Abstract
Utilizing the Citrobacter rodentium (CR)-induced transmissible murine colonic hyperplasia (TMCH) model, we provide mechanistic basis of changes in β-catenin/APC/CKIε leading to progression and/or regression of hyperplasia in vivo. In response to CR-induced TMCH, crypt lengths increased significantly between Days6–27 post-infection, followed by a steep decline by Day34. β-Cat45/total β-catenin were elevated on Day1 post-infection, preceding changes in crypt length, and persisted for 27days before declining by Day34. Importantly, cellular CKIε and β-catenin co-immunoprecipitated and exhibited remarkable parallel changes in kinetics during hyperplasia/regression phases. β-catenin, phosphorylated at Ser33,37 and Thr41 (β-cat33,37/41), was low till Day12, followed by gradual increase until Day27 before declining by Day34. GSK-3β exhibited significant Ser9-phosphorylation/inactivation at Days6–12 with partial recovery at Days27–34. Wild type (wt) APC (p312) levels increased at Day6 with transient proteolysis/truncation to p130 form between Days12–15; p312 reappeared by Day19 and returned to baseline by Day34. The kinetics of β-Cat45/β-catenin nuclear accumulation and acetylation (Ac-β-CatLys49) from Days6–27, followed by loss of phosphorylation/acetylation by Day34 was almost identical; Tcf-4 co-immunoprecipitated with β-Cat45/β-catenin and localized immunohistochemically to β-Cat41/45-positive regions leading to elevated cyclin D1 expression, during the hyperproliferative, but not regression phases of TMCH. CKIε mediated phosphorylation of β-Cat45, resulting in stabilization/nuclear translocation of β-Cat45 may be critical for maintaining proliferation at Days6–27. Reversal of GSK-3β phosphorylation and APC changes may be equally critical during the regression phase from Days27–34.
Keywords: Colon, Hyperproliferation, Hyperplasia, Murine, In Vivo, Colon Cancer, Bacterial Infection
Introduction
The normal intestinal epithelium maintains a very tight balance among proliferation, differentiation, migration and cell death to ensure perpetual renewal. Tumorigenesis occurs when these mechanisms become uncoupled, resulting in hyperproliferation at the cost of differentiation/apoptosis. Hyperproliferation of colonic crypt cells has been shown to be a risk factor for colorectal cancer (CRC) [1,2]. Sporadic CRCs are generally associated with mutations in the Apc (adenomatous polyposis coli) gene and, to a lesser extent, in the β-catenin-encoding gene. Both APC and β-catenin are components of the Wnt signaling pathway which controls the specification, maintenance and activation of intestinal stem/progenitor cells. Deregulation of this pathway due to either genetic or epigenetic defects can potentially result in the development of familial and/or sporadic epithelial cancers [3].
The cellular β-catenin resides mainly at sites of intercellular (adherens) junctions where it interacts with E-cadherin and α-catenin. In the absence of Wnt signal, the cytoplasmic pool of β-catenin is limited and undergoes rapid degradation when associated with axin and APC via sequential phosphorylation, first at Ser45 (β-cat45) by casein kinase I (CKI) and then at Ser33,37/Thr41 by glycogen synthase kinase-3β (GSK-3β) leading to targeted ubiquitination through E3 ubiquitin ligase, β-transducing repeat containing protein (βTrCP) and proteasomal degradation [4–8]. As a consequence, Wnt target genes are not up-regulated. When the Wnt ligand binds its cognate Frizzled/LRP5,6 receptor, Axin is recruited to the plasma membrane leading to either its degradation and/or dissociation from the multiprotein complex. Concomitantly, Wnt signaling promotes inactivation of GSK3β causing β-catenin to accumulate in the cytoplasm. Stabilized β-catenin interacts with transcription factors of the T-cell factor/lymphoid enhancing factor (TCF/LEF) family to form a bipartite complex [9,10]: TCF/LEF factors provide the DNA-binding specificity, while β-catenin provides transactivation domains. This bipartite complex then leads to the expression of Wnt target genes, such as c-myc and cyclin D1 [11,12], well known for their role in cell proliferation and oncogenesis. β-Catenin participates in transactivation by recruiting two other transcriptional co-factors, CBP/p300 acetyltransferase and the chromatin-remodeling protein Brg-1, to TCF target gene promoters [13–15].
Nuclear accumulation of β-catenin is considered a hallmark of activated canonical Wnt signaling. Accumulation of nuclear β-catenin is observed in the crypt stem cell/progenitor compartments at the bottom of normal adult crypts in small and large intestines [16,17]. Recent studies in rodents illuminate the role of canonical Wnt signaling in proliferation of normal intestinal epithelium during development and adult stages of life [18]. Given that hyperproliferation is one of the earliest changes noted in the epithelium of humans at high risk for colon cancer [1,2], it is important to delineate signaling mechanisms which drives hyperproliferation, including components of the Wnt signaling pathway.
We have used the transmissible murine colonic hyperplasia (TMCH) model which involves the earliest molecular and functional changes that place the colon at risk for subsequent development of carcinoma [19]. TMCH is caused by Citrobacter rodentium (CR) infection (a naturally occurring disease of laboratory mice), and is characterized by epithelial cell hyperproliferation in the distal colon [20]. We have shown previously that TMCH exhibits both functional and molecular changes typical of the earliest stages of neoplastic transformation: increases in levels of cellular/nuclear β-catenin in the colonic crypts of mice infected with CR, preceding hyperplasia and upregulation of down-stream targets of β-catenin, including cyclin-D1 and c-myc [21]. The latter changes in β-catenin levels are critical for maintaining an elevated cell census during TMCH, since reduced cellular abundance of β-catenin leads to abrogation of hyperproliferation/hyperplasia [22]. Alterations in β-catenin abundance and nuclear translocation were not due to mutational inactivation of wild type (p312) APC [23]. More recently, we described a novel mechanism of β-catenin activation during TMCH: Ser9 phosphorylation and inactivation of GSK-3β leading to stabilization, nuclear translocation and DNA binding of both unphosphorylated and β-cat45 [24].
Hyperplasia associated with TMCH generally regresses by 4–6 weeks post infection in Swiss-Webster mice. Thus, in the current study, we examined for the first time changes in β-catenin/APC/CKIε, not only during the extended hyperplastic phase of TMCH, but also during the recovery (regression) phase of TMCH. Results of the current study show that: i) hyperplasia persists until day 27, despite lack of bacterial attachment to colonic mucosa beyond day 9 [22], suggesting possible expansion of stem/progenitor cell population in response to CR infection which needs to be examined in future studies, ii) both un-phosphorylated and Ser45-phosphorylated β-catenin are elevated during long-term proliferative phase of TMCH, associated with surprising truncation of wtAPC, and iii) reappearance of wtAPC p312 along with partially active GSK-3β towards the end of the proliferative phase of TMCH; this may contribute towards degradation of β-catenin at later time points, coinciding with abrogation of hyperplasia between days 27–34. These findings suggest that the self-limiting characteristic of TMCH in outbred mice may require switching off of aberrant Wnt signaling. Thus the TMCH model in outbred mice represents an excellent model for investigating cellular changes in the colonic crypts associated with intestinal infections, which can potentially prime the colonic mucosa towards neoplasia.
