purified Bcl-2, Bcl-XL, Mcl-1, Bcl-w or Bfl-1/A1 protein bind to purified Bid and/or Bim proteins or their BH3 peptides in solution based assays (1-3, 29). Complexes between these anti-apoptotic proteins and BH3-only activators were also detected in isolated mitochondria or total cell lysates (3,29,30). These interactions are functional when investigated in many contexts: in liposomes, mitochondria, or cells, the multi-BH domain anti-apoptotic proteins inhibited tBid/Bim induced Bax or Bak activation events including insertion into membranes, oligomerization and permeabilization (1-3,7,9,29).
As a consequence of the inhibition of BH3-only activators by anti-apoptotic proteins, other BH3-only proteins may act as sensitizers by displacing the activators from anti-apoptotic proteins, thus allowing the activators to bind to Bax/Bak. This group of anti-apoptotic proteins includes Bad, Bik, Bmf, Hrk, Bnip3, and Noxa. Consistent with this role, BH3 peptides from these sensitizers did not activate Bax or Bak in MOM liposomes or isolated mitochondria, and did not trigger cytochrome c release or apoptosis in cells (1-3,7). However, they did bind to anti-apoptotic Bcl-2 family proteins neutralizing them and releasing the inhibition of the activator BH3 peptides or proteins. As a consequence, the released activator BH3 sequences bound to Bax or Bak to trigger cytochrome c release and apoptosis (1-3, 7). A prediction is that the intact BH3-only sensitizer proteins may function similarly to their corresponding peptides, but reconstitution studies using purified full length BH3-only sensitizers have not yet been carried out.
In addition to activation by BH3-only pro-apoptotic proteins, previously activated Bax/Bak can also activate itself. This phenomenon, termed auto-activation, was first suggested by Ruffolo and Shore, who noted that an activated Bak mutant potently induced conformation change and oligomerization of non-activated Bak. They suggested that this mechanism may propagate the activation initiated by BH3-only proteins (31). We recently showed auto-activation may also be relevant for Bax: an extended peptide including the BH3 motif and downstream residues of Bax can activate purified Bax in both mitochondrion and a liposome based system. Further analyses showed that liposome-bound Bax activated soluble Bax suggesting that membrane binding causes a conformation change in Bax exposing the extended peptide. Therefore, membrane-bound Bax can activate cytosolic Bax in vitro (32). However, we have not yet determined whether it is membrane-bound monomeric, oligomeric Bax or both that mediate auto-activation.
Cross-activation of Bax and Bak has also been observed. Bak BH3 peptide triggered Bax conformation change in presence of mitochondria and caused membrane insertion (33). Bak oligomerization was observed in Bax expressing cells after ATP depletion but not in Bax knockout cells, suggesting that in that system Bak activation requires Bax (34). In contrast, Bax oligomerization occurred in the Bak knockout cells after the same treatment, suggesting that Bax can be activated independent of Bak. Bif-1 (a protein that is not a Bcl-2 family member; see Section 3.1) selectively binds to Bax but not Bak and may be required for Bax activation in some circumstances (35,36). In Bif-1 expressing cells both Bax and Bak were activated after drug treatment, and in Bif-1 deficient cells, both Bax and Bak activation were abolished, suggesting that in these cells Bak activation may require Bax activation (36).
Auto- and cross-activation of Bax and Bak are difficult to explain within the current version of the direct activation model, where apoptosis proceeds only when the capacity of anti-apoptotic proteins to “titrate out” activator BH3-only proteins has been exceeded. If this were the case, then no excess “free” anti-apoptotic proteins would be available to inhibit activated Bax and Bak, particularly when generated through auto- and cross-activation. This prediction was not supported by experiments showing that Bcl-2 inhibited the auto-activation of Bax and Bak (31,32). Furthermore, if Bcl-2 and Bcl-XL must be inactivated by BH-3 only proteins prior to activation of Bax or Bak and anti-apoptotic proteins cannot bind and inhibit Bax/Bak directly, then auto-activation would mean that any activation of Bax or Bak would ultimately kill the cell. One solution to this problem would be if Bax/Bak auto-activation was limited to oligomers in which oligomerization by BH3 proteins is self limiting. In this case apoptosis could still be regulated if the initiation of each oligomer required a BH3 activator.
Another prediction of the direct activation model is that most anti-apoptotic proteins are either unoccupied or bound by activator BH3-only proteins in non-stressed cells. This prediction was at odds with the data that Mcl-1 and Bcl-XL bind to Bak in dividing 293T cells (37), and that Mcl-1 forms a complex with Bax in 2B4 cells before treatment with dexamethasone (3). However, it must be kept in mind that cells in culture are abnormal, stressed due to high oxygen and usually growing on plastic therefore it would not be surprising if they are partially activated for apoptosis.
The direct activation model also proposes that the apoptosis inhibitors are in a stable complex with activator BH3-only proteins and that this is what allows non-apoptotic cells to survive; stress-induced increases in sensitizer BH3-only proteins are required to displace the activators from the inhibitors. However, Bcl-w can be inactivated by integration into the mitochondrial membrane triggered by binding to Bim, suggesting that Bim interaction with Bcl-w is dynamic and disabling, directly contrary to the prediction of the direct activation model (38). We also found that tBid binds to Bcl-2 transiently; however, in this case, the binding activates Bcl-2 by changing its conformation. The tBid did not associate with the conformationally altered Bcl-2 after activating it (11), again demonstrating that anti-apoptotic proteins do not function exclusively as passive “traps” for BH3 activators.
3. Displacement model
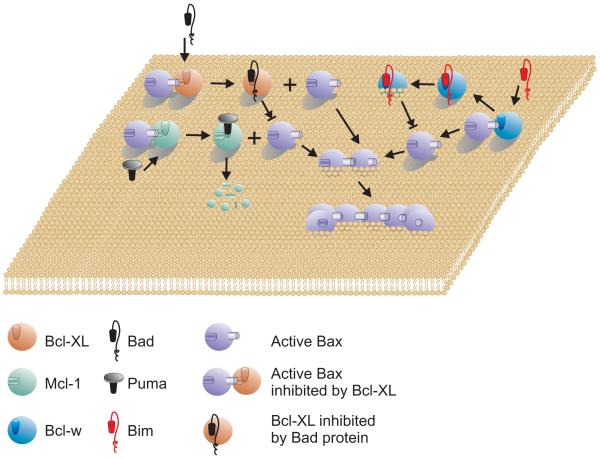
The main competing model to explain the regulation of apoptosis by Bcl-2 family members is displacement: the salient feature is that the multi-BH pro-apoptotic proteins Bax and Bak are constitutively active and must be continuously bound and inhibited by multi-BH anti-apoptotic proteins for cells to survive (Figure 2). In apoptotic cells, BH3-only proteins displace Bax and Bak from anti-apoptotic proteins such as Mcl-1 and Bcl-XL. Since Bax or Bak can be inhibited by more than one anti-apoptotic protein, all the relevant anti-apoptotic proteins must be inhibited for apoptosis to proceed. Furthermore, BH3-only proteins display selectivity for their targets, so more than one BH3-only protein may be required for inhibit all relevant anti-apoptotic proteins. The released Bax and Bak are active apoptosis inducers that eventually oligomerize and permeabilize the mitochondrial membrane. As shown in Figure 2, the BH3 binding renders anti-apoptotic proteins non-functional by (a) displacing Bax or Bak (shown for Bcl-XL and Bax), (b) inducing a non-functional membrane integrated topology that can no longer bind Bax or Bak (shown for Bcl-w and Bim) or (c) enabling proteosome mediated degradation (shown for Mcl-1 and PUMA).
