Abstract
To better understand the relationship between mindfulness and depression, we studied normal young adults (n=27) who completed measures of dispositional mindfulness and depressive symptomatology, which were then correlated with: a) Rest: resting neural activity during passive viewing of a fixation cross, relative to a simple goal-directed task (shape-matching); and b) Reactivity: neural reactivity during viewing of negative emotional faces, relative to the same shape-matching task. Dispositional mindfulness was negatively correlated with resting activity in self-referential processing areas, while depressive symptomatology was positively correlated with resting activity in similar areas. In addition, dispositional mindfulness was negatively correlated with resting activity in the amygdala, bilaterally, while depressive symptomatology was positively correlated with activity in the right amygdala. Similarly, when viewing emotional faces, amygdala reactivity was positively correlated with depressive symptomatology and negatively correlated with dispositional mindfulness, an effect that was largely attributable to differences in resting activity. These findings indicate that mindfulness is associated with intrinsic neural activity and that changes in resting amygdala activity could be a potential mechanism by which mindfulness-based depression treatments elicit therapeutic improvement.
Keywords: Mindfulness, Depression, Amygdala, Emotion, Default network
Practices that cultivate mindfulness have recently been incorporated into therapies for depression. These practices, which include mindfulness meditation, are designed to develop the capacity to focus attention on present moment experiences in an open and receptive manner (Brown, Ryan, & Creswell, 2007).
Enhancing mindfulness is a central component of Mindfulness Based Cognitive Therapy (MBCT) and has been shown to prevent the relapse of major depression in patients who have experienced multiple depression episodes (Kingston, Dooley, Bates, Lawlor, & Malone, 2007; Ma & Teasdale, 2004; Teasdale, et al., 2000). Furthermore, MBCT has been shown to be more effective than maintenance anti-depressant therapy in reducing residual depressive symptoms and improving quality of life (Kuyken, et al., 2008). Based on the success of this approach, MBCT has also been explored as a potential treatment during the acute phase of depression, demonstrating beneficial effects in two nonrandomized trials (Eisendrath, et al., 2008; Kenny & Williams, 2007).
A critical question raised by these findings concerns the mechanisms by which mindfulness-treatments impact depressive symptoms. At a neural level, major depressive disorder is associated with dysfunction in multiple interconnected limbic and cortical structures that are thought to be part of a functional circuit governing and producing affective state (Drevets, Price, & Furey, 2008; Mayberg, 2003). One of these structures, the amygdala, is critically involved in fear related processing and exhibits greater resting activity in patients suffering from depression. Thus, measurements of glucose metabolism or cerebral blood flow while the subject is resting in the scanner reveal higher levels of amygdala activity amongst depressed subjects than controls (Clark, et al., 2006; Drevets, et al., 1992). In addition, the magnitude of resting amygdala metabolism is positively correlated with the degree of depressive symptomatology (Abercrombie, et al., 1998; Drevets, et al., 2002; Drevets, et al., 1992; Saxena, et al., 2001). As these high levels of resting amygdala activity normalize with the remission of depression (Clark, et al., 2006; Drevets, et al., 1992), it appears that elevated levels of resting amygdala activity are a marker of the depressed state.
This relationship between elevated resting amygdala activity and depressive symptomatology suggests that one mechanism by which mindfulness training may reduce depression relapse and symptoms is by quelling heightened resting amygdala activity. Therefore, in this study, we examined the neural correlates of dispositional mindfulness and depressive symptomatology in a non-clinical sample during passive sensory viewing (fixation cross) relative to a simple goal-directed control task (shape-matching). The logic for this approach is that areas that are correlated with both dispositional mindfulness and depressive symptomatology are potential neural sites where mindfulness training could be affecting depression related processes.
Neuroimaging studies of depressed patients also indicate that amygdala reactivity, as opposed to resting amygdala activity, is implicated in depression. Studies of amygdala reactivity to negative emotional stimuli have shown that the amygdala is hyper-reactive during the depressed state (Sheline, et al., 2009; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007) and that amygdala activity normalizes with either successful pharmacotherapy (Fu, et al., 2004; Sheline, et al., 2001) or cognitive behavioral therapy (Fu, et al., 2008; Siegle, Carter, & Thase, 2006). Thus, it is also possible that the relationship between mindfulness and depression may be characterized by greater amygdala reactivity to threatening cues (instead of, or in addition to, resting amygdala activity), so an additional analysis explored the relationship of dispositional mindfulness and depressive symptomatology to amygdala reactivity during the viewing of threatening and fearful faces (relative to the same shape-matching control task).
In addition, we also examined neural correlates of depression and mindfulness in medial prefrontal and parietal regions, which have been shown to be involved in self-reflective processing (Lieberman, in press) and are often activated during resting states (Gusnard, Akbudak, Shulman, & Raichle, 2001). Of particular relevance for the present study, rumination on negative aspects of the self has been associated with greater risk for depression (Nolen-Hoeksema, 2000) as well as greater medial PFC activity (Ray et al., 2005). In contrast, training in mindfulness meditation, which emphasizes developing a non-judgmental meta-awareness of the self, was associated with decreased medial PFC activity, relative to controls, in a self-referential task (Farb, et al., 2007). Therefore, we hypothesized that mindfulness and depression would have contrasting effects on medial PFC during rest, with mindful individuals showing reduced activity in self-reflective neural regions at rest.
Methods
Participants
Twenty-seven UCLA undergraduates (16 females) participated in the study for $20. Participants identified themselves as Asian (39%), Caucasian (29%), Latino (18%), African American (7%), or “other” (7%). Prospective participants were excluded through a structured telephone interview if they had serious physical or mental health problems (e.g. “Has a doctor ever told you that you have a serious physical/mental health problem?”), were receiving current treatment from a mental health professional, were using mental health-related medication (e.g., Prozac), or were pregnant/breast feeding. Participants also met the following functional magnetic resonance imaging (fMRI)-related inclusion criteria: a) were right handed; b) were not claustrophobic; and c) had no metal in their bodies (dental fillings were allowed). All procedures were approved by the UCLA Institutional Review Board.