Materials and Methods
TMCH
Swiss-Webster mice were given an overnight culture of C. rodentium (CR) mixed with drinking water, as described previously [21–28]. Animals were euthanized at 0, 1, 3, 6, 9, 12, 20, 27, 34-Days-post-infection and distal colons removed. The characteristic findings of TMCH were invariably present: a grossly thickened distal colon, with no other changes noted in the remainder of the colon or within the peritoneal cavity. Crypt length increased significantly with no obvious increase in epithelial or submucosal inflammatory cell numbers.
Isolation of intact colonic crypts, sub-cellular fractionation, Western Blotting, Immunoprecipitation (IP) and Kinase Assays
Distal colonic crypts were isolated for length measurement and processed for biochemical assays as described previously [21–28]. Briefly, distal colons were attached to a paddle and immersed in Ca2+-free standard Krebs-buffered saline (in mM: 107 NaCl, 4.5 KCl, 0.2 NaH2PO4, 1.8 Na2HPO4, 10 glucose, and 10 EDTA) at 37°C for 10–20 min, gassed with 5% CO2-95% O2. Individual crypt units were then separated from the submucosa/musculature by intermittent (30 s) vibration into ice-cold potassium gluconate-HEPES saline (in mM: 100 potassium gluconate, 20 NaCl, 1.25 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 5 sodium pyruvate) and 0.1% BSA. For IP studies, crypt cytosolic or nuclear extracts were normalized for protein concentration and incubated O/N with appropriate antibodies as described previously [23,24,27,28]. Control experiments were performed by carrying out the immunoprecipitations in the presence of the immunizing peptides, or with control IgG antisera.
Activity assay for GSK-3β was carried out after immunoprecipitating isolated crypt extracts from the distal colons of uninfected (normal) and 6, 12, 20, 27, 34-Days post-infected mice with anti-GSK-3β. Kinase reactions in the immuno-precipitates were performed in a total volume of 40 μl of kinase buffer containing 3.75 μg of GSK-3β substrate: YRRAAVPPSPSLSRHSSP-HQ (pS) EDEEE, as described previously [24]. During GSK-3β assay, nonphosphorylated glycogen synthase peptide acted as negative control. Nonspecific 32P incorporation was subtracted from values obtained with the phospho-glycogen synthase peptide.
Immunohistochemistry
IHC (immuno-histochemistry) for phospho β-catenin (β-cat41/45) primarily recognizing β-catenin phosphorylated at Ser45 or Tcf-4 and IMF (immuno-fluorescence) for PCNA (proliferating cell nuclear antigen) was performed on 5-μm-thick frozen sections from distal colons of normal and TMCH mice (day 6–day 34) utilizing the HRP labeled polymer conjugated to secondary antibody using Envision+System-HRP (DAB; DakoCytomation, Carpinteria, CA) with microwave accentuation as described [28]. Antibody controls included either omission of the primary antibody or detection of endogenous IgG staining with goat anti-mouse or anti-rabbit IgG (Calbiochem, San Diego, CA). The visualization was carried out either via light microscope (IHC) or confocal microscopy.
Results
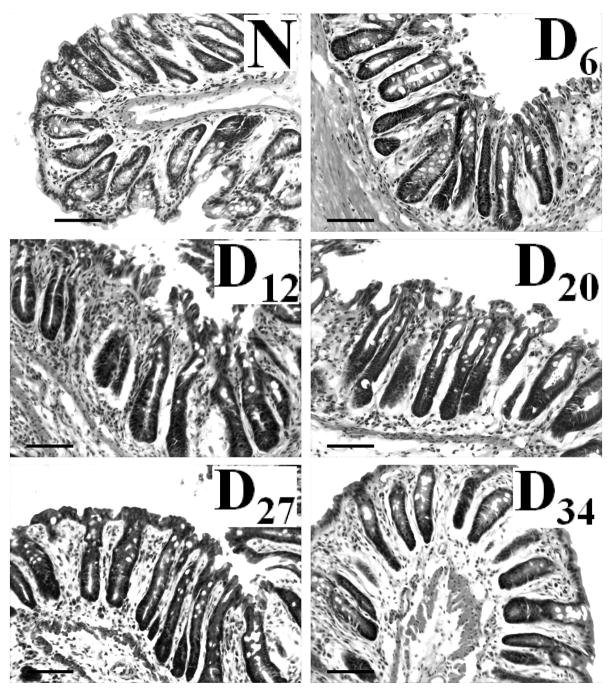
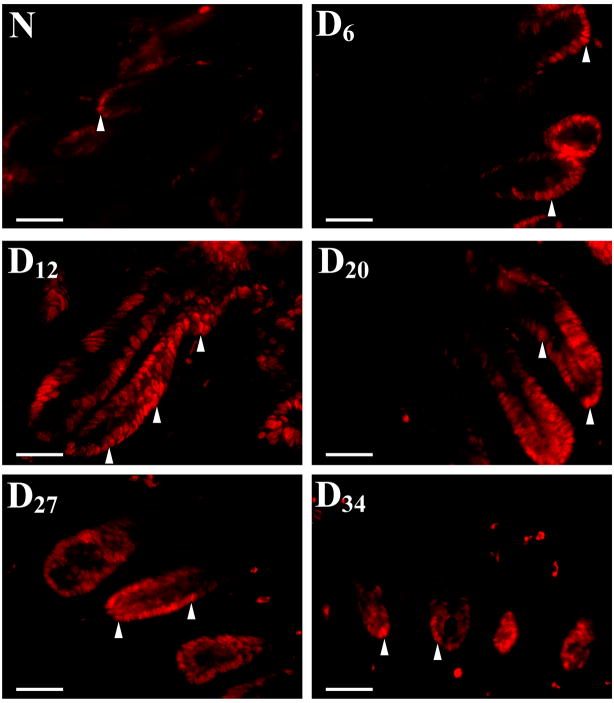
Sequential analysis of H&E stained transverse sections revealed almost 2-fold increase in crypt length by 6 days’ post-infection with continued hyperplasia till Day 27 which finally declined by Day 34 (Figure 1, Table 1). To determine if these increases in crypt length were due to increased proliferation of colonic epithelial cells, we next examined colonic crypt sections for PCNA staining by immunoflourescence as a marker for proliferation. Representative sections from distal colons of uninfected, normal (N), mice and from Days 6–34 post-infected mice are shown in Fig 2. In normally proliferating crypts, only cells at the base exhibited nuclear staining (Fig 2). At Day 6 and particularly at Day 12 TMCH, intense nuclear immunoreactivity extended throughout the longitudinal crypt axis (Fig 2). Intense PCNA nuclear immunoreactivity persisted till Day 27 before returning to base line by Day 34 (Fig 2). Cells positive for nuclear staining for PCNA/crypt were counted (Table 1). Data presented in Table 1 confirms the IMF findings presented in Fig 2 correlating strongly with changes in crypt hyperplasia during TMCH (Figure 1).
Figure 1. Relative hyperproliferation/hyperplasia of colonic crypts during TMCH in Swiss outbred mice, compared to that in non-infected (N) mice.
H&E of transverse sections of distal colons from non-infected (N) and infected (Days 6–34) mice, demonstrating the relative length of the colonic crypts. Bar = 100 μm (n=5).
Table 1.