Figure 2.
The Displacement Model
Anti-apoptosis proteins such as Bcl-XL, Mcl-1 and Bcl-w bind to and thereby inhibit active forms of Bax/Bak (for clarity only Bax is shown, in this model Bax and Bak are assumed to function similarly). BH3 only proteins initiate membrane permeabilization by displacing active Bax from the anti-apoptosis proteins by direct binding. The active Bax then inserts into the membrane and oligomerizes (the order is not specified by the model and was selected arbitrarily). Binding of BH3 proteins inhibit anti-apoptosis proteins by various mechanisms: Bcl-XL by sequestration, Bcl-w by embedding it in the membrane and Mcl-1 by initiating degradation. For simplicity each BH3 protein is shown binding a single anti-apoptosis protein however, the model proposes hierarchies of interaction based on the relative affinities of the interactions. Moreover, an active Bax displaced from Bcl-XL may be bound by Mcl-1 or some other anti-apoptosis protein. Therefore, initiating membrane permeabilization requires inactivating all of the relevant anti-apoptosis proteins. For simplicity the legend includes only one example of inhibition of Bax.
In support of the displacement model, complexes of Bak with various anti-apoptotic proteins are present in dividing cells in culture. Selectivity has been demonstrated by the fact that Bak in the membrane fraction can be co-immunoprecipitated with Mcl-1 and Bcl-XL but not Bcl-2, Bcl-w or A1 (37,39,40). Consistent with this, a Bak BH3 peptide has high affinity for purified Mcl-1 and Bcl-XL proteins but low affinity for Bcl-w and Bcl-2 when measured in vitro using the Biacore system (37). A mutation in the BH3 motif of Bak that reduced the binding affinity of the peptide to Mcl-1 abolished Bak-Mcl-1 co-immunoprecipitation in cell lysate, suggesting that the BH3 motif of Bak is required for the binding with the anti-apoptotic partner (37). Bak has also been proposed to be held in check by other binding partners such as VDAC2 (41). However, the stoichiometry of the interaction and the relative amounts of the endogenous proteins in cells has not been reported. Therefore, it is difficult to assess the physiological relevance of Bak binding partners.
The status of Bax as a constitutively active protein that must be inhibited for cell survival is less clear. The original identification of Bax as membrane bound protein that binds to Bcl-2 in non-stressed cells (42) is an artifact of the detergent used for cell lysis, as non-ionic detergents induce an activated conformation of Bax (43). In fact Bax behaves as a monomeric protein after solubilization with CHAPS, a detergent that does not alter Bax conformation or authentic binding interactions (44,45). However, even using these detergent conditions discordant results have been obtained: Bax was not co-immunoprecipitated with Mcl-1 and Bcl-XL in one report (37), but was co-immunoprecipitated with Mcl-1 by another group (3). The latter results are difficult to reconcile with cell fractionation data from many laboratories indicating that Bax is either primarily cytosolic or loosely bound to membranes. Moreover, crosslinking studies are also consistent with Bax being primarily monomeric in dividing cells (45). Thus, it is difficult to see how a monomeric soluble protein is being ‘held in check’ by binding to anti-apoptotic proteins.
The second critical feature of the displacement model is that BH3 only proteins disrupt the interaction between multi-domain pro-apoptotic and anti-apoptotic proteins. The displacement of Bax from anti-apoptotic proteins was documented even before the displacement model was formally proposed. Bad was initially identified as a protein that competes with Bax for binding to Bcl-XL (46). The displacement model also explains how insertion of Bax into mitochondrial membranes by a Bad BH3 peptide occurs in the presence of Bcl-XL or Bcl-2. However, Bad BH3 does not cause Bax to insert into membranes in the absence of prior binding to an anti-apoptotic protein (5). Thus, it may be that Bad contributes to signal integration by functioning to augment a prior signal that activated Bax that was inhibited by Bcl-XL or Bcl-2. In this regard it is often difficult to ascertain just how healthy the cultured cells used in most of these experiments are (see above). Moreover, the use of transformed cells which may be more dependent on anti-apoptosis proteins for continued survival may also affect the results obtained (1).
Bak has also been shown to be activated after displacement: in cells that overexpress Noxa, binding of Mcl-1 to Bak is reduced as Mcl-1-Noxa complexes form (37). Here Noxa displaces Bak from Mcl-1 by binding directly, since a Noxa mutant that does not bind to Mcl-1 does not displace Bak. However, since Noxa does not bind to Bcl-XL, the released Bak can still be bound and inhibited by Bcl-XL, if present. As predicted, in cells expressing both Mcl-1 and Bcl-XL, apoptosis is not induced by overexpression of Noxa alone. However, when Noxa was co-expressed with an engineered BH3-only protein that binds to Bcl-XL, apoptosis was induced. Knocking out Bcl-XL also sensitizes these cells to Noxa killing. In contrast, loss of Bcl-2 had no effect on Noxa killing, consistent with the finding that Bcl-2 did not sequester Bak in these cultured cells. These data indicate that all the relevant anti-apoptotic proteins that engage the multi-BH pro-apoptotic protein must be neutralized before apoptosis can take place. However, which anti-apoptotic protein is relevant may differ according to the cellular context, as Bcl-2 binds to and inhibits Bid induced activation of Bak in mitochondria from human epithelial KB cells (31). In adenovirus E1A infected cells, Bak released from Mcl-1 forms a complex with E1B 19K, a viral Bcl-2 homolog (39). Thus, the conclusion that Bcl-2 does not sequester activated Bak cannot be generalized at this moment.