Individual Difference Measures
Depressive symptomatology was assessed using the Beck Depression Inventory (BDI, α = 0.88), which was the primary measure used in studies documenting the effectiveness of MBCT (Williams, Russell, & Russell, 2008). Dispositional mindfulness was assessed using the Mindful Attention Awareness Scale (MAAS; Brown and Ryan, 2003) (e.g., “I find it difficult to stay focused on what’s happening in the present,” all items were reverse scored; α = 0.78). This measure has good psychometric properties and is sensitive to the effects of mindfulness training (Brown, et al., 2007; Chambers, Lo, & Allen, 2008). In addition, participants also completed other measures previously associated with amygdala activation or the MAAS, including the Spielberger Trait Anxiety Inventory (α = 0.91), a 10-item measure of neuroticism, drawn from the International Personality Item Pool (α = 0.86), and the Public Self-Consciousness subscale of the Self-Consciousness Scale, a 7-item measure assessing one’s self-awareness as a social object (α = 0.70).
Experimental Paradigm
As part of an affect labeling and processing study (Lieberman, et al., 2007), participants viewed three different sets of stimuli: 1) a fixation cross; 2) shapes; 3) faces displaying emotional expressions (Figure 1). When viewing shapes or faces, participants performed a matching task between a target at the top of the screen and a pair of stimuli presented at the bottom of the screen. For shape matching, participants were instructed to choose the shape at the bottom of the screen that matched the target presented at the top of the screen and for face matching they chose the face from the pair at the bottom that expressed the same emotion as the target face at the top of the screen. To prevent habituation of amygdala response to the viewing of negative emotional expressions (fear and anger), 20% of the trials in the affect matching condition used faces with a positive emotional expression (happiness or surprise). Faces were selected from a standardized set of images (Tottenham, et al., 2009) and consisted of an equal number of male and female faces.
Figure 1.
The three different forms of stimuli viewed by the participants. A) Passive viewing of the fixation cross. B) Shape Match; from the pair of shapes at the bottom of the screen, participants chose the shape that best matched the target at the top of the screen. C) Affect Match; from the pair of faces at the bottom of the screen, participants chose the face that had the matching facial expression.
Task blocks began with a 3-second instruction cue notifying the participant to perform either the shape-matching or face-matching task, which was followed by 10 randomized trials, each 5 seconds in length, resulting in task blocks that were 50 seconds in length. In addition to these two tasks, participants completed four other tasks for which analyses were reported separately (Creswell, Way, Eisenberger, & Lieberman, 2007). The six task blocks were separated by a fixation crosshair, which remained on the screen for 10 seconds, and served as the baseline. The participants completed two runs; the shape-matching task and the affect matching task occurred once in each run. The participants responded via a button box as soon as they were sure of the correct answer. The stimuli remained on the screen for the entire 5-second trial.
Data Acquisition and Analysis
Data were acquired on a Siemens Allegra 3-T head-only scanner. Head movements were restrained with foam padding and surgical tape placed across each subject’s forehead. For each subject, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo; repetition time = 5,000 ms; echo time = 33 ms; matrix size = 128 × 128; 36 axial slices 3 mm thick with a 1-mm skip between slices; field of view = 20 cm) was acquired coplanar with the functional scans. Two functional scans were acquired (echo-planar T2*-weighted gradient-echo, repetition time = 3,000 ms, echo time = 25 ms, flip angle = 90°, matrix size = 64 × 64, 36 axial slices 3 mm thick with a 1-mm skip between slices, field of view = 20 cm), each lasting 6 min 18 s. During each functional scan, 126 volumes were collected.
The imaging data were analyzed using statistical parametric mapping (SPM99; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, United Kingdom). Images for each subject were realigned to correct for head motion, normalized into a standard stereotactic space as defined by the Montreal Neurological Institute (MNI), and smoothed with an 8-mm Gaussian kernel, full width at half maximum. For each subject, task conditions were modeled as epochs. Planned comparisons were computed for each subject using the general linear model, with a canonical hemodynamic response function. The resulting contrast images were entered into second-level analyses using a random effects model to allow for inferences at the group level.
For assessment of the resting state, fixation was compared to shape-matching. Typically, such comparisons of active and passive task blocks refer to the differences in activity with reference to the goal-directed task. Hence, the term “deactivations” is often used to describe these differences (Raichle, et al., 2001). To facilitate the connection with resting glucose metabolism studies of depressed subjects, we refer to these differences in reference to the fixation condition and use the term “activations” (Gusnard & Raichle, 2001), though the reader should be mindful that these are relative comparisons. Direct neurophysiological recordings of activity during passive and goal-directed tasks confirm this interpretation of the neuroimaging data, as neural activity has been found to increase during passive tasks in both the medial prefrontal cortex and precuneus (Miller, Weaver, & Ojemann, 2009).
To create a mindfulness variable controlling for related individual differences measures, variables that correlated significantly or marginally significantly with the MAAS were regressed into the MAAS and the standardized residuals were saved. These standardized residual values were then entered as a regressor in a random effects whole-brain group analysis, comparing neural activity during shape matching with neural activity during passive fixation. Results are reported according to the voxel of peak activation among each identified cluster of activation. The correction for multiple comparisons in whole-brain analyses was carried out using an uncorrected p value of 0.005 combined with a cluster-size threshold of 20 voxels (Forman, et al., 1995). To identify activation clusters that spatially overlapped in both the correlation with dispositional mindfulness as well as depressive symptomatology, the Marsbar toolbox was used (Brett, Anton, Valabregue, & Poline, 2002). Each activation cluster was defined as a region of interest and then a subtraction was performed to reveal voxels that were conjointly related to each variable. Marsbar was used to then extract the mean parameter estimates from this ROI and subsequent statistical analyses were performed in SPSS 14.0 (Chicago, IL). All coordinates are reported in MNI coordinate space.
Results
Initial Analyses
Initial zero-order correlational analyses were performed to examine the interrelationship between the individual difference measures. Although scores on the MAAS and the Beck Depression Inventory (BDI) were not significantly related, there was a trend in the negative direction (r = −0.29, p = 0.14), as would be expected (Brown & Ryan, 2003). Scores on the MAAS were negatively related to public self-consciousness (r = −0.51, p < 0.01) and were marginally higher in male subjects (t(25)= 1.79, p = 0.09). There was no relationship between the MAAS and either neuroticism (r = −0.24, ns) or the STAI (r = −0.13, ns). To examine correlations between mindfulness and neural activity, we examined correlations between neural activity and: 1) self-reported dispositional mindfulness and 2) a residualized dispositional mindfulness variable, in which gender and public self-consciousness were regressed into the MAAS and the standardized residuals were then used as the regressors.
