Abstract
The free radical reaction of polyunsaturated fatty acids with molecular oxygen leads to hydroperoxides as the first stable products. From linoleic acid the two conjugated diene hydroperoxides at carbons 9 and 13 were considered the only primary products until the recent discovery of the bis-allylic 11-hydroperoxide. The 11-carbon is the site of the initial hydrogen abstraction, and the 11-hydroperoxide is formed without isomerization of the 9,10 and 12,13 cis double bonds. In the autoxidation reaction bis-allylic hydroperoxides are obtained only in the presence of an efficient antioxidant, for example, α-tocopherol. The antioxidant functions as a hydrogen atom donor, necessary to trap the fleeting bis-allylic peroxyl radical intermediate as the hydroperoxide. Understanding of the mechanism of formation of bis-allylic hydroperoxides has led to increased appreciation of the central role of the intermediate peroxyl radical in determining the outcome of lipid peroxidation.
Keywords: bis-allylic, hydroperoxide, peroxyl radical, linoleic acid, antioxidant, autoxidation
Introduction
The mechanism of the autoxidation of polyunsaturated fatty acids as a radical chain reaction was established more than half a century ago (e.g., refs. [1–4]). Soon after followed the elucidation of the role of antioxidants as agents that break the radical chain [5], and the identification of secondary transformation products of the primary hydroperoxides through enzymatic and non-enzymatic transformations, the latter an active area of research that continues to unravel novel enzymes and products (e.g., refs [6–10]).
Within the process of lipid peroxidation three partially overlapping phases of radical reactions can be distinguished: initiation, propagation, and termination (Fig. 1). In the initiation phase reactions prevail that form and expand the pool of radicals. During the propagation phase the chain reaction between fatty acid radicals and molecular oxygen leads to the formation and accumulation of the primary hydroperoxide products. Reactions between radicals leading to non-radical products dominate during the termination phase.
Fig. 1. Three phases of the autoxidation process.
Following the initial hydrogen abstraction (initiation) the radical cycles between the fatty acyl (pentadienyl) radical L• and the peroxyl radical (red). As the net result of the propagation phase, oxygen and the fatty acid are fed into the cycle to give the hydroperoxide as the product (blue). Note that the peroxyl radical (red) goes on to give the hydroperoxide (blue) upon hydrogen atom transfer from a new molecule of fatty acid (LH, blue) which in turn becomes a pentadienyl radical (L•, red), ready to pick up oxygen to form the next peroxyl radical. Termination of the process occurs when either two radicals react giving non-radical products, or when an antioxidant (AH) reduces the peroxyl to a hydroperoxide while being transformed into a stable radical (A•). For sake of simplicity, only the reacting pentadiene is shown in this and the following figures.
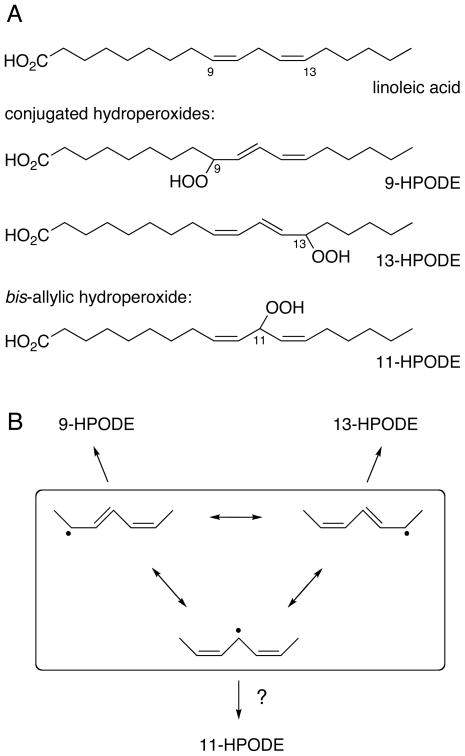
In this review an emphasis will be put on the discovery and mechanism of formation of the long sought-after bis-allylic 11-hydroperoxide of linoleic acid1 autoxidation. The formation of 9-, 11-, and 13-hydroperoxides is expected based on the three mesomeric structures for the pentadienyl radical of linoleic acid that implicate localization (and therefore reactivity with O2) of the radical at carbons 9, 11, and 13 (Fig. 2). But in contrast to the two well-known 9- and 13-hydroperoxides that are easily identified in autoxidation reactions, the 11-hydroperoxide as the third presumptive primary product had defied isolation and proven elusive for decades [11]. It turned out that the instability of the intermediate bis-allylic peroxyl radical is the crucial factor why this hydroperoxide has proven so difficult to identify and isolate. Therefore, in order to be able to better explain the mechanistic basis for formation of the bis-allylic hydroperoxide, the concept of radical reactions as reversible and competing reactions will be introduced in the following section. This will set the stage for understanding of how the rate constants of different and competing reactions are a determining factor for the product outcome during lipid autoxidation.