Binding of anti-apoptosis proteins by their BH3-only ligands inactivates the anti-apoptotic proteins by multiple mechanisms. Noxa binding to Mcl-1 not only displaced Bak, from but also induced Mcl-1 degradation (37) dependent on proteasome activity (48). The E3 ubiquitin ligase, Mule/ARF-BP1, catalyzes the polyubiquitination of Mcl-1 (49). This enzyme contains a BH3 motif that binds to Mcl-1 but not to Bcl-XL or Bcl-2. This specificity accounts for the rapid turnover of Mcl-1 compared to other anti-apoptotic proteins. However, the exact sequence of these competitive binding events is not known. Does Noxa bind to Mcl-1 first to displace Bak and then Mule bind to Mcl-1 to displace Noxa? Interestingly, binding of the activator Bim BH3 peptide prevented Mcl-1 binding to Mule and Mcl-1 subsequent degradation, suggesting that Mule may not be able to compete with all BH3-only proteins for Mcl-1(50). Perhaps Mule only displaces sensitizer BH3-only proteins like Noxa that bind Mcl-1 more weakly than activator BH3 proteins like Bim, as indicated by the relative affinities of the respective BH3 peptides (2,3, 29). However, if sensitizers bind Mcl-1 with lower affinity than activator proteins then very high levels of expression of the sensitizer might be required to be effective. These data suggest that a hybrid of the displacement model (“Bak becomes active after Mcl-1 disappears”) and the direct activation model (“activator and sensitizer proteins both kill the cell, but require different mechanisms”) best explain certain results.
In contrast to the consequence of Mcl-1 displacement, binding of Bcl-XL by Bad did not cause Bcl-XL degradation, but rather translocation and insertion into the mitochondrial membrane. It is not clear if Bad remains bound to Bcl-XL in this membrane integrated conformation to prevent Bcl-XL from binding to Bax or Bak (51). Translocation of Bcl-XL to mitochondria was also observed after UV induced apoptosis, but only after Mcl-1 was degraded. (48) Perhaps in this case, the relevant BH3 protein binds more tightly to Mcl-1 first, initiating Mule-1 dependent degradation. After Mule has displaced the BH3 protein from Mcl-1, it is free to bind to Bcl-XL. Similar to the induced membrane translocation of Bcl-XL by Bad, binding of Bim to Bcl-w induced Bcl-w insertion into the mitochondrial membrane, with neutralization of the anti-apoptosis activity (38). However, in this experiment, Bim was tethered to Bcl-w and thus could not diffuse away; it is unclear if a Bim protein free to dissociate from Bcl-w would provide ongoing inactivation of Bcl-w.
Whatever mechanism leads to inactivation of the anti-apoptotic proteins, co-immunoprecipitation experiments with a series of BH3-only proteins demonstrated that specific BH3-only proteins have different anti-apoptotic proteins as preferred targets (29,52). For examples, Bad bound to both Bcl-2 and Bcl-XL but not to Mcl-1; Bik bound strongly to Bcl-XL but not to Bcl-2 or Mcl-1; and Noxa only bound to Mcl-1 not to Bcl-2 or Bcl-XL. The exceptions were Bim and Puma that bound to all three anti-apoptotic proteins. Selective targeting of anti-apoptosis proteins by BH3-only proteins was also seen in binding studies using specific BH3 peptides (2,3, 29). Therefore, Bim or Puma should neutralize all of the anti-apoptotic proteins and hence be the most effective apoptosis inducers, whereas Bad or Noxa would be partial inducers since each neutralizes only a subset of the anti-apoptotic proteins. These predictions were in fact confirmed by experiments in cells where multiple anti-apoptotic proteins are expressed (29). Interestingly a combination of Noxa and an engineered protein with the Bad BH3 motif induced apoptosis as effectively as Bim alone, suggesting that partial inducers with different binding spectra cooperate to neutralize all anti-apoptotic proteins (29). However, the combination of Noxa and Bad BH3 peptides did not induce cytochrome c release from mitochondria isolated from wt or Bcl-2-expressing FL5.12 cells grown in the presence of IL-3 (3). These data suggest that binding of all anti-apoptotic proteins is not sufficient per se to induce apoptosis in all contexts. Presumably these non-apoptotic cells did not contain activated Bak or Bax as required by the displacement model. Instead, the results are more consistent with the direct activation model whereby the Bax/Bak released from binding to Bcl-2 by Noxa and Bad still requires activation by BH3-only activators.
Another prediction of the displacement model is that reducing the binding affinity of a BH3-only protein for its anti-apoptotic targets will release less activated Bax or Bak and result in less apoptosis. However, a Bid mutant (mIII-1) with reduced binding affinity for Bcl-2 and Bcl-XL is as effective as the wild type Bid in mitochondrial and liposomal permeabilization assays (13,27). Furthermore, a Bid mutant (mIII-3) that retained wild type binding affinity for Bcl-2/Bcl-XL but had reduced affinity for Bax showed impaired pro-apoptotic function in these in vitro assays. Moreover, neither mutant counteracted the protection by Bcl-2 in FL5.12 cells following IL-3 withdraw (52). These results can be most easily explained if two activities of Bid are jointly necessary; binding to Bax activates it, while Bid binding to Bcl-2 prevents Bcl-2 from inhibiting activated Bax. Thus, in the experiments described above Bid-mIII-1 bound to Bax activating it, but did not bind to Bcl-2 to prevent inhibition of Bax, whereas Bid-mIII-3 did not activate Bax because of low binding affinity. In this last case, directly contrary to the prediction of the displacement model, Bid-mIII-3 binding to Bcl-2 did not induce apoptosis (13,52). Consistent with this view, we recently demonstrated that Bax mediated liposome permeabilization induced by tBid-mIII-1 was inhibited by membrane-tethered Bcl-2 (53).
Thus detailed examination of the two competing models informs us that each accounts for some, but not all of the experimental results. Some of the discrepancies are due to different assays and cell lines that are used. In this context, the experiments with mitochondria from either normal hematopoietic cells or their leukemic counterparts are informative (1,3): Bax/Bak mediated cytochrome c release by BH3 only peptides from the former follows the direct activation model, whereas the displacement model best explains the latter. Ultimately, the decision to “make a hole” is dependent on conformational changes in Bax and Bak that are controlled by multiple competing factors that have different affinities and concentrations that will determine the outcome of the regulatory interactions with the pore-forming proteins. Furthermore, the concentrations, affinities and conformations of these regulatory proteins themselves differ by subcellular localization. We therefore propose a model which we term “embedding together” that incorporates these features.
4. Embedding Together
The main distinguishing feature of the embedding together model is the recognition that membrane permeabilization does not occur until Bax and/or Bak insert multiple sequences into the MOM lipid bilayer. Therefore, the final and essential stage at which membrane permeabilization is regulated takes place in the bilayer. Furthermore, because large conformational changes accompany membrane insertion, measurements of protein-protein interactions must be assessed in this environment for these changes to be relevant to the final regulated stage of MOMP. Although both direct activation and displacement models accept that there are multiple interactions between anti- and pro-apoptotic proteins, and the proponents of each have measured the affinities of many of these interactions in vitro (1,37), all these measurements are made with either peptides or proteins in solution, or with truncated proteins binding to peptides chemically coupled to artificial surfaces. These quantitative approaches are useful and can suggest, but not confirm physiologically relevant interactions. However, the affinity and on/off rates for these interactions may differ significantly when proteins are full length, in their correct membrane topology, and different conformations ensue. In cells and tissues, support for direct activation and displacement models has been limited to co-immunoprecipitations, in many cases with over-expressed proteins. There are many important protein:protein interactions in which the binding affinity is too low to survive co-precipitation. To accurately predict the biological importance of competing interactions measured in vitro requires determining binding constants with full length proteins in the right context, in solution and on membranes, where appropriate. For example, for tBid interactions with Bax measurements must be made in solution and for proteins bound to membranes because both proteins are found in cytoplasmic and membrane bound forms. However, Bcl-2 and Bak are constitutively bound to membranes therefore binding interactions should be measured in or on membranes. The embedding together model includes interactions in solution and on membranes as multiple parallel equilibriums in which the relative affinities and rate constants dictate the most likely sequence of events (Figures 3 and 4).