The BDI had a similar magnitude of relationship (r = −0.31, p = 0.12) with the residualized measure of mindfulness as it did with the uncorrected measure. Scores on the BDI were positively correlated with both neuroticism (r =0.61, p < 0.001) and trait anxiety (r = 0.63, p < 0.001). There were no gender differences in reported depressive symptomatology (t(25) = 0.40, p = 0.69). There was a wide distribution of depressive symptomatology in this sample, ranging from 0 to 24 on the BDI, with 26% of the participants scoring at or exceeding the cutoff (10) for mild depression.
Neural Analyses
Resting State Analyses (Fixation versus Shape-Matching)
In the fixation condition, relative to shape-matching, there was widespread activation throughout a network of areas (Table 1; Figure 2) that have been referred to as the default network (Gusnard and Raichle, 2001). These included the four areas most commonly included in the default network (Buckner, Andrews-Hanna, & Schacter, 2008): a large midline cluster with a peak in the precuneus/posterior cingulate (−2, −36,48; t=6.48, p <0.005), inferior parietal lobule, bilaterally (−36, −78,40; t=5.03, p < 0.005; and 36, −76,40, 4.49, p < 0.005), dorsomedial prefrontal cortex (BA 9: 0,46,34, t=3.44, p < 0.005), and medial prefrontal cortex (BA10: 2,50,10; t=3.75, p < 0.005). In addition, there was activation in medial and lateral temporal lobe areas associated with the default network (Buckner, et al., 2008) including the left and right amygdala (−22, −6, −16, t =4.84; 22,0, −32, t = 4.42, p < 0.005), the left and right parahippocampal gyrus (−20, −28, −24, t = 5.84; 36, −8, −40, t = 6.29, p < 0.005), and the left (−64, −42, −10, t = 3.97, p < 0.005) and right (rostral: 58, −2, −24, t = 4.49; caudal: 64, −52, 6, t = 4.91, p < 0.005) middle temporal gyrus. The bilateral fusiform gyrus also showed greater activation (30, −92, −10, t=7.00; −32, −94,12, t = 6.33, p < 0.005), which is common when a passive sensory task rather than eyes-closed rest is used as the baseline (Gusnard and Raichle, 2001). Additionally, the right dorsal anterior cingulate cortex (2,12,24, t = 4.15, p < 0.005) and the dorsal striatum (8,18,8, t = 6.26, p < 0.005) were activated.
Table 1.
Baseline neural activation during fixation relative to shape matching..
| Region | Side | Brodmann Area | MNI Coordinate | t | k (voxels) | ||
|---|---|---|---|---|---|---|---|
| Frontal Lobe | |||||||
| Superior frontal gyrus | R | 10 | 28 | 66 | 16 | 3.61 | 52 |
| Superior frontal gyrus | L | 8 | −24 | 36 | 52 | 4.32 | 51 |
| Superior frontal gyrus | L | 9 | −34 | 48 | 32 | 3.54 | 20 |
| Superior frontal gyrus | 8 | 0 | 46 | 34 | 3.44 | 95 | |
| Superior frontal gyrus | R | 6 | 6 | 26 | 46 | 3.54 | 40 |
| Medial frontal gyrus | L | 9 | −40 | 20 | 34 | 3.63 | 68 |
| Inferior frontal gyrus | R | 44 | 50 | 24 | 26 | 6.10 | 1470 |
| Inferior frontal gyrus | L | 45 | −58 | 24 | 18 | 4.30 | 192 |
| Inferior frontal gyrus | R | 47 | 40 | 34 | −18 | 4.44 | 72 |
| Inferior frontal gyrus | L | 47 | −26 | 52 | −2 | 3.63 | 47 |
| Precentral gyrus | L | 4 | −62 | −4 | 10 | 3.33 | 26 |
| Medial prefrontal cortex | R | 32 | 2 | 50 | −10 | 3.75 | 351 |
| Temporal Lobe | |||||||
| Superior temporal gyrus | L | 42 | −50 | −32 | 12 | 3.72 | 183 |
| Superior temporal gyrus | R | 22 | 62 | −38 | 2 | 5.44 | 393 |
| Middle temporal gyrus | L | 21 | −64 | −42 | −10 | 3.97 | 64 |
| Middle temporal gyrus | R | 21 | 58 | −2 | −24 | 4.49 | 200 |
| Middle temporal gyrus | R | 21 | 64 | −52 | 6 | 4.91 | 263 |
| Parahippocampal gyrus | R | 36 | 36 | −8 | −40 | 6.29 | 3511 |
| Parahippocampal gyrus | L | 35 | −20 | −28 | −24 | 5.84 | 257 |
| Fusiform Gyrus | R | 20 | 40 | −18 | −24 | 5.24 | 220 |
| Parietal Lobe | |||||||
| Precuneus | L | 7 | −2 | −36 | 48 | 6.48 | 462 |
| Posterior Cingulate | L | 23 | −2 | −26 | 36 | 5.62 | 464 |
| Inferior parietal lobule | L | 39 | −36 | −78 | 40 | 5.03 | 171 |
| Inferior parietal lobule | R | 39 | 36 | −76 | 46 | 4.49 | 303 |
| Occipital Lobe | |||||||
| Fusiform gyrus | R | 18 | 30 | −92 | −10 | 7.00 | 1171 |
| Fusiform gyrus | L | 18 | −32 | −94 | −12 | 6.33 | 893 |
| Subcortical and Paralimbic | |||||||
| Dorsal Anterior Cingulate Cortex | R | 2 | 12 | 24 | 4.15 | 239 | |
| Dorsal Striatum | R | 8 | 18 | 8 | 6.26 | 288 | |
| Amygdala | L | −22 | −6 | −16 | 4.84 | 320 | |
| Amygdala | R | 22 | 0 | −32 | 4.42 | 168 | |
| Ventral Midbrain | R | 4 | −14 | −18 | 4.94 | 163 | |
Note. Significance was determined using p < 0.005 with a 20-voxel extent threshold.
Figure 2.
Activations in the Fixation relative to Shape Match contrast. Denoted areas are part of the default network. DMPFC = dorsomedial prefrontal cortex; VMPFC = ventromedial prefrontal cortex; IPL = Inferior Parietal Lobule; LTC = Lateral Temporal Cortex.