Crypt lengths and nuclear positivity for PCNA/crypt
| Days of Infection | Crypt length (μm) | # Cells +ve/crypt |
|---|---|---|
| Uninfected (N) | 163±22.0 | 3.0 |
| Day 6 | 310±31.0* | 15.0 |
| Day 12 | 443±66.0* | 35.0 |
| Day 20 | 300±26.0* | 23.0 |
| Day 27 | 290±23.0* | 17.0 |
| Day 34 | 228±20.0* | 4.0 |
P<0.05; n = 5.
Figure 2. Crypt hyperplasia as measured by proliferating cell nuclear antigen (PCNA) staining.
Immunofluorescent labeling of PCNA as a marker of proliferation (arrow heads) in frozen sections prepared from non-infected (N) and infected (Days 6–34) mice. PCNA labeling correlated with changes in crypt hyperplasia during the time course of TMCH. Bar = 75 μm (n=5).
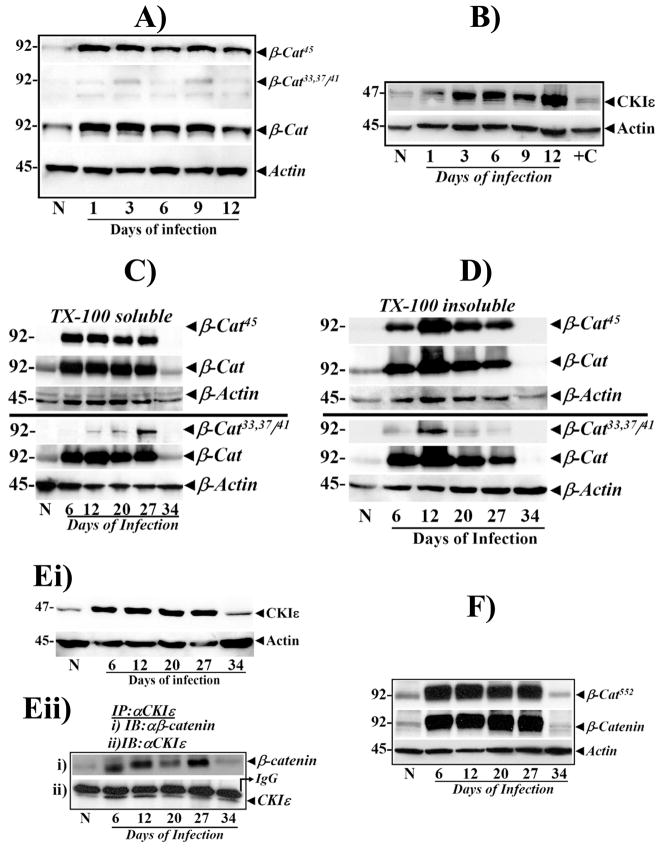
We recently showed that cellular/nuclear levels of total β-catenin and β-cat45 were significantly increased on days 6 and 12 (peak hyperplasia) of TMCH compared to that in the non-infected mice [24], while the levels of β-catenin phosphorylated at Ser33, 37/Th41 (β-Cat33,37,41), did not change [24]. In the present study, we measured kinetics of β-catenin phosphorylation at both early (Days 1–12) and later time periods (Days 20–34) of TMCH and examined moiety-specific phosphorylation of β-catenin. It needs to be clarified that even though observations at days 6 and 12 have been published recently [24], we considered it extremely critical to include these time points in order to understand how changes in the components of the Wnt pathway affect progression and regression phases of TMCH. Ser45-phosphorylation of β-catenin increased as early as day 1 post-infection, preceding gross changes in hyperplasia and persisted until day 12 (Fig 3A). Increases in Ser45-phosphorylation when normalized to total β-catenin levels however exhibited only subtle enhancement in phosphorylation intensity suggesting that increased phosphorylation of β-catenin at Ser45, may be due to a proportional increase in overall β-catenin abundance (Fig 3A). Ser33,37/Thr 41 residues however, failed to show changes in phosphorylation at these time points (Fig 3A), as reported earlier [24]. Since CKIε primes β-catenin for subsequent phosphorylation by GSK-3β, and we have reported significant increase in CKIε expression and activity during Days6 and 12 of TMCH [24], we next measured CKIε levels during days 1–12 of TMCH. Cellular levels of CKIε increased as early as Day 1 post-infection and correlated with increases in β-cat45 (Fig 3B) suggesting that both CKIε-mediated increases in β-cat45 and total β-catenin within a day of CR infection, may regulate its stability and may prime the colonic mucosa for hyperplasia which becomes evident from Day 6 onwards (Table 1; Figs 1, 2).
Figure 3. Relative levels of phosphorylated and total β-catenin at early stages of colonic crypt hyperplasia in relation to cellular CKIε levels.
A.Western blots showing relative levels of phosphorylated and total β-catenin in non-infected (N) and days 1–12 colonic crypt samples. β-Actin was used as loading control (n=5). B. Cellular levels of CKIε in non-infected (N) and Days 1–12 colonic crypt samples. +C is calyculinA-treated HEK293 cells used as positive control. C. Sub-cellular distribution of phosphorylated/total β-catenin in relation to relative levels of CKIε. Crypts isolated from uninfected (N ) and Days 6–34 days post-infected distal colons were fractionated into TritonX-100-soluble (C) and TritonX-100-insoluble (cytoskeletal, D) components and probed with antibodies to β-cat45 or β-cat33,37/41 and total β-catenin (β-cat). β-Actin was used as loading control. E. CKIε expression and co-immunoprecipitation (co-ip) with β-catenin during TMCH. Ei. Cellular crypt extracts prepared from normal uninfected (N) and Days 6–34 post-infected distal colons were probed with anti-CKIε. β-Actin was used as loading control (n=5). Eii. Co-ip with anti-CKIε in N and Days 6–27 colonic crypt extracts followed by blotting with anti-β-catenin and CKIε, respectively. F. Akt and PKA-catalyzed phosphorylation of β-catenin at Ser552. Western blotting of crypt cellular extracts (N and Days 6–34) with an antibody recognizing Ser-552 in β-catenin. β-Actin was used as loading control (n=5).
Sub-cellular distribution of β-catenin during the phases of hyperplasia (Days 6–27) and regression (Days 27–34) was examined. β-Cat45 along with unphosphorylated β-catenin, increased reproducibly in Triton-X-100 soluble fractions at Days6 and 12 of TMCH and remained elevated till Day27 of TMCH, before precipitously declining by Day34 (Fig 3C), mimicking the kinetics of hyperplasia and regression (Figs 1,2). Ser33,37/Thr 41 residues in β-catenin however, failed to show phosphorylation until Day 12 of TMCH (Fig 3C), suggesting lack of GSK-3β action on β-cat45 during early phases of hyperplasia. Interestingly, β-cat33,37/41 exhibited a gradual increase in cellular abundance from Day12 onwards, peaking at Day 27, followed by a rapid decline by Day 34 (Fig 3C). β-Cat33,37/41 levels were, however, relatively lower than that of β-cat45, suggesting only partial recovery of GSK-3β activity at later time points.