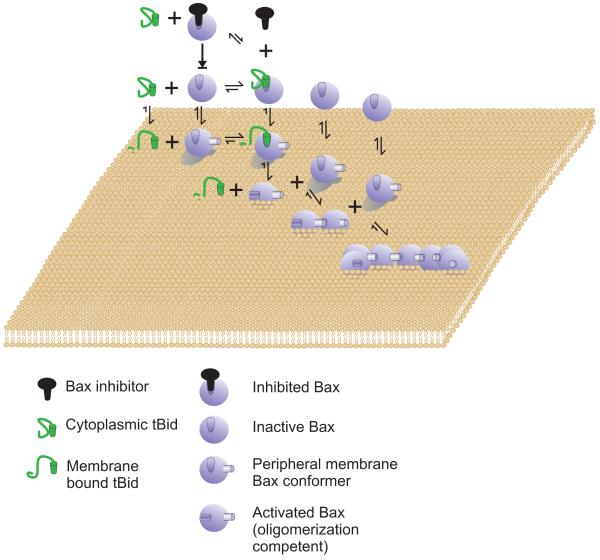
Figure 3.
The Embedding Together Model - Initiation of membrane permeabilization.
Bax is most frequently an inactive cytoplasmic protein. The binding affinity for cytoplasmic Bax for cytoplasmic BH3 proteins (tBid is shown) is low and cytoplasmic Bax is in equilibrium with form that binds with relatively low affinity to the surface of membranes. Therefore, other relatively low affinity interactions that might not survive immunoprecipitation can inhibit Bax in the cytoplasm by shifting the equilibrium away from membrane binding. Bax binds to membranes peripherally concomitant with a conformational change in Bax that increases the affinity of Bax/tBid interaction. In the diagram tBid is the only activator shown however, the model presupposes BH3 only proteins and other proteins that interact with Bax both in the cytoplasm and on the membrane can modulate one or more of the equilibriums shown. In the case of tBid the affinity for membranes is high and binding to membranes results in a conformation change that increases tBid affinity for Bax. The interaction between membrane bound tBid with peripherally membrane bound Bax triggers insertion of Bax into the membrane. The integral membrane form of Bax can recruit additional Bax proteins and oligomerize to permeabilize the outer mitochondrial membrane. The only step that is effectively irreversible is integration into the membrane. The model does not require that any one pro-apoptotic protein perform all of the functions ascribed to tBid, but rather envisions that most will perform only a subset of them. Bak is proposed to function similarly to Bax except that it is expected to bind the different modulators (e.g. tBid) with different affinities, its inactive conformation is constitutively membrane bound and is thus envisioned to be functionally similar to the peripheral membrane form of Bax.
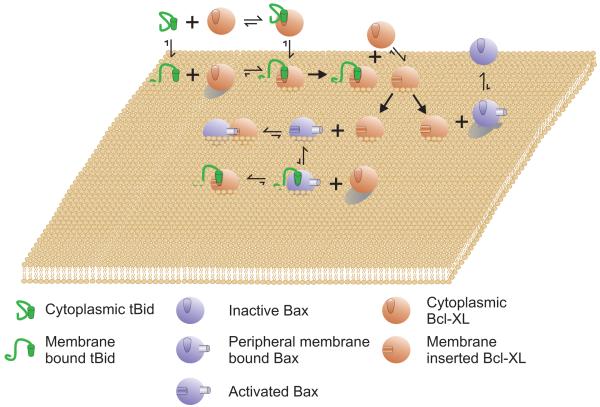
Figure 4.
The Embedding Together Model - Inhibition of membrane permeabilization.
Bcl-XL inhibits both tBid and Bax. Although it may bind to both, the affinity of Bcl-XL for membrane bound tBid is higher than for cytoplasmic tBid. Binding of Bcl-XL to tBid tiggers insertion of Bcl-XL into membranes. Once inserted in membranes Bcl-XL recruits other Bcl-XL proteins to insert into membranes. Bcl-XL/tBid complexes are dead-end structures that neither prevent nor promote apoptosis. Membrane bound Bcl-XL prevents Bax inserting into the membrane by preventing the conformation change in Bax that occurs at the membrane. Membrane bound Bcl-XL also binds to and inhibits the integral membrane (oligomerization competent) form of Bax. The only step that is effectively irreversible for Bcl-XL is integration into the membrane. The model does not require that any one anti-apoptotic protein perform all of the functions ascribed to Bcl-XL, but rather envisions that most will perform only a subset of them. Bcl-2 is proposed to function similarly to Bcl-XL except that it is expected to bind the different modulators (e.g. tBid/Bax) with different affinities. As Bcl-XL competes with Bax/Bak in all steps it has no inactive conformation. Moreover, pro-apoptotic and anti-apoptotic multi-region Bcl-2 family proteins are envisioned as functioning similarly (competitively) except that the anti-apoptotic proteins are unable to support oligomerization and therefore, function as defective constituents of Bax/Bak oligomers inhibiting full oligomerization and membrane permeabilization.
4.1 Regulation of Bax and Bak in membranes
In the embedding model, we propose that Bax and Bak go through multiple, regulated conformational changes before assuming a final conformation (and/or stoichiometry, as discussed below) necessary for pore formation. This conclusion is based on disparate observations from many laboratories exploring different model systems. We demonstrated that when Bax interacts with a membrane surface even without inserting into the membrane it undergoes a transient change in conformation that increases its thermal stability (measured by differential scanning calorimetry) and exposes an amino-terminal epitope (measured by the 6A7 monoclonal antibody (54)) (12). Upon re-isolation Bax reverts to the 6A7 negative conformation, suggesting the two conformers of Bax are in a dynamic equilibrium and that interaction with the membrane surface shifts the equilibrium towards the 6A7 positive conformer sufficiently that it can be trapped by interaction with the antibody. Similarly the 6A7 positive conformer is in equilibrium with a conformer that can insert into membranes but the equilibrium strongly favours the cytoplasmic form (Figure 3). A further interaction with a BH3 molecule like tBid, a specific lipid in the membrane or interaction with another activator (see below) can shift this equilibrium in favour of the conformer that then inserts into membranes. Insertion into membranes is irreversible and another interaction may or may not be required to then “lock in” the 6A7+ conformation. The pro-apoptotic arm of the pathway is illustrated for tBid and Bax not to limit it to these proteins but because these proteins have proven to be more experimentally tractable and therefore there is more experimental evidence to draw upon (Figure 3).