In this same resting-state contrast, the BDI was positively correlated with activation in the right amygdala (22, 0, −28; t = 3.28, p < 0.005, k = 40), medial prefrontal cortex (BA10/11: 4,56, −14; t = 3.37, p < 0.005), inferior frontal gyrus (−42,42,6; t = 3.46, p < 0.005), and several areas in the visual cortex (Table 2; Figure 3). As anxiety related constructs have been related to amygdala activity (Etkin & Wager, 2007), the relationship of both trait anxiety and neuroticism with amygdala activity were examined. Neither variable exhibited a significant correlation with amygdala activity and when they were used as covariates in an assessment of the neural correlates of the BDI, the relationship between BDI score and right amygdala activity remained significant (26, 2, −24, t = 3.76, p < 0.005, k = 55). In terms of areas inversely correlated with BDI scores, only one cluster had a significant negative relationship and that was in the superior frontal gyrus (30,46,38; t=3.34, p < 0.005, k=47).
Table 2.
Areas positively correlated with Beck Depression Inventory Score
| Region | Side | Brodmann Area | MNI Coordinate | t | k (voxels) | ||
|---|---|---|---|---|---|---|---|
| Frontal Lobe | |||||||
| Inferior Frontal Gyrus | L | 46 | −42 | 42 | 6 | 3.46 | 65 |
| Medial prefrontal cortex | R | 10 | 4 | 56 | −14 | 3.37 | 33 |
| Parietal Lobe | |||||||
| Superior Parietal Lobule | R | 7 | 24 | −72 | 56 | 3.07 | 21 |
| Occipital Lobe | |||||||
| Fusiform Gyrus | R | 19 | 26 | −84 | −16 | 3.92 | 131 |
| Fusiform Gyrus | R | 37 | 46 | −56 | −18 | 3.91 | 129 |
| Cuneus | R | 18 | 20 | −94 | 12 | 3.41 | 53 |
| Subcortical | |||||||
| Amygdala | R | 22 | 0 | −28 | 3.28 | 40 | |
Note. Significance was determined using p < 0.005 with a 20-voxel extent threshold.
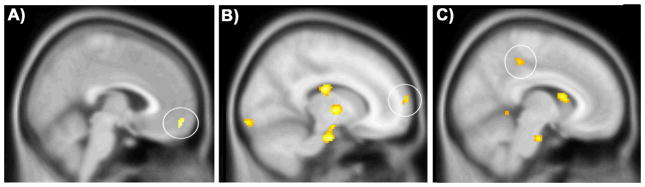
Figure 3.
Correlations of depression and mindfulness with self-referential regions during rest (Fixation versus Shape-Match). Positive correlations with Beck Depression Inventory score in the ventromedial prefrontal cortex (A; 4,56, −14) and negative correlations with dispositional mindfulness in the medial prefrontal cortex (B; 16,68,12) and the posterior cingulate (C; 8, −34,48).
In this same fixation versus shape-match contrast, dispositional mindfulness was negatively correlated with activity in multiple areas (Table 3). The global maximum was centered in the left amygdala/hippocampal transition area (−12, −12, −20; t = 5.22, p < 0.005, k = 301) and extended both rostrally and caudally from this peak. There was also a peak in the right anterior medial temporal lobe (34, 10, −26; t = 3.99, p < 0.005, k = 109) that extended caudally to encompass the rostral amygdala. In addition, the left ventrolateral prefrontal cortex (−44, 34, −10; t = 3.73, p < 0.005, k = 134) was negatively associated with dispositional mindfulness. With respect to areas considered part of the default network, dispositional mindfulness was also negatively related to precuneus activity (2, −48, 56; t = 3.68, p < 0.005, k = 101). As for the positive correlation with dispositional mindfulness, no areas exceeded the statistical threshold for significant activation.
Table 3.
Areas negatively correlated with dispositional mindfulness.
| Region | Side | Brodmann Area | MNI Coordinate | t | k (voxels) | ||
|---|---|---|---|---|---|---|---|
| Frontal Lobe | |||||||
| Inferior Frontal Gyrus | L | 47 | −44 | 34 | −10 | 3.73 | 134 |
| Inferior Frontal Gyrus | L | 44 | −58 | 6 | 6 | 3.59 | 35 |
| Precentral Gyrus | L | 6 | −50 | 0 | 50 | 3.59 | 23 |
| Temporal Lobe | |||||||
| Anterior Medial Temporal Lobe | R | 38 | 34 | 10 | −26 | 3.99 | 109 |
| Medial Temporal Gyrus | R | 21 | 50 | −4 | −26 | 3.83 | 112 |
| Superior Temporal Gyrus | L | 22 | −54 | −6 | −16 | 3.65 | 68 |
| Superior Temporal Gyrus | R | 22 | 66 | −44 | 2 | 3.50 | 38 |
| Occipital and Parietal Lobe | |||||||
| Cuneus | R | 31 | 26 | −66 | 22 | 4.13 | 79 |
| Precuneus | R | 7 | 2 | −48 | 56 | 3.68 | 101 |
| Parietal Operculum | L | 41 | −38 | −18 | 24 | 3.69 | 35 |
| Lingual Gyrus | R | 18 | 26 | −60 | 8 | 3.92 | 102 |
| Fusiform Gyrus | R | 19 | 24 | −56 | −12 | 3.38 | 49 |
| Fusiform Gyrus | R | 19 | 18 | −80 | −14 | 3.05 | 29 |
| Subcortical and Paralimbic | |||||||
| Amygdala/Anterior HPC | L | −12 | −12 | −20 | 5.22 | 301 | |
| Caudate | R | 10 | 12 | 12 | 4.47 | 60 | |
| Hippocampus | L | −26 | −20 | −12 | 3.99 | 79 | |
| Hippocampus | L | 35 | −14 | −32 | −2 | 3.80 | 91 |
| Thalamus | L | −8 | −10 | 14 | 3.80 | 29 | |
| Globus Pallidus | R | 12 | −4 | 0 | 3.30 | 29 | |
| Globus Pallidus | L | −16 | 2 | 2 | 3.28 | 23 | |
Note. Significance was determined using p < 0.005 with a 20-voxel extent threshold.