We next determined levels of cellular β-catenin/β-cat45 in Triton-X-100 insoluble (cytoskeletal) fraction, to determine if increases in the levels of these proteins are perhaps due to their redistribution from cytoskeleton. Significant accumulation of β-cat45 was observed in cytoskeletal fractions (Fig 3D). Changes in β-cat33,37/41 were however, minimal (Fig 3D). These studies suggest that increases in cellular β-catenin/β-cat45 are not secondary to their redistribution from cytoskeletal fraction, thereby corroborating our earlier findings [24]. We next measured cellular levels of CKIε during the regression phase of TMCH. Consistent with our previous findings (Fig 3B) [24], relative levels of cellular CKIε increased significantly at Day 6 of TMCH and persisted till Day 27, before declining rapidly by Day 34 (Fig 3Ei). To confirm whether CKIε-β-catenin association existed at these time points, co-immunoprecipitation (co-ip) studies were performed in crypt cellular extracts. β-Catenin co-immunoprecipitated with CKIε at Days 6 and 12 (Fig 3Eii) as described earlier [24], and continued to exhibit significant co-immunoprecipitation at Days 20 and 27 but not at Day 34 (Fig 3Eii). These changes correlated with increases in relative levels of cellular β-cat45, suggesting continuous priming of β-catenin by CKIε. While CKIε facilitates N-terminal phosphorylation of β-catenin, kinases such as Akt and PKA phosphorylate β-catenin at C-terminal Ser552 (β-cat552) residue which positively regulates β-catenin’s transcriptional function [29,30]. Since we have observed significant activation of PI3-K/Akt pathway during TMCH (data not shown), we next measured presence of β-cat552 in crypt cellular extracts using an antibody that detects endogenous levels of β-catenin only when phosphorylated at Ser 552. As shown in Figure 3F, β-cat552 increased reproducibly in Triton-X-100 soluble fractions at day 6 of TMCH and remained elevated till day 27 before precipitously declining by day 34 suggesting that elevation in β-cat552 along with β-cat45/total β-catenin may be required in maintaining extended proliferatory activity of distal colonic epithelium during TMCH.
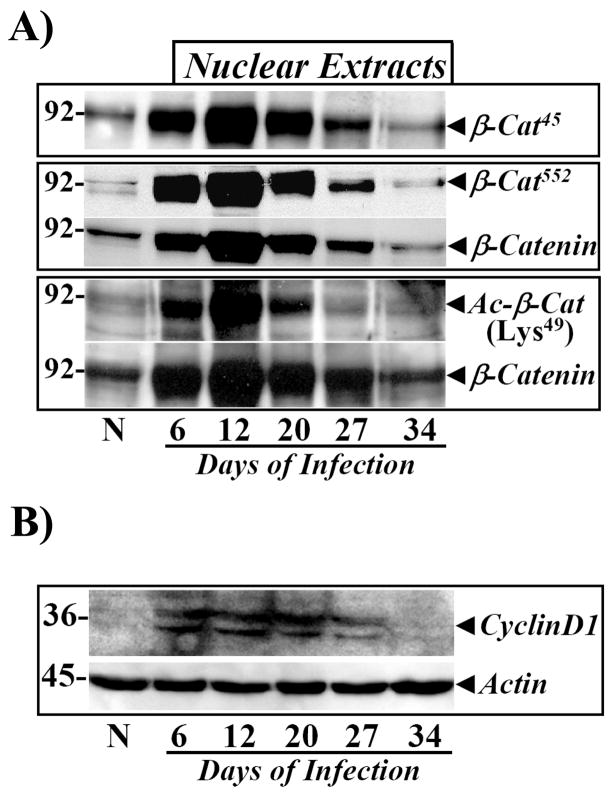
β-Catenin/β-cat45/β-cat552 and Tcf-4 undergo nuclear translocation and co-immunoprecipitate in colonic crypts
To determine whether stabilization of cellular β-catenin/β-cat45/β-cat552 leads to nuclear accumulation, we measured levels of total and phosphorylated β-catenin in the nuclei. Relative levels of total as well as β-cat45 and β-cat552 increased significantly in the nuclei from Days 6–27, followed by a rapid decline to basal levels at Day 34 (Fig 4A). We recently reported that β-cat45 in the nuclei co-immunoprecipitates with a transcriptional-coactivator protein CBP which possesses an integral acetyl transferase activity [24]. Therefore in the current study, we directly measured relative levels of acetylated β-catenin, using an antibody specific for β-catenin acetylated at lysine 49 (Ac-β-cat49). Relative levels of Ac-β-cat49 increased significantly at Day 6 of TMCH and persisted until Day 27, paralleling changes in β-catenin/β-cat45/β-cat552 kinetics during the progression/regression phases of TMCH (Fig 4A). These studies suggest that acetylated β-catenin/β-cat45/β-cat552 may regulate transcriptional coactivation in association with Tcf-4 during TMCH. As a functional readout of changes measured in phosphorylation/acetylation of β-catenin, relative levels of cyclin D1 were measured. The levels of cyclin-D1 increased significantly at Days 6 and 12 of TMCH and remained elevated until Day 27, before complete attenuation by Day 34 (Fig 4B), thereby coinciding with regression of hyperplasia (Figs 1,2; Table 1).
Figure 4. Nuclear accumulation of phosphorylated and acetylated β-catenin in relation to cellular cyclin-D1.
A. Crypt nuclear extracts prepared from normal and Days 6–34 post-infected distal colons were probed with antibodies for β-cat45, β-cat552, total (β-Cat) and acetylated β-catenin (Ac-β-CatLys49), respectively. B. Changes in cyclin-D1 levels parallel proliferation kinetics during TMCH. Cellular crypt extracts prepared from uninfected (N) and Days 6–34 post-infected distal colons were analyzed by blotting with antibody against cyclin-D1. β-Actin was used as loading control (n=5).
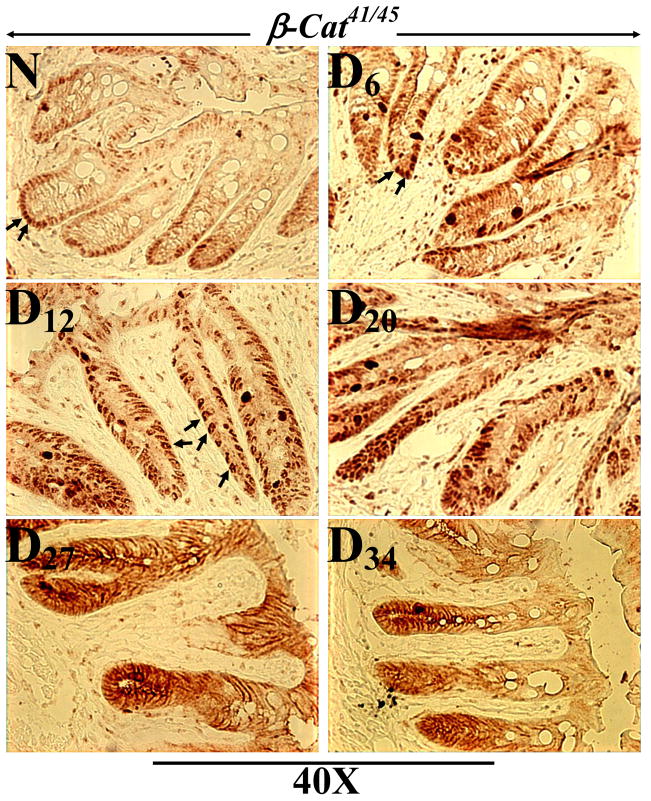
To confirm changes in intracellular distribution of phosphorylated β-catenin during the progression/regression phases of TMCH, IHC studies were performed in frozen sections from distal colons of uninfected (N) and TMCH mice. As shown in Fig 5A, sections from normal colon, stained with anti-phospho β-cat41/45 but exhibited relatively weak staining at the base of the crypt. However, sections from Days 6, 12 and 20 of TMCH colon exhibited significant increase in cytoplasmic and nuclear staining (Fig 5), which correlated with biochemical studies (Figs 3,4). Interestingly at Days 27 and 34, most of the staining was restricted to the lateral membranes (Fig 5) suggesting that this pool of β-catenin may not be involved in signaling. In Supplementary Figure 1, sections counterstained with hematoxylin, to stain the nuclei, are shown.