Recently, we demonstrated that when myc −/− cells are exposed to apoptotic stimuli, Bax adopts a membrane topology in which both the alpha 5/6 helices and the alpha-9 carboxyl-terminal tail-anchor inserted into membranes. In this conformation, Bax is in a low molecular weight complex that does not form pores in membranes. The basic topology of this membrane embedded conformation is the same that Bax adapts in its pore forming conformation in myc +/+ cells (10). However, in myc −/− mitochochondrial membranes, Bax does not bind to the 6A7 monoclonal, and thus a myc regulated activity is required for this “activating” conformational change. Together these results strongly suggest that Bax undergoes two independent conformation changes (insertion into membranes, exposure of the 6A7 epitope) prior to oligomerization and permeabilization of membranes. These changes may occur in either order and each is likely to change the affinity of Bax for the different molecules that regulate membrane permeabilization. The embedding together model does not limit the interactions that regulate membrane permeabilization to Bcl-2 family proteins.
Consistent with this view, the action of an intracellular isoform of clusterin also indicates that there are multiple points where membrane-bound Bax conformation is regulated (55). Clusterin binds specifically to 6A7 positive Bax and inhibits subsequent oligomerization. Thus in one of the possible simplified linear sequences of changes in Bax, translocation leads to integration which leads to a second conformational change which leads to oligomerization. The myc dependent factor is required for going from the second to the third step, and clusterin inhibits the transition between third and fourth states. However, because the embedding together model assumes multiple dynamic equilibria, changes at any step propagate to the others. As a consequence, decreasing clusterin expression by silencing RNA enhances spontaneous apoptosis, and over expressing clusterin decreases apoptosis. Clusterin does not inhibit apoptosis in Bax−/− cells or apoptosis mediated by Bak (55). Significantly in an animal model of myc induced tumorigenesis, over expression of clusterin enhances tumor formation, indicating that in the context of deregulated myc with no other exogenous apoptotic stimuli, Bax may exist in a myc-regulated partially activated state that sensitizes cells to apoptosis. Inhibition of this sensitization by clusterin therefore enhances myc mediated transformation.
Recently, other non-Bcl-2 family membrane bound proteins have been reported to regulate Bax activation positively. Bif-1/endophilin B is a 47 kilodalton protein with a cytoplasmic and partly mitochondrial constitutive distribution that interacts with Bax via its amino terminal domain (56). During apoptosis, it forms homodimers in the cytoplasm, then translocates to mitochondria (36). The interacting domain on Bax was not identified, but Bax bound to Bif-1 was not co-immunoprecipitated with 6A7, indicating the possibility of competing binding sites. Over-expression of Bif-1 increased membrane translocation and activation of Bax, but had no affect on the constitutive translocation of the ΔS184 mutation of Bax suggesting a role for Bif-1 in recruiting Bax to MOM. Knockdown of Bif-1 decreased Bax 6A7 conformational change and migration of Bax to mitochondria after exposure to chemotherapy. Finally, embryonic fibroblasts from Bif-1 −/− mice had greatly delayed apoptosis. Thus Bif-1 seems to act early in the series of conformational changes that enable the translocation and activation of Bax. In contrast, several other cytoplasmic proteins have been identified that bind to the C and/or N terminus of Bax to function as a clamp, preventing Bax activation and apoptosis (57).
Two other factors seem to enhance later steps in the pathway. MAP-1 is a 39 kilodalton protein found in a yeast two-hybrid screen for Bax interacting proteins (58). It has a degenerate 8 amino acid BH3 like domain that is required for interaction with Bax, Bcl-2 and Bcl-XL. In cell lines, Bax only interacts with MAP-1 after induction of apoptosis (in assays in which CHAPS is used to lyse cells). When NP-40 is used, apoptosis induction is not necessary but NP-40 changes Bax and may also change MAP-1 conformation to a more apoptotic one (58). Decreasing MAP-1 expression by silencing RNA in MCF-7 cell leads to decreased apoptosis induced by multiple stimuli (59), and was associated with less conformationally changed and membrane-bound Bax. This suggests that the role of MAP-1 is to shift the equilibrium of Bax conformers sufficiently to “lock in” the 6A7 conformational change after it has been induced by an activator molecule.
Apoptosis associated speck-like protein (ASC) is a p53 regulated protein (60) that binds to Bax constitutively in the cytoplasm of untreated cells, but is localized to mitochondria and can be co-immunoprecipitated by 6A7 after induction of apoptosis. Decreasing ASC expression by silencing RNA decreased Bax translocation and the 6A7 conformational change after apoptosis, suggesting ASC and MAP-1 function similarly.
Traditionally p53 has been recognized to affect apoptosis by acting as a transcription factor that binds to DNA in a sequence specific manner with many target genes that regulate apoptosis. However, recent data from several groups has indicated that p53 has an additional and possibly direct role in regulating mitochondrial physiology relevant to apoptosis by directly interacting with Bcl-2 family members that mediate MOMP.
The first evidence of this direct role was suggested by a report that a fraction of p53 localizes to the mitochondria in tumour cell lines undergoing p53 dependent apoptosis. The relevance of mitochondrial localization was supported by a report that the increased apoptotic activity of a natural polymorphic variant of p53 was due to increased nuclear export and mitochondrial localization (61). The equivalent transcriptional activation properties of the two variants indicated a clear disassociation between apoptotic and transcriptional activity. Another naturally occurring mutation in p53 underscores the separability of these functions even more dramatically: the L25Q/W26S mutant is transcriptionally inactive, localized to the nucleus and does not mediate apoptosis. However, when this mutant is retained in the cytoplasm by blocking nuclear import, it localizes to the mitochondria and mediates apoptosis (62).
There is controversy as to the direct target of p53 at mitochondria. Leu et al (2004)(63) reported co-immunoprecipitation of p53 with endogenous Bak but not Bax (nor Bcl-xl as noted below), interaction of p53 synthesized by in vitro translation with Bak not Bax (nor Bcl-xl) and binding of recombinant GST-p53 to Bak synthesized by in vitro translation. Finally, addition of p53 to enriched mitochondria caused oligomerization of Bak and release of cytochome c from Bak +/+ mitochondria but cytochrome c was not released from Bak −/− mitochondria. By contrast Chipuk et al (2004)(62) demonstrated that isolated cellular p53 or recombinant p53 activated recombinant Bax to release cytochrome c from mitochondria enriched cellular fractions. A Bax requirement for the p53 activity in the mitochondria is at odds with the result from Leu et al since the mitochondria from mouse liver contain Bak (64). Furthermore p53 caused oligomerimization of Bax in liposomes (as detected by BMH cross linking) and permeabilized liposomes. Despite this demonstrated function of p53 to activate Bax in highly purified systems, no direct interaction between Bax and p53 was detected by co-immunoprecipitation. These seemingly disparate results may be partly explained by different cellular sources of mitochondria in which either Bak or Bax are dominant (eg hepatic vs fibroblast mitochondria) and the fact that post translational modifications of p53 such as mono-ubiquination (61) and phosphorylation (65) may allow p53 to bind differentially to Bax, Bak or Bcl-XL.