To examine the unique relationship of mindfulness to neural activity in the fixation versus shape match contrast, the residualized dispositional mindfulness measure (controlling for gender and public self-consciousness) was used as a regressor in a random effects whole-brain analysis. The residualized dispositional mindfulness variable was negatively correlated with activity in multiple subcortical areas, including both the right (24, 2, −20, t=4.09, p < 0.005, k=367) and left amygdala (−20, 2, −20, t=3.73, p<0.005, k=31), the hippocampus bilaterally (−28, −20, −10, t = 3.81; 42, −18, −22, t = 3.90, p < 0.005), and the thalamus bilaterally (−22, −20,14, t = 3.69; 14, −6,2, t = 4.64, p < 0.005). Cortically, there was a significant negative correlation of residualized dispositional mindfulness with midline clusters of activation in the medial prefrontal cortex (BA10: 16,68,12, t = 3.40, p < 0.005) and the posterior cingulate (8, −34,48, t = 3.42), as well as with several clusters in the temporal cortex and visual cortex (Table 4; Figure 3). In contrast to these negative correlations, the residualized dispositional mindfulness variable was positively related to a single cluster in the right orbitofrontal gyrus (18,40, −14, t=3.88, p < 0.005).
Table 4.
Areas negatively correlated with dispositional mindfulness residualized for gender and public self-consciousness.
| Region | Side | Brodmann Area | MNI Coordinate | t | k (voxels) | ||
|---|---|---|---|---|---|---|---|
| Frontal Lobe | |||||||
| Superior Frontal Gyrus | R | 10 | 16 | 68 | 12 | 3.40 | 23 |
| Middle Frontal Gyrus | L | 46 | −40 | 40 | 16 | 3.27 | 30 |
| Inferior frontal gyrus | L | 44 | −58 | 8 | 6 | 4.38 | 32 |
| Orbitofrontal Gyrus | R | 11 | 22 | 26 | −20 | 3.76 | 64 |
| Temporal Lobe | |||||||
| Temporal Pole | R | 38 | 54 | 10 | −26 | 5.12 | 143 |
| Middle temporal gyrus | L | 21 | −48 | −4 | −24 | 4.25 | 155 |
| Parahippocampal Gyrus | L | 34 | −12 | −12 | −22 | 3.56 | 28 |
| Anterior Transverse Temporal Area | L | 41 | −38 | −22 | 24 | 3.64 | 83 |
| Parahippocampal gyrus | L | 27 | −16 | −34 | −4 | 3.92 | 37 |
| Occipital Lobe | |||||||
| Superior Occipital Gyrus | L | 19 | −12 | −86 | 38 | 3.35 | 38 |
| Middle Occipital Gyrus | L | 19 | −26 | −82 | 20 | 3.66 | 206 |
| Cuneus | R | 31 | 24 | −62 | 10 | 3.54 | 51 |
| Cuneus | R | 31 | 28 | −64 | 22 | 3.5 | 122 |
| Cuneus | L | 17 | −8 | −76 | 16 | 3.41 | 23 |
| Lingual Gyrus | R | 18 | 16 | −98 | −10 | 3.69 | 161 |
| Lingual Gyrus | L | 17 | −8 | −92 | 16 | 3.47 | 133 |
| Lingual Gyrus | L | 18 | −6 | −76 | 0 | 3.40 | 56 |
| Fusiform Gyrus | R | 37 | 24 | −62 | −22 | 3.58 | 148 |
| Fusiform Gyrus | L | 37 | −28 | −64 | −22 | 3.56 | 113 |
| Fusiform Gyrus | R | 18 | 34 | −90 | −14 | 3.39 | 45 |
| Subcortical and Paralimbic | |||||||
| Amygdala/Anterior HPC | R | 24 | 2 | −20 | 4.09 | 367 | |
| Amygdala | L | −20 | 2 | −20 | 3.73 | 31 | |
| Posterior Cingulate | R | 23 | 8 | −34 | 48 | 3.42 | 73 |
| Caudate Body | L | −18 | −10 | 28 | 4.59 | 209 | |
| Hippocampal formation | L | 42 | −18 | −22 | 3.90 | 20 | |
| Hippocampal formation | L | −28 | −20 | −10 | 3.81 | 30 | |
| Thalamus | R | 14 | −6 | 2 | 4.64 | 120 | |
| Thalamus | L | −22 | −20 | 14 | 3.69 | 42 | |
| Cerebellum | 0 | −48 | −4 | 3.71 | 63 | ||
Note. Significance was determined using p < 0.005 with a 20-voxel extent threshold.
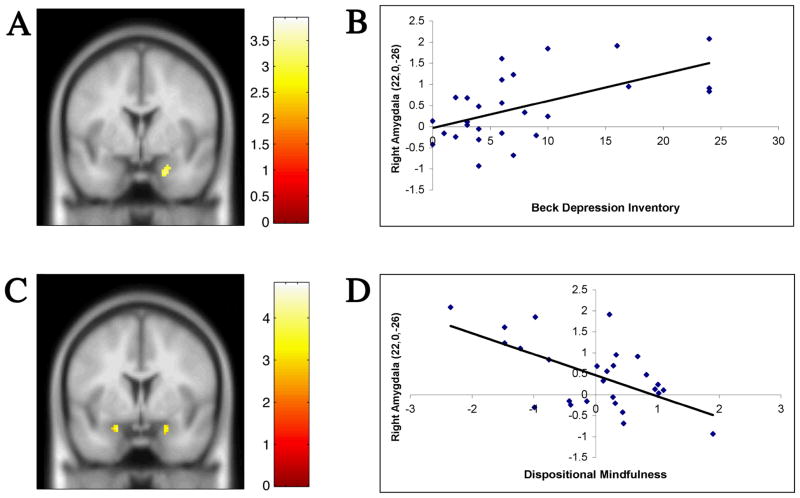
To identify areas that correlated with dispositional mindfulness as well as depressive symptomatology and thus might be involved in both processes, clusters of activation from each respective correlation map were assessed for spatial overlap. Only one area, the right amygdala, had a cluster (22, 0, −26, k = 20) possessing a significant relationship with both variables. This 20 voxel cluster was positively correlated with BDI scores and negatively correlated with the residualized dispositional mindfulness variable (Figure 4). To determine the degree to which shared variance accounted for the relationship of depressive symptomatology and dispositional mindfulness, each variable was entered into a multiple regression with this 20 voxel right amygdala cluster as the dependent variable. Both the residualized dispositional mindfulness variable (β = −0.47, t = −3.18, p < 0.005) and the BDI (β = 0.43, t = 2.87, p < 0.01) were significantly related to activity in this cluster. Thus, each variable was uniquely associated with amygdala activity. In addition, the mean activation for this ROI was not significantly different between males and females, according to an independent samples t-test (t(25)= 1.1, p = 0.29).