Fig. 2. Conjugated and bis-allylic hydroperoxides.
(A) 9-HPODE and 13-HPODE are the well-known conjugated hydroperoxides formed in the autoxidation of linoleic acid. Formation of 11-HPODE was presumed based on the mesomeric structures of the intermediate pentadienyl radical (B) but remained an elusive product until its first isolation in 2000 [25].
Role of the peroxyl radical in the autoxidation of fatty acids
The initial hydrogen abstraction from the pentadiene is essentially irreversible and commits the fatty acid to a reaction with oxygen one way or other. In the case of linoleic acid, hydrogen abstraction occurs at the methylene group (C11) in between the 9,10cis and 12,13cis double bonds and gives a fatty acid radical (pentadienyl radical) that is delocalized over carbons 9 through 13 (Fig. 3, step 1) [12]. Addition of oxygen to the carbon-centered radical is “diffusion-controlled” meaning that it is exceedingly fast, and the rate of reaction is only limited by how fast oxygen can get to the fatty acid radical; but there is essentially no energy barrier for formation of the peroxyl radical (step 2) [13]. (This is true for addition of oxygen to all three carbons 9, 11, and 13, but in this section we will only consider formation of the conjugated hydroperoxides 9-HPODE and 13-HPODE.) The reaction with oxygen is reversible, such that the peroxyl radical can lose O2 and revert back to the carbon radical. This reaction of the peroxyl radical is called β-fragmentation since the bond that breaks is in β-position to where the radical is located [14]. Still, depending on the reaction conditions, peroxyl radicals have a considerable half life which ranges from milliseconds to seconds, opening up the possibility to follow different reaction pathways [15]. Competing with β-fragmentation is the trapping of the peroxyl radical as the hydroperoxide product by transfer of a hydrogen atom, i.e., the peroxyl radical abstracts a hydrogen atom from an available bis-allylic methylene group (step 3). This is the rate-limiting (i.e., slowest) step of the radical reactions during autoxidation [12]. Nevertheless, it is the dominating reaction for the peroxyl radical during the early stages of autoxidation, and it is the step that propagates the chain by passing the radical on to the next molecule of fatty acid.
Fig. 3. A central role for the peroxyl radical in fatty acid autoxidation.
The peroxyl radical is a rather stable entity with a considerable half-life (milliseconds to seconds), and accordingly, it has several possibilities for further reaction; see the main text for further explanations. Of interest for the formation of bis-allylic hydroperoxides is the competition between β-fragmentation of the peroxyl and its reduction to the hydroperoxide, see Fig. 4.
The role of an antioxidant during lipid peroxidation is to reduce the peroxyl radical to the hydroperoxide before it can propagate the radical chain (Fig. 3, step 4) [5, 16, 17]. Good antioxidants like α-tocopherol (vitamin E) are efficient hydrogen donors and are transformed into rather stable radicals that do not initiate or propagate a radical chain reaction (although there are exceptions where the tocopheroxyl radical can become a pro-oxidant [18–20]). Note that antioxidants do not react directly with molecular oxygen or with fatty acid radicals since reaction of the carbon radical with molecular oxygen is several orders of magnitude faster than hydrogen transfer from the antioxidant.
