Abstract
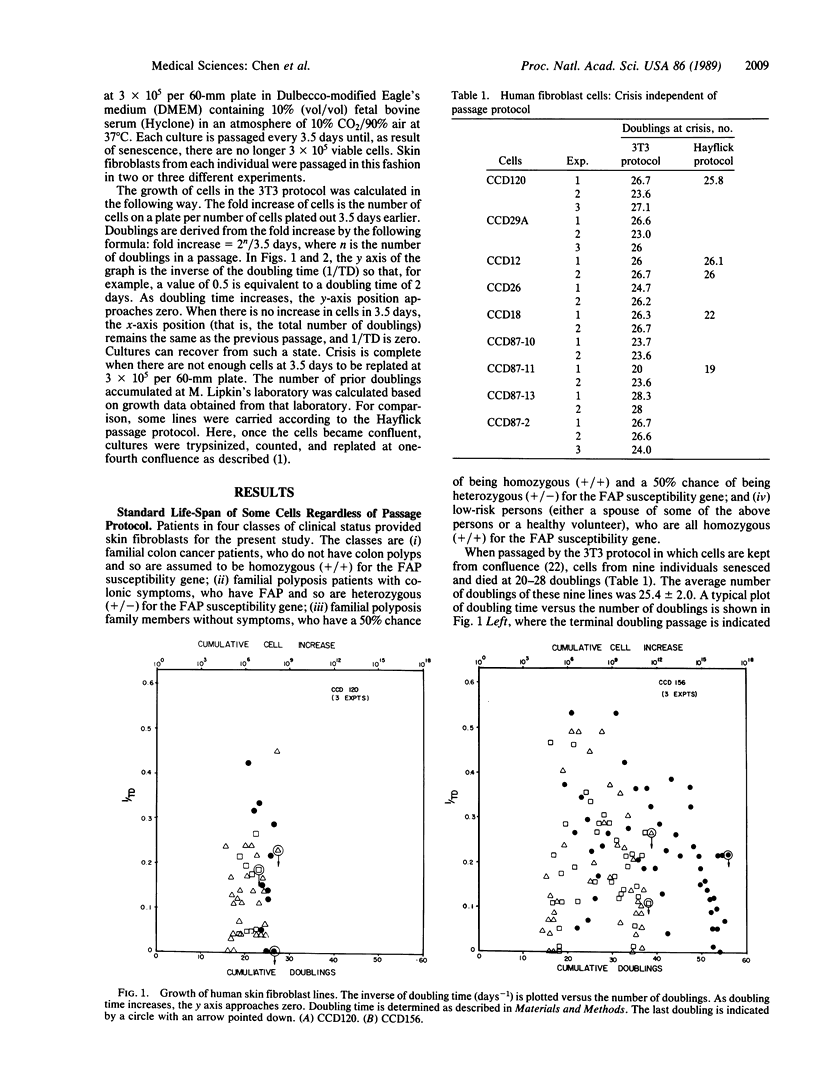
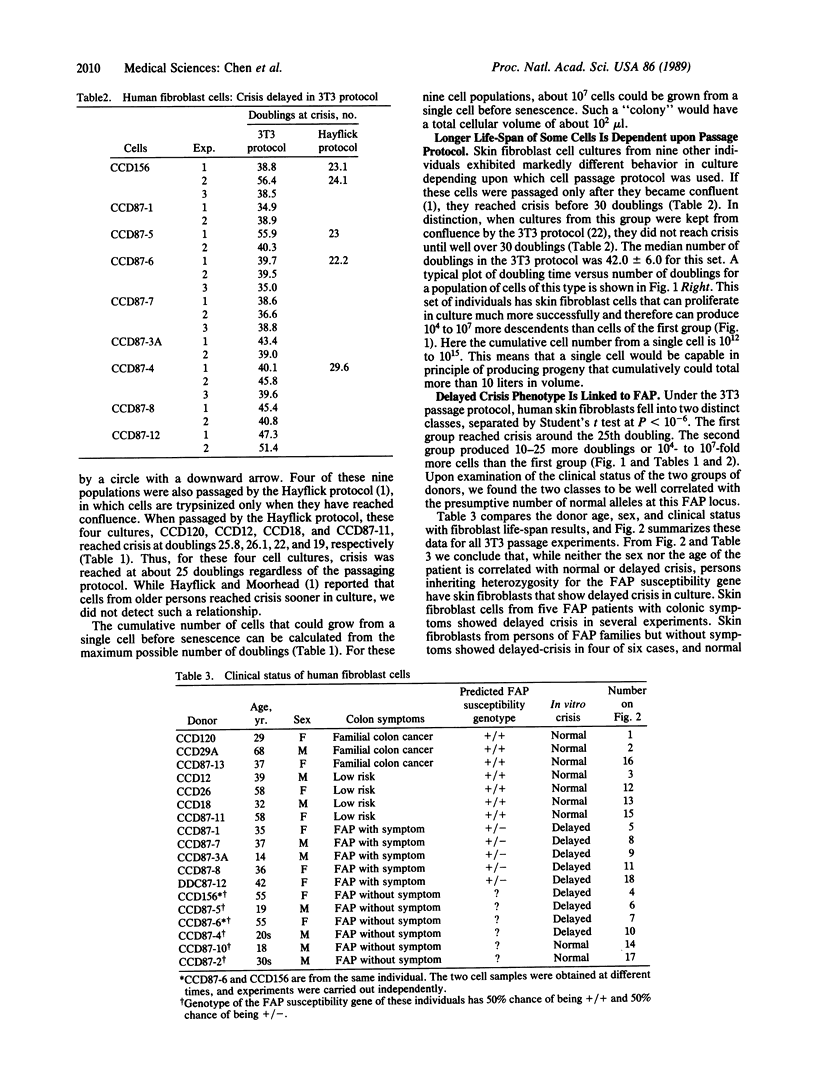
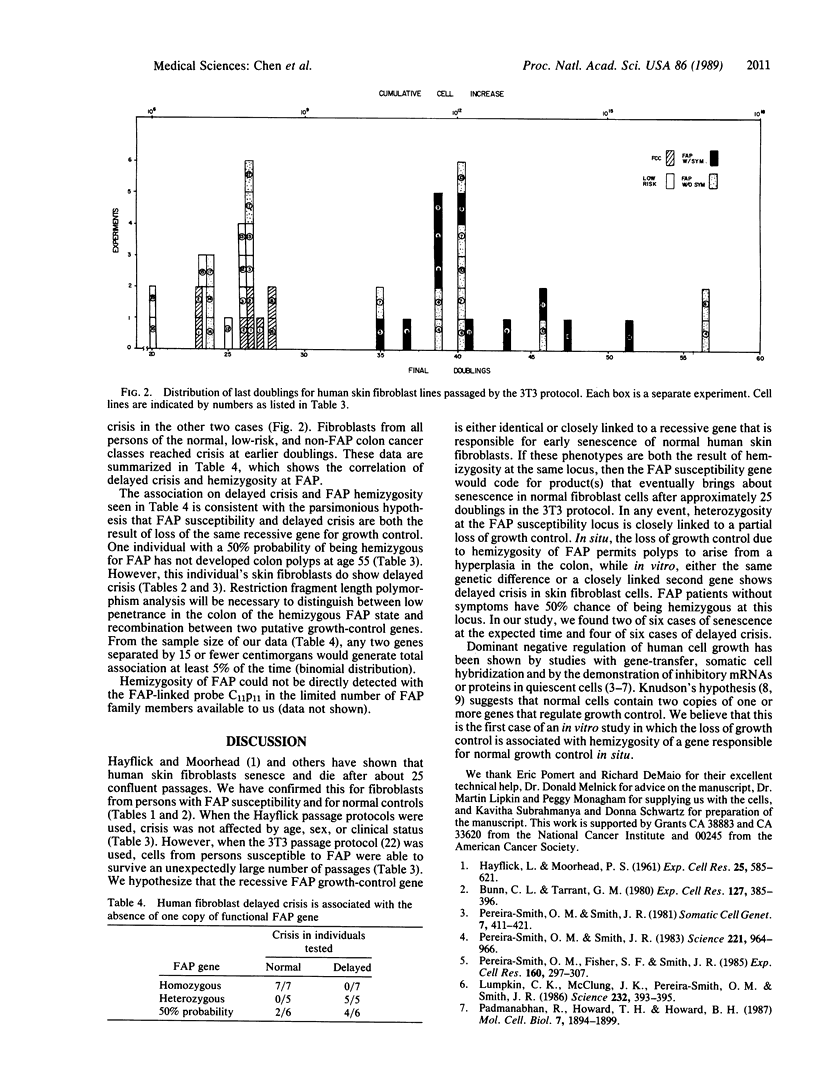
Normal human fibroblast cells have not been reported to escape crisis--that is, they die after about 24 doublings in culture. We have been studying the growth properties of skin fibroblast cells from persons in families with familial adenopolyposis of the colon (FAP). An individual hemizygous at the FAP locus will develop hyperplasia of the colonic epithelium followed by colonic polyps, both at an early age. Polyps themselves still retain a single functional FAP allele. A mutation or deletion in this allele in a polyp is hypothesized to lead to further loss of growth control; thus, a tumor is formed. We found that the in vitro life-span of skin fibroblast cells from FAP individuals and from some asymptomatic children were markedly extended when compared with normal individuals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Bunn C. L., Tarrant G. M. Limited lifespan in somatic cell hybrids and cybrids. Exp Cell Res. 1980 Jun;127(2):385–396. doi: 10.1016/0014-4827(80)90443-7. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Friedman E., Gillin S., Lipkin M. 12-O-Tetradecanoylphorbol-13-acetate stimulation of DNA synthesis in cultured preneoplastic familial polyposis colonic epithelial cells but not in normal colonic epithelial cells. Cancer Res. 1984 Sep;44(9):4078–4086. [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. J., Olschwang S., Monpezat J. P., Lefrançois D., Jagelman D., Petrelli N. J., Thomas G., Feinberg A. P. Concerted nonsyntenic allelic loss in human colorectal carcinoma. Science. 1988 Aug 19;241(4868):961–965. doi: 10.1126/science.2841761. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Pereira-Smith O. M., Smith J. R. Existence of high abundance antiproliferative mRNA's in senescent human diploid fibroblasts. Science. 1986 Apr 18;232(4748):393–395. doi: 10.1126/science.2421407. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Sasaki M., Sugio K., Sato C., Iwama T., Ikeuchi T., Tonomura A., Sasazuki T., Miyaki M. Loss of constitutional heterozygosity in colon carcinoma from patients with familial polyposis coli. Nature. 1988 Jan 21;331(6153):273–277. doi: 10.1038/331273a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Howard T. H., Howard B. H. Specific growth inhibitory sequences in genomic DNA from quiescent human embryo fibroblasts. Mol Cell Biol. 1987 May;7(5):1894–1899. doi: 10.1128/mcb.7.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Fisher S. F., Smith J. R. Senescent and quiescent cell inhibitors of DNA synthesis. Membrane-associated proteins. Exp Cell Res. 1985 Oct;160(2):297–306. doi: 10.1016/0014-4827(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Evidence for the recessive nature of cellular immortality. Science. 1983 Sep 2;221(4614):964–966. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Expression of SV40 T antigen in finite life-span hybrids of normal and SV40-transformed fibroblasts. Somatic Cell Genet. 1981 Jul;7(4):411–421. doi: 10.1007/BF01542986. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