Figure 4.
Left panel: coronal sections showing the activations in the Fixation relative to Shape Match contrast for the positive correlation with depressive symptomatology (A) and the negative correlation with the dispositional mindfulness variable (C). In the right panel, scatterplots depict the correlation of the mean parameter estimates for the 20 voxel right amygdala cluster (22, 0, −26) with depressive symptomatology (B; r = 0.57, p < 0.005) and residualized dispositional mindfulness (D; r = −0.60, p < 0.005).
Reactivity Analyses (Affect Matching relative to Shape Matching)
To examine the relationship of depressive symptomatology and dispositional mindfulness with amygdala reactivity, the right amygdala ROI (described above) was used to extract values from the affect matching relative to shape matching contrast (Figure 5). Consistent with predictions, the dispositional mindfulness variable was negatively correlated with amygdala activation (r = −0.53, p < 0.005) when viewing threatening and fearful faces, relative to shape-matching. Similarly, BDI scores were positively correlated with amygdala activation in this contrast (r = 0.63, p < 0.001). Thus, the right amygdala showed the same pattern of relationship with both depressive symptomatology and the residualized dispositional mindfulness variable in the reactivity analysis as it did in the resting state analysis.
Figure 5.
Amygdala Reactivity. Scatterplots of the relationship between activity in the right amygdala and either dispositional mindfulness (A; r = −0.53, p < 0.005) or depressive symptomatology (B; r = 0.63, p < 0.005) when viewing negative emotional faces, relative to shape matching.
Critical for the interpretation of these results is to remember that the amygdala reactivity observed in this analysis stems from comparing affect matching with a neutral, goal-directed task, as is typically done in the field. However, according to the resting analyses, there is also greater amygdala activity when passive fixation is compared to shape matching. This gives rise to the question as to whether or not there is amygdala activation during affect matching that exists over and above that during resting activity. In other words, is the amygdala activity seen in the affect match versus shape match contrast more attributable to amygdala activation over and above passive fixation or is it more attributable to the deactivation from passive fixation during shape matching? To disentangle these alternative interpretations, amygdala response to the viewing of emotional faces was compared to the passive viewing of the fixation cross (as opposed to shape-matching). As in the previous contrast, the same right amygdala ROI defined in the fixation versus shape-matching contrast was used to extract parameter estimates. There was no significant relationship between the residualized dispositional mindfulness measure and amygdala activation (r = − 0.26, p = 0.18) in this affect match versus fixation contrast (Figure 6). Thus, when amygdala reactivity is assessed relative to a passive rather than goal-directed task, the relationship with the dispositional mindfulness measure is no longer significant. This indicates that for those either high or low in dispositional mindfulness there were relatively similar degrees of amygdala activation when viewing emotional faces relative to passive viewing of a fixation cross. In other words, when the measure of resting activity is subtracted from the measure of reactivity, there is not a relationship between the residualized dispositional mindfulness variable and amygdala activity, indicating that the association between dispositional mindfulness and amygdala reactivity is largely attributable to the differences in resting activity. More specifically, because there were no mindfulness related differences in amygdala activation when affect matching was compared to passive fixation, but there were robust differences when affect matching was compared to shape matching, the relationship of mindfulness to amygdala activation in the affect matching versus shape matching contrast would appear to be explained by the difference in amygdala activity between fixation and shape matching.
Figure 6.
Right amygdala activation (22,0, −26) to viewing affective faces, shapes, and fixation stimuli as a function of dispositional mindfulness (A) or depressive symptomatology (B). Denoted on the graphs are the mean parameter estimates (±SEM) for the ROI across the respective contrasts. Although the data were analyzed as correlations to take full advantage of the distributions, they are graphed here as discrete variables to facilitate visual interpretation. The residualized mindfulness variable was split at the median, while Beck Depression Inventory scores were divided into those that exceeded the threshold for mild depression (n = 7) and those who scored below this threshold (n = 20). Panel A reveals that a large portion of the apparent amygdala reactivity differences associated with residualized dispositional mindfulness (leftmost bars) can be attributed to resting state differences rather than actual reactivity. Panel B suggests that the relation of depressive symptoms to apparent amygdala reactivity can be attributed both to actual reactivity and resting state differences. ** = p < 0.005; * = p < 0.05.
With respect to depressive symptomatology, there was a positive correlation between BDI scores and amygdala activation (r = 0.44, p = 0.02) in the affect matching versus fixation contrast (Figure 6). This suggests that the association of depressive symptomatology with amygdala activity in the affect matching versus shape-matching contrast was a reflection of both greater amygdala response to the viewing of emotional faces as well as greater deactivation during shape matching, relative to fixation. However, the effect in the latter condition was more robust.
Discussion
During passive sensory viewing relative to cognitive engagement in a simple goal-directed task dispositional mindfulness and depressive symptomatology exhibited opposite, overlapping relationships with activity in only one area of the brain, the right amygdala. This association of greater depressive symptomatology with greater relative baseline amygdala activity is consistent with resting glucose metabolism and cerebral blood flow studies of depressed patients showing that amygdala activity is correlated with depression severity (Abercrombie, et al., 1998; Clark, et al., 2006; Drevets, et al., 1992) and suggests that the association of dispositional mindfulness with levels of amygdala activation in this contrast may have clinical relevance.
In addition to these resting state differences, greater depressive symptomatology was associated with greater amygdala reactivity to the viewing of emotional faces. Conversely, dispositional mindfulness was associated with reduced amygdala reactivity to the viewing of emotional faces. This association of dispositional mindfulness and depressive symptomatology with differences in reactivity appeared to be largely driven by differences in the resting state, underscoring the psychological importance of intrinsic neural activity. Hence, studying the relationship between mindfulness and resting neural activity is likely to be a valuable approach for understanding the mechanisms by which mindfulness has salutary effects.