Thus, the recent identification of multiple mitochondria associated BH3 and non-BH3 proteins that bind to and enhance the activation of Bax is congruent with membrane binding being an important step in the regulation of Bax. Consistent with this hypothesis, while Bax and tBid can permeabilize liposomes with no other components necessary, a trypsin sensitive factor promotes cytochrome c release from mitochondria (66).
Finally, experiments with viral suppressors of apoptosis also indicate that there are multiple stages at which Bax conformational changes can be regulated. The cytomegalovirus product vMIA is an inhibitor of apoptosis that constitutively localizes to the outer mitochondrial membrane (67) where regulates both mitochondrial bioenergetics (68) and prevents mitochondrial outer membrane permeabilization by Bax (69). Paradoxically, this happens after vMIA recruits Bax from the cytosol to mitochondria causing full membrane insertion as well as the preliminary steps of oligomerization (68). Furthermore, mitochondrial vMIA was able to mediate the membrane translocation of the Bax Δ1-37 mutant that does not otherwise translocate to mitochondria after apoptotic stress. It appears that vMIA sequesters Bax and prevents permeabilization at the mitochondrial membrane after it induces insertion and oligomerization of the protein. The sequestered Bax cannot be activated by tBid or other apoptotic stimuli again suggesting that that there are multiple, regulated conformational changes during Bax activation. The surprising aspect of the vMIA mechanism of action is that it apparently enhances the two early steps, but inhibits the final step.
There is also evidence that Bak undergoes multiple conformational changes during activation. Both endogenous and viral proteins have been identified that inhibit Bak activation. Cheung et al (41) reported that the second isoform of the voltage dependent anion ion channel (VDAC-2) binds constitutively to Bak in outer mitochondrial membranes. VDAC-2 over expression prevented the oligomerization and spontaneous conformational change in Bak. As expected, the absence of VDAC-2 in embryonic fibroblasts rendered them more susceptible to apoptosis. Presumably VDAC-2 competes with tBid and other BH3 protein(s) for the BH3 binding domain of Bak as VDAC-2 releases Bak if tBid is present. Moreover, a tBid mutant that does not bind to Bak does not release Bak from VDAC-2, suggesting that tBid interacts directly with Bak instead of VDAC-2. Bak bound to VDAC-2 may be in an inactive conformation since lysis of cells with NP-40 (expected to activate Bak) but not CHAPS reduced the co-immunoprecipitation of Bak with VDAC-2.
In the absence of VDAC-2, a portion of Bak undergoes a conformational change detected by altered protease sensitivity. However, this change was not detectable by BMH cross-linking, and only a fraction of the Bak oligomerizes into larger complexes. This suggests that full activation of Bak requires additional conformational changes, and that VDAC-2 inhibits a step prior to oligomerization. It also suggests that VDAC-2 ‘restrains’ only a small fraction of the Bak in the cell, a result inconsistent with the displacement model. Similar to Bax, viral inhibitors of apoptosis have been identified that bind to Bak. The vaccinia F1L protein suppresses apoptosis during viral infection and co-immunoprecipitates in cells with Bak, but not Bax, Bcl-2, Bcl-xl or Mcl-1 (70,71). In mitochondria derived from mouse embryonic fibroblasts F1L prevented the tBID induced conformational change as detected by an epitope-specific monoclonal antibody, Bak oligerimization, crosslinking of Bak by BMH, and release of cytochrome c. In Jurkat and HeLa cells there is a small fraction of Bak that is oligomerized without any distinct apoptotic stimulus. However, it is unclear how ‘normal’ or ‘healthy’ these cells are as transformation makes cells ‘addicted’ to inhibitors of apoptosis. Expression of F1L protein did not noticeably decrease this “pre-activated” fraction. Thus F1L appears to inhibit Bak by sequestration in normal cells. In this sense, F1L forms a dead-end complex with “incompletely” activated Bak in membranes, similar to vMIA that forms a dead-end complex with Bax in membranes.
Taken together, these studies show that there are multiple conformations of Bax and Bak, and several endogenous and viral proteins have different affinities for these states. Unlike the direct activation and displacement models, the embedding together model would expect multiple conformational states that could be regulated by factors that would either enhance or retard these transitions.
4.2 Bcl-2 family protein interactions lead to ‘activation’
Another feature of the embedding together model is based on the observation that, although composed of distinct sequences, many of the Bcl-2 family proteins are similar structurally. Therefore, we predict that they form similar (although not identical) structures in membranes and that the structural consequences of interactions between Bcl-2 family proteins have more in common than implied by either the direct activation or displacement models. We suggest that binding with BH3-only proteins does not simply displace binding interactions with either other BH3 proteins or activated Bax/Bak. Based on our data, we postulate that BH3 proteins cause conformational changes that can activate both pro- and anti-apoptotic multi-domain Bcl-2 family members. Thus, we have reported that when tBid binds to Bcl-2 in mitochondria it causes a conformational change such that Bcl-2 helices 5 and 6 which are normally cytoplasmic become embedded in the membrane (72). This conformational change appears necessary for at least a subset of anti-apoptotic Bcl-2 functions because for several Bcl-2 mutants embedding of helix 5 in the membrane is strongly correlated with prevention of apoptosis in vivo (11) and in vitro (11,32). However, there was no correlation between Bax binding in solution and prevention of apoptosis. Persistent binding of tBid to the conformation altered, functional wild type Bcl-2, was not observed consistent with the “kiss and run” model proposed for Bax activation by tBid (73). Thus it is possible that for both Bcl-2 and Bax, embedding in the membrane changes the conformation of the proteins thereby reducing the affinity for BH3 protein binding and shifting the equilibrium towards releasing tBid. The “vacant” binding site on Bcl-2 may now be ready to bind to Bax or Bak. A further prediction of our model is that the affinity of Bcl-2 for Bax (and other BH3 containing proteins) differ if measured in the “pre-activated conformation” in solution and without the complete carboxyl-terminal insertion sequence (1,37) compared to the physiologic, activated conformation in membranes. In contrast to our results with tBid (and Bim peptide), Bad peptide has been reported to cause the insertion and integration of Bcl-XL into mitochondrial membranes concomitant with inactivation of Bcl-XL. However, in these experiments there is generally a large excess of peptide. Therefore, it will be important to examine the effects of the binding of BH3 proteins to multiple anti-apoptotic proteins using full length proteins in a simplified in vitro system to further examine the relationship between conformation and function.