Figure 5. Immunohistochemistry of phosphorylated β-catenin in frozen sections.
Frozen sections prepared from uninfected (N) and Days 6–34 post-infected mouse distal colons were stained with antibody for β-catenin phosphorylated at Thr41/Ser45 and analyzed with light microscopy (Magnification = 40X; n=3).
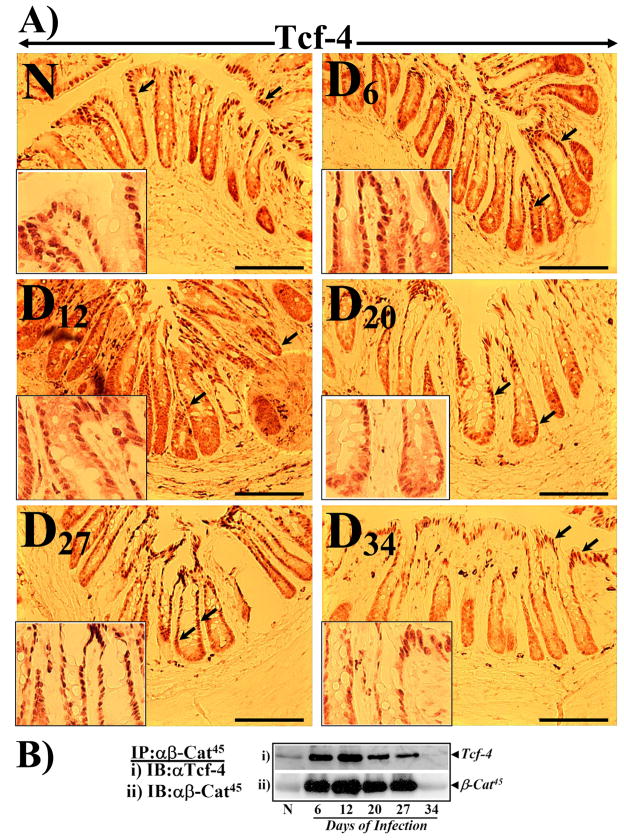
Transcriptional coactivation by β-catenin is achieved through interaction with Tcf-4. We have recently shown that both unphosphorylated β-catenin and β-cat45 interact with Tcf-4 with equal affinity [24]. In the current study, we performed IHC with anti Tcf-4 to determine if Tcf-4 localizes to the regions positive for β-cat41/45 staining. As shown in Fig 6A, Tcf-4 immunoreactivity in the normal colon was restricted to the surface of the crypt (see inset) as reported earlier [31]. At Days 6–27 however, almost the entire longitudinal crypt axis including the crypt base exhibited Tcf-4 nuclear staining (Fig 6A). In particular, at Day 12 of TMCH (peak hyperplasia), crypt base exhibited both diffused cytoplasmic and nuclear staining while staining in the remaining 2/3rd of the crypt was predominantly nuclear (Fig 6A). This pattern of Tcf-4 staining correlated very well with β-cat41/45 staining recorded at the same time point suggesting that β-cat41/45 may co-localize with Tcf-4 in these compartments thereby modulating transcriptional activity during crypt hyperplasia. Supplementary Figure 2 represents counterstaining with hematoxylin to stain the nuclei. Since IHC data with anti-β-cat41/45 predominantly showed membrane staining at days 27 and 34, we used anti-β-cat45 for immunoprecipitation studies. Co-immunoprecipitation with anti-β-cat45 followed by blotting with anti-Tcf-4 exhibited significant interaction of β-cat45 with Tcf-4 at Days 6–27 before declining at Day 34 (Fig 6B). These associations correlated with changes in cyclin-D1 expression during the progression/regression phases of TMCH.
Figure 6. Immunohistochemistry of Tcf-4 in frozen sections and co-immunoprecipitation with β-cat45.
A. Frozen sections prepared from uninfected (N) and Days 6–34 post-infected mouse distal colons were stained with anti-Tcf-4 and analyzed by light microscopy. In normally proliferating crypts, Tcf-4 staining was restricted to mature colonocytes at the crypt surface (arrows; expanded in inset). At Days 6–27, almost the entire longitudinal crypt axis exhibited positive immunoreactivity (arrows; expanded in inset). At Day 34, Tcf-4 staining returned to crypt surface paralleling staining pattern recorded in uninfected crypts (magnification = 20X; n=3). B. Co- immunoprecipitation was performed in N and Days 6–34 crypt nuclear extracts with anti-β-cat45 and immune complexes were probed for Tcf-4 (Bi) and β-cat45 (Bii).
Role of APC and GSK-3β during hyperplasia/regression phases of TMCH
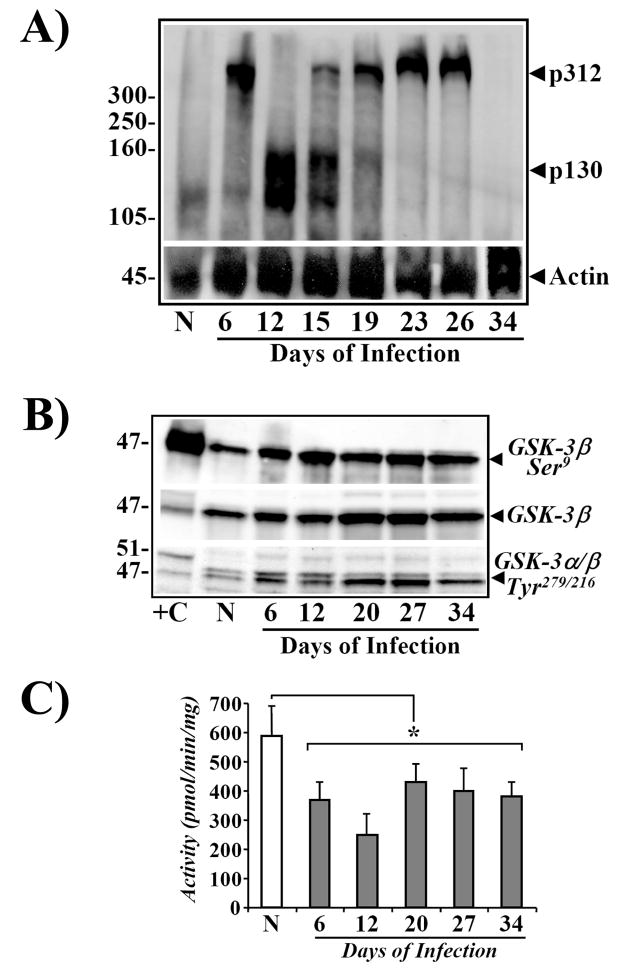
Cytosolic levels of free β-catenin are regulated by APC protein. We have previously shown that increases in cellular abundance of β-catenin at Day 12 were not due to either mutational activation of β-catenin or loss of function of APC protein [23]. In the current studies, we examined cellular levels of APC during both the progression/regression phases of TMCH, by Western blot analysis. A significant increase in cellular levels of p312 was measured at Day 6 followed by appearance of a 130kDa (p130) band at Day 12 of TMCH (Fig 7A). p130 levels, however, started to decline by Day 19 with dramatic reappearance of p312 at Days 19 and 24 which peaked at Day 26; p312 levels returned to baseline at Day 34 (Fig 7A). These findings suggest that truncation followed by reappearance of p312 may be a homeostatic response of the colonic epithelium to perhaps counter the changes in levels of activated β-catenin. The significance of truncation (loss of function?) of APC between Days 12–15 however, remains unknown, but may contribute to the exaggerated increase in cellular β-catenin at Day 12; these intriguing possibilities need to be examined in future studies.