Resting State Activity
The focus on the resting state in this study stems from recent research indicating that unconstrained, passive sensory processing exhibits reliable and predictable deactivations that become apparent when contrasted with a wide variety of cognitive tasks (Gusnard & Raichle, 2001). The constellation of interconnected areas identified using such methods are typically referred to as the default network and generally include the precuneus/posterior cingulate, dorsal and ventral medial prefrontal cortices, and lateral posterior cortices as well as medial temporal lobe areas such as the amygdala and hippocampus (Buckner, et al., 2008; Gusnard & Raichle, 2001; Shulman, et al., 1997). In this study, each of these areas were identified in the fixation versus shape-match contrast (Table 1), supporting the validity of our methods for delineating a resting state.
In spite of the full expanse of the default network exhibiting relative activation in this contrast, only medial portions of it were associated with either depressive symptomatology or dispositional mindfulness. Although the amygdala was the sole area for which the significantly correlated clusters spatially overlapped, portions of the medial prefrontal cortex positively correlated with depressive symptomatology and negatively correlated with residualized dispositional mindfulness (Figure 3).
Tasks that require self-reflection, such as the assessment of the self-descriptiveness of traits (Kelley, et al., 2002) or making judgments about one’s feelings (Ochsner, et al., 2004), reliably activate medial prefrontal cortex and frequently the posterior cingulate, as well (Lieberman, 2007). As Beck et al. (1979) identified negative attitudes towards the self (e.g. “I am a failure”) as a primary driver of depression-inducing and depression-maintaining cognitions, the BDI is designed to measure these self-relevant cognitions. Hence, the positive correlation of the BDI with medial prefrontal activation at rest is consistent with individuals who are higher in depressive symptomatology engaging in greater self-relevant processing when not engaging in a goal-directed task.
Dispositional mindfulness was negatively correlated with resting activation in closely related medial prefrontal and parietal self-referential areas (Figure 3). The lower levels of resting activation in those high in mindfulness might indicate that they are less likely to engage in self-focused processing at rest. Potentially, these lower levels of activation within neural areas processing self-relevant information may indicate that the attachment of thoughts and feelings to the self is less robust in those high in mindfulness. If so, this would be consistent with a core skill trained in MBCT (Segal, Williams, & Teasdale, 2002), which is to develop a meta-cognitive awareness that allows patients to change their relationship with their depression promoting cognitions. More speculatively, in so far as intrinsic neural activity across time and situations represents a “self” (Gusnard and Raichle, 2001), the reduced levels of such neural activity in those high in mindfulness may also be related to the concept of “no-self” in Eastern contemplative traditions or “dying to self” in Western contemplative traditions.
Amygdala Activity and the Resting State
A critical issue in the depression literature has been the relationship between studies showing heightened intrinsic resting amygdala activity in depression (usually PET glucose metabolism studies) and studies showing depressed patients have heightened amygdala reactivity, as assessed by comparing emotional stimuli to neutral stimuli (Whalen, Shin, Somerville, McLean, & Kim, 2002). The data presented herein underscore the importance of the baseline condition in interpreting results of such studies. Typically, it is assumed that when contrasting evocative emotional stimuli to neutral stimuli, the greater amygdala activation is due to activation by the evocative stimuli rather than deactivation to the neutral stimuli. Consistent with this model, amygdala activation had a robust positive correlation with depressive symptomatology in the affect match versus shape match contrast as well as a robust negative correlation with dispositional mindfulness. However, when passive viewing of the fixation cross was used as the baseline for the viewing of emotional faces, there was no relationship between amygdala activation and dispositional mindfulness and only a weak relationship with depressive symptomatology. In light of the robust relationship of these psychological variables with amygdala activation in the fixation relative to shape matching contrast, these data suggest that the differences seen in the affect match versus shape match contrast are not primarily a result of differences in reactivity to emotional stimuli, but rather a reflection of differences in deactivation from resting baseline during shape-matching.
Because blood oxygen level dependent fMRI analyses are dependent on relative comparisons between two tasks, it is not possible to determine whether dispositional mindfulness and depressive symptomatology are more closely associated with differences in amygdala activity during passive fixation or shape matching. Our interpretation is that lower dispositional mindfulness (and higher depressive symptomatology) is associated with greater resting amygdala activity during the passive fixation condition and comparable levels of amygdala activity during shape-matching. This would be consistent with PET studies that have found a positive correlation between resting amygdala glucose metabolism and depression severity (Abercrombie, et al., 1998; Drevets, et al., 2002; Drevets, et al., 1992; Saxena, et al., 2001). It would also be consistent with two recent fMRI studies that measured absolute levels of amygdala activity and found that carriers of a risk allele for depression had higher levels of amygdala blood flow at rest (Canli, et al., 2006; Rao, et al., 2007). However, with the methodology used in the present study, it cannot be conclusively determined that there isn’t mindfulness related differences in amygdala activity during shape-matching. Ultimately, methodological approaches (e.g., perfusion MRI) that don’t use a relative comparison will be necessary to conclusively address this question.
Amygdala Activity and Attention: Functional Implications
An important question raised by the present findings concerns the potential functional effects of higher resting amygdala activity as well as why it is associated with both mindfulness and depression. One potential explanation may lie in the amygdala’s involvement in modulating moment-to-moment vigilance (Davis & Whalen, 2001). The amygdala is richly interconnected with nearly the entire cortical mantle (Amaral, Price, Pitkanen, & Carmichael, 1992) and can use these connections to direct attention to the processing of emotionally relevant stimuli. Accordingly, amygdala lesions prevent this facilitation of attention to aversive emotional stimuli (Anderson & Phelps, 2001) as well as the normal heightening of activation in the face responsive regions of the visual cortex during the viewing of fearful faces (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). Consistent with this role for the amygdala in orienting and narrowing attentional focus, activation of the amygdala in normal subjects by non-emotional stimuli can induce a state of hyper-vigilance that biases subsequent processing towards negative stimuli (Herry, et al., 2007). It would seem then that the higher levels of baseline amygdala activity seen in the present study might be associated with depressive symptomatology, in part, because of a biasing effect upon both internal and external information processing that emphasizes the negative, contributing to the feelings of worthlessness and failure characteristic of depression. Conversely, according to this model, lower levels of baseline amygdala activity may be associated with a less biased and broader attentional stance. This would be consistent with the negative correlation of dispositional mindfulness scores with amygdala activity, as high scores on this measure reflect a greater proclivity to engage in an open and receptive attentional style (Jha, Krompinger, & Baime, 2007), which may reflect less amygdaloid drive upon exteroceptive (and interoceptive) information processing.