According to the embedding model the induced conformational change that accompanies membrane integration of helix 5-6 allows Bcl-2/XL to bind to membrane embedded Bax/Bak monomers/dimers, preventing further oligomerization of Bax/Bak (Figure 4). These anti-apoptotic proteins themselves do not form large oligomers in membranes in the activated conformation. Thus Bcl-2/XL act as oligomerization defective forms of Bax/Bak. In this context, it is interesting to note that activated Bcl-2/Bcl-XL and Bax all insert parts of helix 5,6 and 9 into the membrane, are all initially found as monomers or dimers in the membrane (10,51), and can form ion conducting channels in artificial lipid bilayers that requires the alpha 5-6 helices (74). However, when the pore size is assayed with larger molecules, the pores formed by Bcl-XL are considerably smaller than those formed by large Bax oligomers (Lin et al, in preparation). This suggests a simple physical model whereby the oligomerized proteins form the borders of an aqueous channel in the lipid bilayer, and the channel size is a function of the number of monomers in the channel wall. Precise measurement of the stoichiometry of Bax oligomers is therefore required to understand the structure of this pore.
We hypothesize that the conformational change that leads to large detergent resistant oligomer formation by Bax/Bak is due to domain swapping, perhaps of alpha 5-6 helices among adjacent monomers. Accordingly, the anti-apoptotic proteins cannot mediate large pore formation because the conformational changes these proteins undergo in membranes are sufficiently different. Mapping the precise binding surfaces in membranes between monomers in Bax oligomers that mediate domain swapping, as well as in Bcl-2/Bcl-XL homodimers and Bcl-2/Bcl-XL-Bax heterodimers that cannot form large oligomers is critical to understanding this process (32,53). In this context, it is interesting to note that Bcl-XL and Bcl-2 can be converted to pro-apotoptic proteins like Bax by caspase cleavage at a site that removes the BH4 region and the unstructured loop (75,76). Similarly, binding of the orphan steroid receptor nur77 converts Bcl-2 to a pro-apoptotic protein (77).We would therefore predict these changes result in an altered Bcl-2/Bcl-XL where domain swapping compatible with oligomerization occurs in membranes, and that other mutants of Bcl-2/XL that could swap alpha 5-6 helices similarly would also promote apoptosis. Conversely, Bax/Bak mutants that cannot form large oligomers by domain swapping would not induce apoptosis.
In our model, we also acknowledge that the cytoplasmic form of Bcl-XL and Bcl-2 before helix 5/6 insertion retain other important anti-apoptotic functions such as sequestering BH3 only proteins (Figure 4). It is possible that sequestering pro-apoptotic BH3 molecules may be the ancestral function of anti-apoptotic proteins, as it is preserved in C. elegans, where ced-9 prevents apoptosis by sequestering ced-4. In this case, the BH-3 protein egl-1 displaces ced-4, thereby acting as a “sensitizer” according to the direct activation model. However, ced-9 does not prevent Bax mediated MOMP (78). It is possible therefore that ced-9 does not go through the same conformational change the after binding to egl-1, as Bcl-2 does after binding tBid. Thus the conformational change induced by BH3 protein binding that results in anti-apoptotic proteins binding to the membrane embedded forms of Bax/Bak, may be a more recent and potent boost to function necessitated by the evolution of multi-domain pore-forming pro-apoptotic proteins in metazoans (79). The relative proportion of these two binding activities (BH3 protein binding with and without the embedding in membranes conformational change) may mediate the variation in overall anti-apoptotic function between the different anti-apoptotic proteins in cells (80).
One significant consequence of the recognition of multiple, regulated conformations of both pro- and anti-apoptotic members of the Bcl-2 family that determine transition from inactive to active forms is that these represent novel targets for drug development. Several agents that target the known sites of interaction between pro- and anti-apoptotic proteins (e.g. BH3 mimetics) are already in clinical development (81), and would be predicted to be active by all three of the models that we have discussed here. However, the embedded together model would also predict that allosteric regulators of the transitions between conformational states represent attractive “drugable” targets that may offer greater specificity. Mapping the surfaces of these conformation-specific domains in the physiologically relevant context of membranes is therefore an important priority (32, 53, 82).
5. Conclusion
Some years ago a prescient apoptosis researcher (83) noted that despite the enormous amount of progress (and publications) on apoptosis that have appeared since the original description of the phenomenon in 1972 (84), we are still at the stage of phenomenological description of the mechanisms involved. Since then, the network of combinatorial interactions between different BH3 proteins, and multi-domain anti- and pro-apoptotic Bcl-2 family members has been described leading to two current, testable models that each incorporate some of the observations presented here. We have postulated how recent findings from our laboratories and others can be used to reconcile and integrate aspects of these previous models into a new model for the molecular mechanism of MOMP that recognizes the relevance of multiple interactions between proteins and membranes to control conformational switches. The utility and heuristic value of our model will be determined by multiple novel predictions as noted above, and by pointing out relevant physiological interactions that can be measured to allow progress in quantitative modeling of the overall apoptotic process (e.g. 85) that would pass the critical eye of a critical amateur engineer fixing a transistor radio (83)!
References
- 1.Letai A, Bassik MC, Walensky LD, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Certo M, Del Gaizo Moore V, Nishino N, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carton PF, Gallenne T, Bougras B, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Walensky LD, Kung AL, Escher I, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 8.Cartron PF, Arokium H, Oliver L, et al. Distinct domains control the addressing and the insertion of Bax into mitochondria. J Biol Chem. 2005;280:10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 9.Terrones O, Antonsonn B, Yamaguchi H, et al. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- 10.Annis MG, Soucie EL, Dlugosz PJ, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dlugosz PJ, Billen LP, Annis MG, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yethon JA, Epand R, Leber B, et al. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- 13.Desagher S, Osen-Sand A, Nichols A, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskes R, Desagher S, Antonsonn B, et al. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Terradillos O, Montessuit S, Huang DC, et al. Direct addition of BimL to mitochondria does not lead to cytochrome c release. FEBS Lett. 2002;522:29–34. doi: 10.1016/s0014-5793(02)02871-5. [DOI] [PubMed] [Google Scholar]
- 18.Marani M, Tenev T, Hancock D, et al. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada H, Quearry B, Ruiz-Vela A, et al. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 21.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 22.Zha J, Wewiler S, Oh KJ, et al. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Han Z, Wyche JH, et al. Helix 6 of tBid is necessary but not sufficient for mitochondrial binding activity. Apoptosis. 2003;8:277–289. doi: 10.1023/a:1023676906857. [DOI] [PubMed] [Google Scholar]
- 24.Kim TH, Zhao Y, Ding WX, et al. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol Biol Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong XM, Choi J, Franzin CM, et al. Conformation of membrane-associated proapoptotic tBid. J Biol Chem. 2004;279:28954–28960. doi: 10.1074/jbc.M403490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh KJ, Barbuto S, Meyer N, et al. Conformational changes in BID, a pro-apoptotic BCL-2 family member, upon membrane binding. A site-directed spin labeling study. J Biol Chem. 2005;280:753–767. doi: 10.1074/jbc.M405428200. [DOI] [PubMed] [Google Scholar]