Figure 7.
A. Increases in cellular levels of APC during TMCH. Western blots showing relative levels of APC in non-infected (N) and Days 6–34 post-infected mouse distal colonic crypt samples. Triton X-100-solubilized total crypt extracts were analyzed by blotting with anti-APC N-terminal antibody.25 Protein loading was normalized to β-Actin (n=6). B. Phosphorylation status of GSK-3α/β during TMCH. Western blots showing relative levels of phosphorylated and total GSK-3α/β in non-infected (N) and Days 6–34 post-infected mouse distal colonic crypt samples. Triton X-100-solubilized total crypt extracts were analyzed by blotting with antibodies detecting either Ser-9-phosphorylated (GSK-3β-Ser9) or Tyr-279/216-phosphorylated GSK-3α/β and total GSK-3β. +C, mouse brain extract used as positive control. C. Measurement of GSK-3β activity during TMCH. Crypt cellular extracts from N and Days6–34 post-infected colons were immunoprecipitated with anti-GSK-3β and activity was measured in the immune-complexes with phospho-GSK-3β substrate. *P<0.05 over control; n = 2.
Levels and activity of GSK-3β were also measured to determine whether lack of β-cat33,37/41 at early time points could be due to changes in GSK-3β phosphorylation/inactivation. We recently reported significant Ser9-phosphorylation of GSK-3β leading to 40–70% decrease in its activity [24]. In the current study, we further examined both Ser and Tyr phosphorylation of GSK-3β and also measured its activity in crypt cellular extracts during progression/regression phases of TMCH. Relative levels of Ser 9-GSK-3β increased significantly both at Days 6 and 12 of TMCH, as reported earlier [24], and surprisingly persisted during the regression stages, suggesting that inhibition of GSK-3β activity may not play a major role in dictating the observed changes in β-catenin levels during the regression phase. Interestingly, relative levels of total GSK-3β also increased albeit modestly, during TMCH. In addition to Ser phosphorylation, GSK-3 also undergoes tyrosine phosphorylation at residues 279 (GSK-3α) and 216 (GSK-3β) which leads to GSK-3α/β activation [32]. Since we measured a gradual increase in levels of β-cat33,37/41 on Days 12–27 (Fig 3B) suggesting a partial recovery of GSK-3β activity, we measured Tyr phosphorylation of GSK-3 in cellular extracts of colonic crypts. Relative levels of tyrosine-phosphorylated GSK-3α/β increased significantly at Days 6 and 12 of TMCH, and high levels of Tyr 216-phosphorylated GSK-3β persisted until Day 34 (Fig 7B). To confirm if this led to any reversal in GSK-3β inhibition, we measured GSK-3β activity in anti-GSK-3β-immune complexes. We observed significant inhibition of GSK-3β activity at Days 6 and 12 of TMCH (Fig 7C) as reported earlier [24]. At Day 20, we observed partial recovery of GSK-3β activity (Fig 7C); however, the activity failed to recover completely suggesting that partial recovery may be sufficient to modulate Ser33,37/Thr41 phosphorylation of β-cat45. These cellular changes in GSK-3β and an increase in the levels of CKIε may play an essential role in modulating relative levels of both β-cat45 and total β-catenin during TMCH. The reappearance of p312, along with partially active GSK-3β, may facilitate β-catenin degradation coinciding with abrogation of hyperplasia leading to mucosal recovery from TMCH at Day 34. Thus, it appears that a complicated interplay between cellular changes in some key elements within the Wnt signaling pathway, probably dictates the length of the hyperplastic response of colonic crypts to CR infection in Swiss-Webster mice; the reversal of these changes results in eventual loss of hyperplasia.
Discussion
Activation of β-catenin is recognized as a critical factor in tumorigenesis. Several pathways of β-catenin activation have been delineated, e.g. APC mutations, Wnt signaling and gain-of-function mutations in β-catenin. We previously described novel mechanisms of β-catenin activation in vivo using the transmissible murine colonic hyperplasia (TMCH) model: Ser9 phosphorylation and inactivation of GSK-3β leading to stabilization, nuclear translocation and DNA binding of Ser45-phosphorylated/total β-catenin [24]. As a continuation of these studies, we now present a complicated interplay between cellular/nuclear changes in β-catenin, APC and GSK-3β beyond Day 12 of TMCH, which likely dictates the sustained hyperplastic response of the distal colonic crypts from Days 6–27, culminating in sharp reversal/attenuation of the hyperplastic response at Day 34.
Specifically, utilizing the TMCH model, we demonstrate that: i) Hyperplasia persists until Day 27 despite lack of bacterial presence; ii) β-Cat45 along with unphosphorylated β-catenin, increase by Day 1 post-infection preceding gross changes in crypt morphology and persists until Day 27 of TMCH before regressing by Day 34; iii) Cellular CKIε levels increase and co-immunoprecipitate with β-catenin at these time points correlating with changes in cellular β-cat45 and regression of hyperplasia; iv) Ser33,37/Thr41 residues in β-catenin (β-cat33,37/41), remained unphosphorylated at Days 6 and 12 of TMCH, suggesting lack of GSK-3β action on β-cat45; v) Cellular β-cat33,37/41 levels however, gradually increased at Day 20 and peaked by Day 27 before declining to basal levels by Day 34; vi) Cellular GSK-3β remain phosphorylated at Ser9 and exhibits only partial recovery despite subtle increases in Tyr-216 phosphorylated-GSK-3β at these time points; Cellular levels of total GSK-3β exhibited only modest increase; vii) Wild type APC (p312) expression increases at Day 6 followed by appearance of truncated p130APC at Day 12 TMCH; p130 disappears at Day 19 with concomitant reappearance of p312 which peaks by Day 26 before returning to baseline by Day 34; viii) Cellular β-cat45/β-cat552/β-catenin stabilize, translocate to the nucleus, undergo acetylation at Lys-49, co-immunoprecipitate with Tcf-4 and localize immunohistochemically within Tcf-4-positive regions; ix) Cyclin-D1 expression increases significantly from Day 6–27, before returning to baseline levels by Day 34 correlating with changes in β-catenine/Tcf-4 and with hyperplastic/regression phases of colonic crypt growth in response to CR.
Epithelial renewal in crypts requires coordinated series of events involving proliferation, differentiation and migration towards the intestinal surface. Pluripotent stem cells at the crypt base generate progenitors that occupy the lower third of the colonic crypt. Cells in the amplification compartment divide approximately every 12 hr. In the mid crypt region, the cells differentiate into one of the functional cell types of the colon. At the epithelial cell surface, cells undergo apoptosis and/or extrusion into the lumen. The entire process takes approximately 3–5 days in mice [33]. Proliferation kinetics during TMCH demonstrated significant increase in PCNA immunoreactivity through the length of the crypt axis from Days 6–27, with a peak extension of proliferative zone at Day 12 of TMCH suggesting that altered cytokinetics induced by bacteria may foster an environment which promotes expansion of the proliferative zone during TMCH. Thus, TMCH-associated hyperproliferation/hyperplasia mimics aberrant Wnt signaling wherein constitutive signaling through the canonical Wnt pathway may impose a crypt progenitor phenotype on rapidly proliferating colonocytes.