Another possibility for reaction of peroxyl radicals ensues if the autoxidizing fatty acid contains three or more double bonds (Fig. 3, step ). In these fatty acids the peroxyl radical can react with the additional double bond and form an internal or cyclic peroxide (endoperoxide) [6, 21]. Endoperoxide formation occurs non-enzymatically in the formation of isoprostanes, a class of autoxidation products derived from arachidonic acid that is being used as a reliable marker for lipid peroxidation and oxidant stress in biological systems [22]. A similar reaction is catalyzed by the enzyme cyclooxygenase during prostaglandin synthesis [23].
It is important to understand that the peroxyl radical can perform additional reactions that compete with formation of the primary hydroperoxide product. Whichever reaction proceeds with the highest rate constant will be the preferred reaction (albeit not exclusive) and dominate product formation. The rate constants for the particular reactions depend on the reaction conditions (e.g., temperature, solvent or lipid film), available reagents (antioxidant, double bonds, concentration of radicals), and, as will be highlighted further below, the structure of the peroxyl radical itself [24].
Discovery of the bis-allylic 11-hydroperoxide
The first isolation of the bis-allylic 11-hydroperoxide (11-HPODE) from an autoxidation of linoleic acid methyl ester was reported in 2000 [25]. The autoxidation reaction followed a standard protocol including 5% α-tocopherol in order to suppress secondary transformation of the primary hydroperoxide products [26]. Under these conditions, 11-HPODE accounted for about 5–10% of the hydroperoxide products. The 11-HPODE was isolated using a combination of RP- and SP-HPLC and characterized by UV, GC-MS, and 1H-NMR spectroscopy [25]. The bis-allylic hydroperoxide does not share the typical diene chromophore of the conjugated 9- and 13-hydroperoxides (λmax around 235 nm) but instead it has prominent end absorbance around 205 nm. Treatment of 11-HPODE with mild acid induces quantitative transformation to the conjugated 9- and 13-HPODE, enabling quantification of the ensuing diene chromophore using UV spectroscopy.
The antioxidant was shown to be a critical reagent in the autoxidation reaction since the bis-allylic hydroperoxide was only formed in the presence of α-tocopherol and undetectable when absent [25]. Subsequently it was shown that with increasing concentrations of the antioxidant 11-HPODE becomes equally abundant to 9-HPODE or 13-HPODE, respectively [27]. Besides the strict requirement of a strong antioxidant for its formation, the facile isomerization into the conjugated hydroperoxides together with a less prominent UV absorbance and co-elution with other products on HPLC are features of 11-HPODE that may have contributed to its elusive character [25]. In addition to autoxidative transformation of polyunsaturated fatty acids in the presence of α-tocopherol, bis-allylic hydroperoxides can also be formed by total chemical synthesis or photosensitized (singlet) oxidation of polyunsaturated fatty acids [28–30].
How is the bis-allylic 11-hydroperoxide formed?
Why is the bis-allylic 11-hydroperoxide only formed when the antioxidant α-tocopherol is present in the autoxidation reactions? The answer to this question relates directly to the stability of the intermediate bis-allylic peroxyl radical (Fig. 4). As explained above, one of the possibilities for reaction of the peroxyl radical is β-fragmentation, the loss of O2 and reversion back to the pentadienyl radical. It turned out that the rate of β-fragmentation of bis-allylic peroxyl radicals is more than 3 orders of magnitude faster than for conjugated peroxyls [24, 27]. As a consequence, the corresponding bis-allylic hydroperoxide can only be formed if trapping of the peroxyl radical as the hydroperoxide is faster than its β-fragmentation. In reactions without α-tocopherol, trapping of peroxyl radicals occurs through hydrogen abstraction from a methylene, but this reaction is rather slow (see Fig. 1). Still, it is fast enough to compete with β-fragmentation of the slower fragmenting conjugated peroxyl radicals, whereas β-fragmentation of the bis-allylic peroxyl by far outcompetes hydrogen abstraction from the fatty acid methylene. However, if a good hydrogen donor like α-tocopherol is present in the reaction, the antioxidant can transfer its hydrogen atom fast enough to the bis-allylic peroxyl radical such that the corresponding hydroperoxide product is formed2. In fact, the more antioxidant is present in the autoxidation reaction, the more prominent the bis-allylic hydroperoxide becomes as a product [27].