Clinical Implications
As meditation training elicits increases in MAAS scores that are closely coupled with decreases in BDI scores (Chambers, et al., 2008), the present finding of a linear relationship between dispositional mindfulness and baseline amygdala activity suggest a potential neural mechanism by which mindfulness in general, and MBCT, in particular, may reduce depressed mood and depression relapse. Bremner et al (1997) showed that heightened resting amygdala activity was predictive of pharmacologically induced depression relapse. Thus, in so far as the between-subjects correlational data presented here applies to the within-subject changes following mindfulness training, increases in mindful awareness may lead to corresponding decreases in baseline amygdala activity. Thus, increases in mindfulness could lower baseline amygdala activity below the threshold for triggering depression relapse or depressed mood. As the findings presented here are correlational, experimental studies with mindfulness training interventions are needed to causally implicate resting state amygdala activity in the mindfulness-depression link. Nonetheless, the present findings provide an initial indication that incremental increases in mindful awareness may lead to corresponding decreases in resting state amygdala activity and emphasize the utility of examining baseline amygdala activity.
Methodological Considerations
Although the dispositional mindfulness measure was associated with the amygdala bilaterally, the correlation with BDI was limited to the right amygdala. This association of the right amygdala with depressive symptomatology is consistent with some clinical studies (Abercrombie, et al., 1998; Clark, et al., 2006) while others have found that activity in the left amygdala is more closely associated to depressive state (Drevets, et al., 2002; Drevets, et al., 1992). This discordance is not limited to resting studies, as activational studies have found depressive state associated with the left amygdala (Fu, et al., 2004) the right amygdala (Fu, et al., 2008) or both the right and left amygdala (Sheline, et al., 2001).
One potential factor contributing to these laterality differences that has not been extensively studied in the context of depression is sex differences. The lateralized effects of sex upon amygdala function have been most persuasively documented in studies of emotionally enhanced memory, where the left amygdala is more closely associated with memory for arousing material in males and the right amygdala is more closely associated with such memory in females (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Canli, Desmond, Zhao, & Gabrieli, 2002). Functional connectivity analyses in the resting state indicate that this difference in memory may arise from sex-related differential connectivity patterns of the right and left amygdala (Kilpatrick, Zald, Pardo, & Cahill, 2006). However, we did not find any sex differences in activity in the right amygdala ROI, which is consistent with a recent meta-analysis of 148 emotional processing studies (Sergerie, Chochol, & Armony, 2008). Thus, it would appear that the right lateralized amygdala correlation with mood state is not simply a function of there being a greater number of females in this sample.
In light of the role of the amygdala in anxiety and anxiety disorders (Etkin & Wager, 2007), it is important to consider the degree to which the effects reported here are driven by anxiety related factors or are specific for mindfulness and depressive symptomatology. Trait anxiety itself was not associated with baseline amygdala activity. Statistically controlling for its effects upon the relationship between amygdala activity and mindfulness or depressive symptomatology had negligible effects, which is consistent with this measure being more strongly associated with amygdala reactivity to subliminal (Etkin, et al., 2004) or unattended fearful faces (Bishop, Duncan, & Lawrence, 2004; Dickie & Armony, 2008). In addition, neuroticism, a trait measure of the proclivity to experience negative affect, was not associated with baseline amygdala activity and when included as a covariate did not appreciably change the results, which is consistent with studies that examined the relationship between neuroticism and resting glucose metabolism (Deckersbach, et al., 2006; Kim, Hwang, Park, & Kim, 2008). Thus, dispositional mindfulness, as well as depressive symptomatology, were uniquely related to baseline amygdala activity in this sample.
Conclusion
In summary, dispositional mindfulness and depressive symptomatology show opposite relationships with resting activity in the right amygdala, indicating that this may be a potential mechanism linking mindfulness-based treatments with reductions in depressed mood and relapse risk. That resting state activity differences largely explained the differences in emotional reactivity underscores the importance of understanding intrinsic activity within the brain. Perhaps it is fitting that mindfulness, a practice focused on observant contemplation rather than action, is associated with neural activity when an individual is just “being” rather than “doing.”
Acknowledgments
This research was supported by a National Institute of Mental Health (NIMH) postdoctoral fellowship to BMW as part of the University of California at Los Angeles Health Psychology Program as well as by NIH grants AG030309 and R21MH07152.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo.
References
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9(14):3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala. New York: Wiley-Liss; 1992. pp. 1–67. [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery D. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Archives of General Psychiatry. 1997;54(4):364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–237. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learning and Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences U S A. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research. 2008;32(3):303–322. [Google Scholar]
- Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Research. 2006;146(1):43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Miller KK, Klibanski A, Fischman A, Dougherty DD, Blais MA, et al. Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depression and Anxiety. 2006;23(3):133–138. doi: 10.1002/da.20152. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Research. 2008;162(1):51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology Biochemistry and Behavior. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendrath SJ, Delucchi K, Bitner R, Fenimore P, Smit M, McLane M. Mindfulness-based cognitive therapy for treatment-resistant depression: a pilot study. Psychotherapy and Psychosomatics. 2008;77(5):319–320. doi: 10.1159/000142525. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27(22):5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitve, Affective, and Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kenny MA, Williams JM. Treatment-resistant depressed patients show a good response to Mindfulness-based Cognitive Therapy. Behaviour Research and Therapy. 2007;45(3):617–625. doi: 10.1016/j.brat.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hwang JH, Park HS, Kim SE. Resting brain metabolic correlates of neuroticism and extraversion in young men. Neuroreport. 2008;19(8):883–886. doi: 10.1097/WNR.0b013e328300080f. [DOI] [PubMed] [Google Scholar]
- Kingston T, Dooley B, Bates A, Lawlor E, Malone K. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychology and Psychotherapy. 2007;80(Pt 2):193–203. doi: 10.1348/147608306X116016. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. New York, NY: McGraw-Hill; (in press) [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clinics of North America. 2003;13(4):805–815. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, et al. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62(6):600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biological Psychiatry. 2001;50(3):159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale J. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York: Guilford Press; 2002. [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G, Fiez J, Corbetta M, Buckner R, Miezin F, Raichle M, et al. Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry. 2002;7(4):234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Williams JM, Russell I, Russell D. Mindfulness-based cognitive therapy: further issues in current evidence and future research. Journal of Consulting and Clinical Psychology. 2008;76(3):524–529. doi: 10.1037/0022-006X.76.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]