We have shown previously that epithelial hyperplasia in the distal colon of outbred Swiss-Webster mice infected with C. rodentium is associated with alterations in CKIε that influences β-catenin signaling [24]. In the current study, we once again observed dramatic increases in CKIε abundance and co-immunoprecipitation with β-catenin which paralleled increases in cellular levels of β-cat45 (see Fig 3) during progression/regression phases of TMCH. CKIε [34,35] along with CKIγ [36], and Casein Kinase II [37] have been im plicated as positive regulators of the Wnt signaling pathway. This canonical pathway is regulated by several switches serving to ensure that levels of β-catenin are commensurate with the requirements of the cell during mitogenesis. Recent studies have shown that CKIε functions as one of the switches to direct Dishevelled from the c-Jun N-terminal kinase pathway to the β-catenin pathway thereby positively regulating Wnt signaling [38]. We observed constitutive phosphorylation of β-catenin at Ser45 which partitioned equally into Triton-X-soluble and insoluble fractions along with unphosphorylated β-catenin. Changes in β-cat33,37/41 were however minimal, exhibiting only a gradual increase at later time point of hyperplasia (see Fig 3). These studies suggest that priming of β-catenin at Ser45, probably by CKIε, occurs in vivo as reported earlier [24]. However, β-cat45 does not effectively enter the degradation pathway during the period of sustained proliferatory activity of the colonic epithelium. It is tempting to speculate therefore that upregulation of CKIε expression/activity during TMCH, probably through Wnt, may be critical in the pathogenesis of colonic hyperplasia. However, a cause-and-effect relationship needs to be established in future studies.
APC has many functions; the most prominent is regulation of β-catenin-mediated gene transcription in response to Wnt signaling. Loss of APC leads to de-regulated β-catenin and subsequent neoplasia. However, the increases in β-catenin cellular abundance in TMCH are neither due to mutational activation of β-catenin nor due to dysfunction related to APC protein [23]. In the current study, we measured cellular levels of APC during the progression/regression phases of TMCH in order to understand how changes in APC expression affect β-catenin function. As reported earlier, we observed significant increase in wtAPC (p312) at Day6 of TMCH followed by the surprising appearance of a truncated molecule (p130) at peak hyperplasia. Interestingly, p130 disappeared with concomitant increase in p312 at later time points (see Fig 7A). Such changes in APC expression are novel and have not been previously reported in native epithelium. The increase in APC may represent a homeostatic response by the colonic epithelium to regulate cellular levels of β-catenin during TMCH. However, despite reappearance of p312 at Day 19, which peaked by Day 26, β-cat45/β-catenin did not return to baseline levels until Day 34 suggesting that other cellular signaling events may be linked to non-entry of β-cat45 into the degradation pathway [24].
Indeed, GSK-3β was phosphorylated at Ser9 throughout the 34 days of TMCH, albeit at lower levels towards the end, while total levels of GSK-3β exhibited only modest increase at these time points (see Fig 7B). It has been shown recently that transgenic mice overexpressing kinase-inactive GSK-3β develop mammary tumors with overexpression of β-catenin and cyclin-D1 [39]. Similarly, transgenic mice overexpressing PKCβII exhibit significant decrease in GSK-3β activity [40]. Thus, phosphorylation and inactivation of GSK-3β in vivo is not unprecedented and corroborates earlier findings [24]. GSK-3β also undergoes phosphorylation at Tyr 216 which along with phosphorylation at Tyr 279 for GSK-3α, activates the enzyme [32]. We measured significant phosphorylation of GSK-3α/β at Tyr 279 and 216 respectively during the course of TMCH. Despite these post-translational changes which activate the enzyme, GSK-3β exhibited only partial recovery at later time points (see Fig 7C). It is tempting to speculate that this partially active GSK-3β may be sufficient to promote gradual increase in β-cat33,37/41 at these time points and along with wtAPC, may be instrumental in facilitating β-catenin degradation leading to regression of hyperplasia.
Nuclear accumulation of β-catenin, a hallmark of activated Wnt signaling, results in the induction of a variety of oncogenic target genes which promote cell proliferation and survival. We previously showed that both unphosphorylated and β-cat45 translocated to the nucleus and interacted with Tcf-4 [24]. In the current study, in addition to β-cat45/β-catenin, we also observed significant nuclear accumulation of Akt and PKA-catalyzed β-cat552 suggesting the involvement of Wnt-independent pathway in modulating cellular hyperplasia during TMCH. Tcf-4 has been shown to exhibit highly restricted expression pattern related to the development stage of the intestinal epithelium [31]. In the adult mice, Tcf-4 immunoreactivity is restricted to the crypt surface, away from the proliferatory zone [31]. In the uninfected mice, the pattern of Tcf-4 staining in the colonic crypts was consistent with earlier findings [31]. At Days6–27 however, Tcf-4 staining was observed to extend downwards to areas positive for β-cat45 staining. This was confirmed in a biochemical assay where β-cat45 co-immunoprecipitated with Tcf-4 (see Fig 6B). To our knowledge, this has not been previously reported. Thus, Tcf-4 may work in conjunction with β-cat45 to affect target gene upregulation thereby modulating hyperplasia during proliferative phases of TMCH.
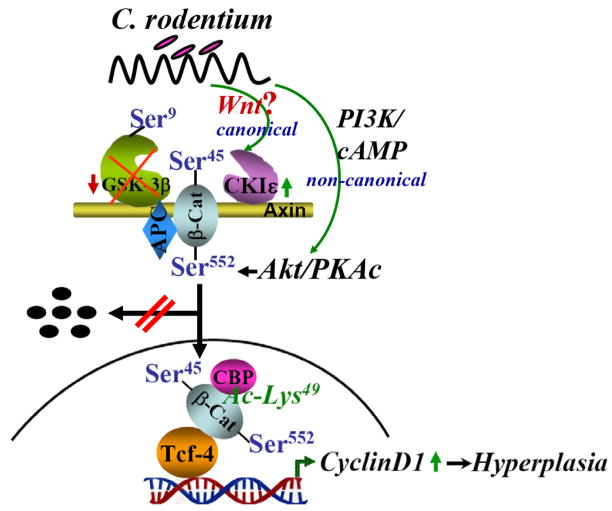
We have shown previously that CREB-binding protein (CBP) co-immunoprecipitates with β-ca45/β-catenin leading to significant increases in acetylation of these catenins [24]. Since CBP binds and acetylates β-catenin at Lys 49 [13,14], we utilized an antibody which detects endogenous β-catenin only when acetylated at Lys 49. Relative levels of Lys-49-acetylated β-catenin increased significantly during proliferative phases of TMCH just as observed with changes in β-cat45/β-cat552/β-catenin; the reverse was measured during regression. A dramatic upregulation of cyclin-D1 expression was measured at days which correlated with changes in Lys-49-acetylated β-catenin levels and increases in cell census during TMCH; a cause-and-effect relationship however, will need to be examined in future studies. Figure 8 describes proposed mechanism of hyperplasia during TMCH.