Fig. 4. Mechanistic basis for the formation of conjugated and bis-allylic hydroperoxides.
Following hydrogen abstraction from the bis-allylic methylene oxygen adds to all three reactive positions (9, 11, and 13) of the mesomeric pentadienyl radical to form the corresponding peroxyl radicals. However, loss of oxygen (β-fragmentation) from the bis-allylic peroxyl (C11) is much faster than from the conjugated peroxyls (C9 and C13). As a consequence, the bis-allylic hydroperoxide (11-HPODE) can only be formed if a very good H-donor (like α-tocopherol) is included in the autoxidation reaction, whereas 9-HPDOE and 13-HPODE can be formed upon hydrogen abstraction from a fatty acid bis-allylic methylene group.
The concept of kinetic and thermodynamic products
Another mechanistically significant reaction inherent to the reactivity of peroxyl radicals is the rearrangement of the fatty acid carbon chain. As would be expected, the carbon chain arrangement of the starting fatty acid is preserved in the first hydroperoxide products that are isolated form linoleic acid autoxidation, i.e., the conjugated HPODEs have the double bonds in the 10trans,12cis (9-HPODE) or 9cis,11trans (13-HPODE) configuration; the bis-allylic 11-HPODE is 9cis,12cis (cf. Fig. 2A). Since the cis, trans hydroperoxides are the immediate products, they are considered to be formed under kinetic control, i.e., before isomerization of the carbon chain can occur. However, with prolonged autoxidation, and especially in the absence of α-tocopherol, the double bond configuration of the conjugated hydroperoxides rearranges from cis, trans to trans, trans [31, 32]. The apparent driving force for this transformation is the greater thermodynamic stability of the trans, trans conjugated double bond in comparison to the cis, trans double bond. The trans, trans hydroperoxides are formed at the expense of the cis, trans hydroperoxides, and therefore, these products are considered to be formed under thermodynamic control.
β-Fragmentation is an essential step in the isomerization of the double bond geometry of the primary cis, trans conjugated diene hydroperoxide to a trans, trans diene [31, 32]. Isomerization of the double bond is initiated by reversion of the hydroperoxide back to the peroxyl radical followed by β-fragmentation of the peroxyl to the pentadienyl radical; this can occur with the conformation of the carbon chain unchanged (cisoid) or rearranged (transoid) (Fig. 5). The rate of fragmentation into a pentadienyl radical with transoid conformation is about 16-times faster than fragmentation into a cisoid pentadienyl radical (430 s−1 versus 27 s−1 [12]). Thus, following β-fragmentation the original cis, cis pentadiene is preferentially transformed into a cis, trans pentadienyl radical. If oxygen adds at the other end of this pentadienyl radical the resulting peroxyl radical (and the corresponding hydroperoxide) will have the trans, trans-configuration (Fig. 5). Double bond isomerization via β-fragmentation of the peroxyl radical can be suppressed by the presence of an antioxidant (e.g., α-tocopherol) in the reaction because it prevents the hydroperoxide from reverting back to the peroxyl radical [24].
Fig. 5. Proposed mechanism for the isomerization of conjugated hydroperoxides.
Loss of a hydrogen atom reverts the hydroperoxide back to the peroxyl radical, illustrated here for 13-HPODE. β-Fragmentation of the peroxyl radical gives the delocalized pentadiene radical; the rate of β-fragmentation into the cis, trans configurated pentadienyl radical is about 16-times faster than fragmentation into the cis, cis-pentadienyl radical [12]. Therefore, isomerization of the 12,13 bond into the trans configuration is preferred during β-fragmentation. Subsequent addition of molecular oxygen at the other end of the pentadiene gives the 9-hydroperoxide and locks the 10,11 as well as the 12,13 double bonds as trans. Loss of oxygen from the intermediate peroxyl radical (β-fragmentation) and re-oxygenation are essential steps in the isomerization of cis, trans-hydroperoxides to trans, trans-hydroperoxides.