- 27.Oh KJ, Barbuto S, Pitter K, et al. A membrane-targeted BID BH3 peptide is sufficient for high potency activation of BAX in vitro. J Biol Chem. 2006 Sep 20; doi: 10.1074/jbc.M602341200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Grinberg M, Sarig R, Zaltsman Y, et al. BID Homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem. 2002;277:12237–12245. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Yin XM, Chao DT, et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 31.Ruffolo SC, Shore GC. BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem. 2003;278:25039–25045. doi: 10.1074/jbc.M302930200. [DOI] [PubMed] [Google Scholar]
- 32.Tan C, Dlugosz PJ, Peng J, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764–14775. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Wang HG. Bcl-XL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J Biol Chem. 2002;277:41604–41612. doi: 10.1074/jbc.M207516200. [DOI] [PubMed] [Google Scholar]
- 34.Mikhailov V, Mikhailova M, Degenhardt K, et al. Association of Bax and Bak homooligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem. 2003;278:5367–5376. doi: 10.1074/jbc.M203392200. [DOI] [PubMed] [Google Scholar]
- 35.Pierrat B, Simonen M, Cueto M, et al. SH3GLB, a new endophilin-related protein family featuring an SH3 domain. Genomics. 2001;71:222–234. doi: 10.1006/geno.2000.6378. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi Y, Karbowski M, Yamaguchi H, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–9382. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson-Annan J, O’Reilly LA, Crawford SA, et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuconati A, Mukherjee C, Perez D, et al. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leu JI, Dumont P, Hafey M, et al. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 41.Cheng EH, Sheiko TV, Fisher JK, et al. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 42.Oltvai ZN, Milliman CL, Korsmeyer SJ, et al. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 43.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 44.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 45.Antonsson B, Montessuit S, Sanchez B, et al. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 46.Yang E, Zha J, Jockel J, et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 47.Letai A, Sorcinelli MD, Beard C, et al. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–249. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Nijhawan D, Fang M, Traer E, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong O, Gao W, Dhu F, et al. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Warr MR, Acoca S, Liu Z, et al. BH3-ligand regulates access of MCL-1 to its E3 ligase. FEBS Lett. 2005;579:5603–5608. doi: 10.1016/j.febslet.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Jeong SY, Gaume B, Lee YJ, et al. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 2004;23:2146–2155. doi: 10.1038/sj.emboj.7600225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang K, Yin XM, Chao DT, et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 53.Peng J, Tan C, Roberts GJ, et al. tBid elicits a conformational alteration in membrane-bound Bcl-2 such that it inhibits bax pore formation. J Biol Chem. 2006 doi: 10.1074/jbc.M608303200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Kim JK, Edwards CA, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 56.Cuddeback SM, Yamaguchi H, Komatsu K, et al. Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J Biol Chem. 2001;276:20559–20565. doi: 10.1074/jbc.M101527200. [DOI] [PubMed] [Google Scholar]
- 57.Schinzel A, Kaufmann T, Borner C. Bcl-2 family members: integrators of survival and death signals in physiology and pathology. Biochim Biophys Acta. 2004;1644:95–105. doi: 10.1016/j.bbamcr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Tan KO, Tan KM, Chan SL, et al. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J Biol Chem. 2001;276:2802–2807. doi: 10.1074/jbc.M008955200. [DOI] [PubMed] [Google Scholar]
- 59.Tan KO, Fu NY, Sukumaran SK, et al. MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci U S A. 2005;102:14623–14628. doi: 10.1073/pnas.0503524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohtsuka T, Ryu H, Minamishima YA, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 61.Dumont P, Leu JI, Della Pietra AC, et al. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 62.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 63.Leu JI, Dumont P, Hafey M, et al. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 64.Perfettini JL, Kroemer RT, Kroemer G. Fatal liaisons of p53 with Bax and Bak. Nat Cell Biol. 2004;6:386–388. doi: 10.1038/ncb0504-386. [DOI] [PubMed] [Google Scholar]
- 65.Park BS, Song YS, Yee SB, et al. Phospho-ser 15-p53 translocates into mitochondria and interacts with Bcl-2 and Bcl-xL in eugenol-induced apoptosis. Apoptosis. 2005;10:193–200. doi: 10.1007/s10495-005-6074-7. [DOI] [PubMed] [Google Scholar]
- 66.Roucou X, Rostotseva T, Montessuit S, et al. Bid induces cytochrome c-impermeable Bax channels in liposomes. Biochem J. 2002;363:547–552. doi: 10.1042/0264-6021:3630547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldmacher VS, Bartle LM, Skaletskaya A, et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poncet D, Pauleau AL, Szabadkai G, et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J Cell Biol. 2006;174:985–996. doi: 10.1083/jcb.200604069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnoult D, Bartle LM, Skaletskaya A, et al. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc Natl Acad Sci U S A. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wasilenko ST, Banadyga L, Bond D, et al. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J Virol. 2005;79:14031–14043. doi: 10.1128/JVI.79.22.14031-14043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Postigo A, Cross JR, Downward J, et al. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ. 2006;13:1651–1662. doi: 10.1038/sj.cdd.4401853. [DOI] [PubMed] [Google Scholar]
- 72.Kim PK, Annis MG, Dlugosz PJ, et al. During apoptosis bcl-2 changes membrane topology at both the endoplasmic reticulum and mitochondria. Mol Cell. 2004;14:523–529. doi: 10.1016/s1097-2765(04)00263-1. [DOI] [PubMed] [Google Scholar]
- 73.Korsmeyer SJ, Gross A, Harada H, et al. Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb Symp Quant Biol. 1999;64:343–350. doi: 10.1101/sqb.1999.64.343. [DOI] [PubMed] [Google Scholar]
- 74.Schendel SL, Montal M, Redd JC. Bcl-2 family proteins as ion-channels. Cell Death Differ. 1998;5:372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- 75.Cheng EH, Kirsch DG, Clem RJ, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 76.Clem RJ, Cheng EH, Karp CL, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 78.Delavani P, Adrain C, Taylor RC, et al. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 79.Aouacheria A, Brunet F, Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol Biol Evol. 2005;22:2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- 80.Fiebig A, Zhu W, Hollerbach C, et al. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldsmith KC, Liu X, Dam V, et al. BH3 peptidomimetics potently activate apoptosis and demonstrate single agent efficacy in neuroblastoma. Oncogene. 2006;25:4525–4533. doi: 10.1038/sj.onc.1209489. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z, Lapolla SM, Annis MG, et al. Bcl-2 homodimerization involves two distinct binding surfaces, a topographic arrangement that provides an effective mechanism for Bcl-2 to capture activated Bax. J Biol Chem. 2004;279:43920–43928. doi: 10.1074/jbc.M406412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazebnik Y. Can a biologist fix a radio?--Or, what I learned while studying apoptosis. Cancer Cell. 2002;2:179–182. doi: 10.1016/s1535-6108(02)00133-2. [DOI] [PubMed] [Google Scholar]
- 84.Kerr JF, Wylie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janes KA, Albeck JG, Gaudet S, et al. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]