Figure 8. Proposed mechanism of CR-induced hyperplasia.
Both canonical (through Wnt?) and non-canonical pathways may be involved in modulating β-catenin’s stability and nuclear interaction with CBP and Tcf-4 thereby affecting CR-induced hyperplasia.
Thus, while TMCH is a self-limiting disease in Swiss-Webster mice, persistent β-cat45/β-cat552/β-catenin-mediated hyperplasia until Day 27 of TMCH suggests that there may be an expansion of the stem and/or progenitor cell pool during TMCH. Indeed, transgenic mice overexpressing activated β-catenin demonstrate constitutive renewal and aberrant expansion of the stem/progenitor cell pool [41]. This provocative hypothesis is the focus of our ongoing effort to link changes in cell census during TMCH to mucosal priming for neoplasia.
Supplementary Material
Supplementary Figure 1. Counter-staining of frozen sections with hematoxylin to label the nuclei. Frozen sections prepared from the uninfected (N) and Days 6–34 post-infected mouse distal colons previously stained for β-cat45, were counter-stained with hematoxylin to label the nuclei (n = 3; magnification = 20X).
Supplementary Figure 2. Counter-staining of frozen sections with hematoxylin to label the nuclei. Frozen sections prepared from the uninfected (N) and Days 6–34 post-infected mouse distal colons previously stained for Tcf-4, were counter-stained with hematoxylin to label the nuclei (n = 3; magnification = 20X).
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA-099121 and CA131413) and the Crohn’s and Colitis Foundation of America and by funds from the Gastrointestinal Research Interdisciplinary Program (GRIP), University of Texas Medical Branch.
Abbreviations
- APC
adenomatous polyposis coli protein
- CBP
cAMP response element-binding protein-binding protein
- GSK-3β
glycogen synthase kinase 3β
- TMCH
transmissible murine colonic hyperplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Risio M, Lipkin M, Candelaresi G, Bertone A, Coverlizza S, Rossini FP. Correlations between rectal mucosa cell proliferation and the clinical and pathological features of nonfamilial neoplasia of the large intestine. Cancer Res. 1991;51:1917–1921. [PubMed] [Google Scholar]
- 2.Risio M. Mucosal cell proliferation in colorectal neoplasia. In: Rossini F, Lynch PHT, Winawer SJ, editors. Recent Progress in Colorectal Cancer: Biology and Management of High Risk Groups. 1992. pp. 155–158. [Google Scholar]
- 3.Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 4.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadigan K, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 6.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 9.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 10.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 11.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 12.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf D, Rodova M, Miska EA, Calvet JP, Kouzarides T. Acetylation of beta-catenin by CREB-binding protein (CBP) J Biol Chem. 2002;277:25562–25567. doi: 10.1074/jbc.M201196200. [DOI] [PubMed] [Google Scholar]
- 15.Hurlstone AF, Olave IA, Barker N, van Noort M, Clevers H. Cloning and characterization of hELD/OSA1, a novel BRG1 interacting protein. Biochem J. 2002;364:255–264. doi: 10.1042/bj3640255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 17.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 18.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. Wnt/TCF Signature. Gastroenterol. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Barthold SW, Jonas AM. Morphogenesis of early 1, 2-dimethylhydrazine-induced lesions and latent period reduction of colon carcinogenesis in mice by a variant of Citrobacter freundii. Cancer Res. 1977;37:4352–4360. [PubMed] [Google Scholar]
- 20.Barthold SW, Coleman GLGL, Bhatt PN, Osbaldiston GW, Jonas AM. The etiology of transmissible murine colonic hyperplasia. Lab Anim Sci. 1976;26:889–894. [PubMed] [Google Scholar]
- 21.Sellin JH, Umar S, Xiao J, Morris AP. Increased beta-catenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res. 2001;61:2899–2906. [PubMed] [Google Scholar]
- 22.Umar S, Morris AP, Kourouma F, Sellin JH. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif. 2003;36:361–375. doi: 10.1046/j.1365-2184.2003.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umar S, Wang Y, Sellin JH. Epithelial proliferation induces novel changes in APC expression. Oncogene. 2005;24:6709–6718. doi: 10.1038/sj.onc.1208820. [DOI] [PubMed] [Google Scholar]
- 24.Umar S, Wang Y, Morris AP, Sellin JH. Dual alterations in casein kinase I-epsilon and GSK-3beta modulate beta-catenin stability in hyperproliferating colonic epithelia. Am J Physiol. 2007;292:G599–G607. doi: 10.1152/ajpgi.00343.2006. [DOI] [PubMed] [Google Scholar]
- 25.Umar S, Scott J, Sellin JH, Dubinsky WP, Morris AP. Murine colonic mucosa hyperproliferation, I. Elevated CFTR expression and enhanced cAMP-dependent Cl(−) secretion. Am J Physiol. 2000a;278:753–764. doi: 10.1152/ajpgi.2000.278.5.G753. [DOI] [PubMed] [Google Scholar]
- 26.Umar S, Sellin JH, Morris AP. Murine colonic mucosa hyperproliferation. II. PKC-beta activation and cPKC-mediated cellular CFTR overexpression. Am J Physiol. 2000b;278:765–774. doi: 10.1152/ajpgi.2000.278.5.G765. [DOI] [PubMed] [Google Scholar]
- 27.Umar S, Sellin JH, Morris AP. Increased nuclear translocation of catalytically active PKC-zeta during mouse colonocyte hyperproliferation. Am J Physiol. 2000c;279:224–247. doi: 10.1152/ajpgi.2000.279.1.G223. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Guang-Sheng GS, Kourouma F, Umar S. Citrobacter rodentium-induced NF-kappaB activation in hyperproliferating colonic epithelia: role of p65 (Ser536) phosphorylation. British J Pharmacol. 2006;148:814–824. doi: 10.1038/sj.bjp.0706784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 31.Barker N, Huls G, Korinek V, Clevers H. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am J Pathol. 1999;154:29–35. doi: 10.1016/S0002-9440(10)65247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright NA, Irwin M. The kinetics of villus cell populations in the mouse small intestine. I. Normal villi: the steady state requirement. Cell Tissue Kinet. 1982;15:595–609. doi: 10.1111/j.1365-2184.1982.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 34.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 35.Swiatek W, I-Chun T, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of Casein Kinase Iε Activity by Wnt Signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 36.Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol Cell Biochem. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- 37.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 38.Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of disheveled. Mol Cell Biol. 2005;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, Seldin DC. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005;65:5792–5801. doi: 10.1158/0008-5472.CAN-05-1021. [DOI] [PubMed] [Google Scholar]
- 40.Murray NR, Davidson LA, Chapkin RS, Clay Gustafson W, Schattenberg DG, Fieldsl AP. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zechner D, Fujita Y, Hülsken J, Müller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Counter-staining of frozen sections with hematoxylin to label the nuclei. Frozen sections prepared from the uninfected (N) and Days 6–34 post-infected mouse distal colons previously stained for β-cat45, were counter-stained with hematoxylin to label the nuclei (n = 3; magnification = 20X).
Supplementary Figure 2. Counter-staining of frozen sections with hematoxylin to label the nuclei. Frozen sections prepared from the uninfected (N) and Days 6–34 post-infected mouse distal colons previously stained for Tcf-4, were counter-stained with hematoxylin to label the nuclei (n = 3; magnification = 20X).