Bioformation of bis-allylic hydroperoxides
It is a remarkable coincidence that around the time when 11-HPODE was discovered as a product of the autoxidation of linoleic acid, it was also first described as an enzymatic product. The wheat crop fungus Gaeumannomyces graminis harbors an unusual lipoxygenase that contains manganese as its active site metal [33] whereas a non-heme iron as the catalytic center is the rule in all other known LOX enzymes [34]. The fungal LOX also has unprecedented catalytic activity: it forms a mixture of 13R-HPODE and 11S-HPODE when reacting with linoleic acid as substrate. Biosynthesis of both products is initiated by abstraction of the pro-S hydrogen at C-11 of linoleic acid [35]. When 11S-HPODE was used as a substrate, it was converted to 13R-HPODE through β-fragmentation to a pentadienyl radical followed by oxygen rebound; oxygen was subject to partial exchange prior to its reincorporation [35, 36].
The question of whether other, iron-containing LOX enzymes can form bis-allylic hydroperoxides has been investigated only for soybean LOX-1 and the pathogen-inducible LOX from rice leaves [37]. Upon careful examination of the products, it was found that the rice LOX formed about 0.4% of 11-HPODE in addition to the main product, 13S-HPODE, whereas the soybean enzyme did not form any bis-allylic hydroperoxide. More promising candidates for formation of bis-allylic hydroperoxides could be the so-called type-II LOX enzymes from soybean seeds [34]. These enzymes have been shown to form varying mixtures of 9-HPODE and 13-HPODE with partial lack of stereocontrol [38]. The possibility that these non-specific products could have arisen via isomerization of a bis-allylic hydroperoxide has not yet been investigated.
It is likely that the earlier discovered bis-allylic hydroxide 11R-hydroxy-9Z,12Z-octadecadienoic acid (11R-HODE) in the red alga Lithothamnion corallioides is biosynthesized by a LOX enzyme, too, followed by reduction of the presumed hydroperoxide intermediate to the hydroxide [39]. Whether the bis-allylic 11-hydroperoxide is subject to further enzymatic transformation besides reduction has not yet been described.
It has not been established whether bis-allylic hydroperoxides are formed under the conditions of biological autoxidation, e.g., in the LDL particle in human blood. A particle of LDL contains about 6–12 molecules of α-tocopherol on average [18], invoking the possibility for formation of bis-allylic oxygenation products in vivo. The inherent instability of the bis-allylic hydroperoxides and their corresponding hydroxy derivatives will make their identification in biological samples a challenging task for further investigation.
Summary
The bis-allylic 11-hydroperoxide is the third primary product of the autoxidation of linoleic acid. In contrast to the well-known conjugated diene hydroperoxides, bis-allylic hydroperoxides are only formed under autoxidation conditions where an efficient hydrogen atom donor is present. The antioxidant, for example α-tocopherol, is necessary to trap the extremely short-lived bis-allylic peroxyl radical as the hydroperoxide before it reverts back to the carbon radical and molecular oxygen.
The discovery of the bis-allylic hydroperoxide and its mechanism of formation has highlighted the critical role occupied by the peroxyl radical during autoxidation. It is the central branching point from where reaction pathways diverge. Which of these pathways prevails is dependent on the reactions conditions, on additional reagents present, and also on the structure of the fatty acid carbon chain that carries the peroxyl radical.
Acknowledgments
This work was supported by National Institutes of Health grants GM15431 and GM076592.
The abbreviations used are
- HPODE
hydroperoxyoctadecadienoic acid
- LOX
lipoxygenase
Footnotes
For consistency of numbering of the carbon atoms, the chemical reactions will be explained using linoleic acid as the model (9Z,12Z-octadecadienoic acid, C18.2) in the text and figures. Similar reactions occur with higher unsaturated fatty acids like linolenic acid (C18.3) and arachidonic acid (C20.4), although it is important to keep in mind that the additional double bond(s) enable types of reactions that are not possible in linoleic acid.
The rate constants for the reactions involved are as follows. β-Fragmentation of the bis-allylic peroxyl radical occurs at 1.9 × 106 s−1, whereas the same fragmentation of the conjugated peroxyls occurs at 30 s−1 or 400 s−1, respectively, depending on whether a cis, cis- or a cis, trans-pentadienyl radical results [27]. Hydrogen abstraction from the fatty acid methylene by the peroxyl radical (i.e., the propagation of the autoxidation chain) occurs at about 100 M−1 s−1 whereas the antioxidant reaction with α-tocopherol as the hydrogen donor has a rate constant of 3.8 × 106 M−1 s−1 [19].
References
- 1.Bergström S. Autoxidation of linoleic acid. Nature. 1945;156:717–718. [PubMed] [Google Scholar]
- 2.Bolland JL. Kinetics of olefin oxidation. Q Rev, Chem Soc. 1949;3:1–21. [Google Scholar]
- 3.Bergström S, Blomstrand R, Laurell S. On the autoxidation of linoleic acid in aqueous colloidal solution. Acta Chem Scand. 1950;4:245–250. [Google Scholar]
- 4.Bateman L. Olefin oxidation. Q Rev, Chem Soc. 1954;8:147–167. [Google Scholar]
- 5.Ingold KU. Inhibition of the autoxidation of organic substances in the liquid phase. Chem Rev. 1961;61:563–589. [Google Scholar]
- 6.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radical Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 7.Gerwick WH. Epoxy allylic carbocations as conceptual intermediates in the biogenesis of diverse marine oxylipins. Lipids. 1996;31:1215–1231. doi: 10.1007/BF02587906. [DOI] [PubMed] [Google Scholar]
- 8.Grechkin A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog Lipid Res. 1998;37:317–352. doi: 10.1016/s0163-7827(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 9.Tijet N, Brash AR. Allene oxide synthases and allene oxides. Prostaglandins Other Lipid Mediat. 2002;68–69:423–431. doi: 10.1016/s0090-6980(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 10.Schneider C, Niisuke K, Boeglin WE, Voehler M, et al. Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120. Proc Natl Acad Sci US A. 2007;104:18941–18945. doi: 10.1073/pnas.0707148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslbeck F, Grosch W, Firl J. Formation of hydroperoxides with unconjugated diene systems during autoxidation and enzymic oxygenation of linoleic acid. Biochim Biophys Acta. 1983;750:185–193. doi: 10.1016/0005-2760(83)90219-9. [DOI] [PubMed] [Google Scholar]
- 12.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 13.Maillard B, Ingold KU, Scaiano JC. Rate constants for the reactions of free radicals with oxygen in solution. J Am Chem Soc. 1983;105:5095–5099. [Google Scholar]
- 14.Porter NA. Mechanism for the autoxidation of polyunsaturated lipids. Acc Chem Res. 1986;19:262–268. [Google Scholar]
- 15.Marnett LJ. Peroxyl free radicals: potential mediators of tumor initiation and promotion. Carcinogenesis. 1987;8:1365–1373. doi: 10.1093/carcin/8.10.1365. [DOI] [PubMed] [Google Scholar]
- 16.Burton GW, Ingold KU. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vivo. J Am Chem Soc. 1981;103:6472–6477. [Google Scholar]
- 17.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 18.Ingold KU, Bowry VW, Stocker R, Walling C. Autoxidation of lipids and antioxidation by alpha-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc Natl Acad Sci US A. 1993;90:45–49. doi: 10.1073/pnas.90.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowry VW, Ingold KU. The unexpected role of vitamin E (alpha-tocopherol) in the peroxidation of human low-density lipoprotein. Acc Chem Res. 1999;32:27–34. [Google Scholar]
- 20.Upston JM, Terentis AC, Stocker R. Tocopherol-mediated peroxidation of lipoproteins: implications for vitamin E as a potential antiatherogenic supplement. Faseb J. 1999;13:977–994. doi: 10.1096/fasebj.13.9.977. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor DE, Mihelich ED, Coleman MC. Stereochemical course of the autoxidative cyclization of lipid hydroperoxides to prostaglandin-like bicyclo endoperoxides. J Am Chem Soc. 1984;106:3577–3584. [Google Scholar]
- 22.Musiek ES, Yin H, Milne GL, Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40:987–994. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 23.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol. 2007;14:473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratt DA, Mills JH, Porter NA. Theoretical calculations of carbon-oxygen bond dissociation enthalpies of peroxyl radicals formed in the autoxidation of lipids. J Am Chem Soc. 2003;125:5801–5810. doi: 10.1021/ja034182j. [DOI] [PubMed] [Google Scholar]
- 25.Brash AR. Autoxidation of methyl linoleate: identification of the bis-allylic 11-hydroperoxide. Lipids. 2000;35:947–952. doi: 10.1007/s11745-000-0604-0. [DOI] [PubMed] [Google Scholar]
- 26.Peers KF, Coxon DT. Controlled synthesis of monohydroperoxides by α-tocopherol inhibited autoxidation of polyunsaturated lipids. Chem Phys Lipids. 1983;32:49–56. [Google Scholar]
- 27.Tallman KA, Pratt DA, Porter NA. Kinetic products of linoleate peroxidation: rapid β-fragmentation of nonconjugated peroxyls. J Am Chem Soc. 2001;123:11827–11828. doi: 10.1021/ja0169724. [DOI] [PubMed] [Google Scholar]
- 28.Yeola SN, Saleh SA, Brash AR, Prakash C, et al. Synthesis of 10(S)-hydroxyeicoatetraenoic acid: a novel cytochrome P-450 metabolite of arachidonic acid. J Org Chem. 1996;61:838–841. [Google Scholar]
- 29.Schneider C, Boeglin WE, Yin H, Stec DF, et al. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids. 2005;40:1155–1162. doi: 10.1007/s11745-005-1480-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Sun M, Salomon RG. Preparative singlet oxygenation of linoleate provides doubly allylic dihydroperoxides: putative intermediates in the generation of biologically active aldehydes in vivo. J Org Chem. 2006;71:5607–5615. doi: 10.1021/jo0605795. [DOI] [PubMed] [Google Scholar]
- 31.Chan HWS, Levett G, Matthew JA. The mechanism of the rearrangement of linoleate hydroperoxides. Chem Phys Lipids. 1979;24:245–256. [Google Scholar]
- 32.Porter NA, Wujek DG. Autoxidation of polyunsaturated fatty acids, an expanded mechanistic study. J Am Chem Soc. 1984;106:2626–2629. [Google Scholar]
- 33.Su C, Oliw EH. Manganese lipoxygenase. Purification and characterization. J Biol Chem. 1998;273:13072–13079. doi: 10.1074/jbc.273.21.13072. [DOI] [PubMed] [Google Scholar]
- 34.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 35.Hamberg M, Su C, Oliw E. Manganese lipoxygenase. Discovery of a bis-allylic hydroperoxide as product and intermediate in a lipoxygenase reaction. J Biol Chem. 1998;273:13080–13088. doi: 10.1074/jbc.273.21.13080. [DOI] [PubMed] [Google Scholar]
- 36.Oliw EH. Factors influencing the rearrangement of bis-allylic hydroperoxides by manganese lipoxygenase. J Lipid Res. 2008;49:420–428. doi: 10.1194/jlr.M700514-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Oliw EH, Cristea M, Hamberg M. Biosynthesis and isomerization of 11-hydroperoxylinoleates by manganese- and iron-dependent lipoxygenases. Lipids. 2004;39:319–323. doi: 10.1007/s11745-004-1235-1. [DOI] [PubMed] [Google Scholar]
- 38.Schneider C, Schreier P. Catalytic properties of allene oxide synthase from flaxseed (Linum usitatissimum L. ) Lipids. 1998;33:191–196. doi: 10.1007/s11745-998-0195-9. [DOI] [PubMed] [Google Scholar]
- 39.Hamberg M, Gerwick WH, Åsen PA. Linoleic acid metabolism in the red alga Lithothamnion corallioides: Biosynthesis of 11(R)-hydroxy-9(Z),12(Z)-octadecadienoic acid. Lipids. 1992;27:487–493. [Google Scholar]