Abstract
Psoriasis vulgaris is a multi-factorial heritable disease characterized by severe inflammation resulting in poorly differentiated, hyperproliferative keratinocytes. Recent advances in genetic analyses have implicated components regulating the IL-23 and NFκB pathways as risk factors for psoriasis, and advanced our understanding of this complex disease. These inflammatory pathways exhibit increased activity in skin lesions, and promote secretion of various cytokines such as IL-17 and IL-22. Unrestrained, the activated inflammatory cytokine network in psoriasis may trigger a vicious cycle of inflammation and cellular proliferation that ultimately results in lesion formation. These advances in genetic analyses, together with the progress made in targeted biologic therapy, pave the path to tailor treatment based on an individual’s genetic and immunologic profile.
Introduction
Psoriasis vulgaris is a chronic debilitating disease affecting 1–2% of the Caucasian population.1 It is characterized by recurrent episodes of red, scaly, raised skin plaques, which develop within seemingly normal skin and triggered by a large number of factors such as drugs (i.e. beta blockers, anti-malarial drugs)2, stress, physical injury to the skin (the Koebner response), and infection.3
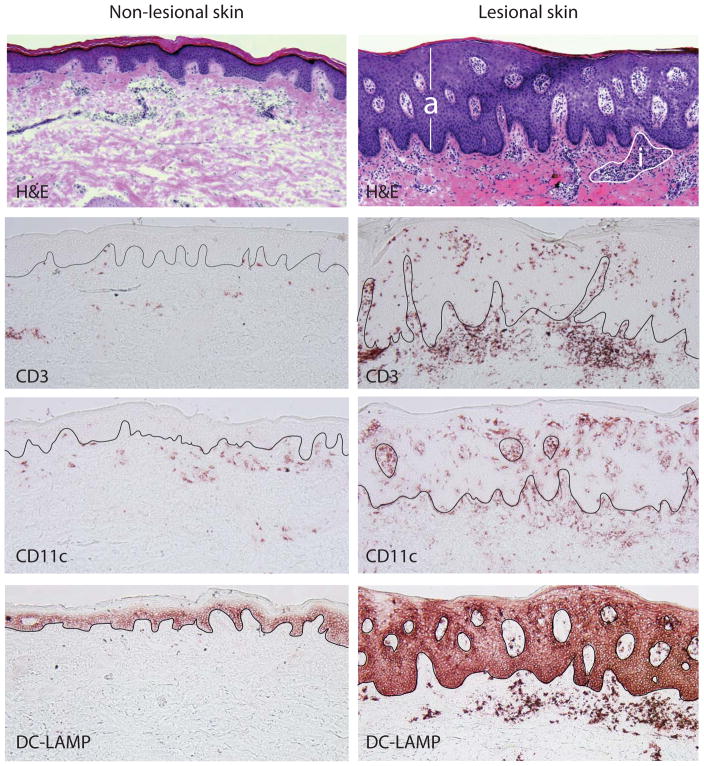
Several defining histologic changes can be observed as lesions develop. These include (1) a thickened epidermis (acanthosis) arising from rapid keratinocyte proliferation, (2) reduced or absent granular layer (hypogranulosis) and retention of nuclei by corneocytes (parakeratosis) as a result of aberrant differentiation of keratinocytes, (3) marked dilation of blood vessels in the papillary dermis causing visible erythema, and (4) a dense inflammatory infiltrate composed of clusters of CD4+ T helper cells and antigen presenting dendritic cells (DCs) in the dermis, and CD8+ T cells and neutrophils in the epidermis.4 (Figure 1)
Figure 1.
Comparative histologic pictures of non-lesional and lesional psoriatic skin demonstrate marked acanthosis (a) and dermal inflammation (i) in psoriasis lesions compared to non-lesional skin (H&E stain). Inflammatory infiltrates in the psoriatic lesion consist of numerous T cells (CD3) as well as dendritic cells (CD11c), many of which are mature (DC-LAMP).
Psoriasis is classified by many as an immune-mediated inflammatory disease (IMID) of the skin. Indeed, the remarkable therapeutic efficacy of a variety of immuno-modulatory agents5–8 have reinforced the vital role of the immune system in psoriasis pathogenesis. Furthermore, a recent explosion of knowledge surrounding emerging T cell, and DC, subsets, have shed more light on specific immune pathways that may be central to lesion formation. The success of targeted therapeutics, as well as advances in genomic analyses, have further implicated these immunological pathways. Here, we review these recent findings and consolidate them into our current understanding of this complex disease.
Clues from Genetic Analyses
Psoriasis is a complex genetic disorder; which means that it is a multi-factorial heritable disease that is influenced by multiple genes and environmental factors.3,9 Several psoriasis associated chromosomal regions (PSORS 1–10) have been identified by conventional family-associated on genetic linkage approach, with PSORS 1, tightly linked to HLA-Cw6 as the most frequent detected allele. 12
However, the full sequencing of the human genome facilitated the identification of single nucleotide polymorphisms (SNPs) that represent subtle coding variations between individuals. These advances provided the means for “mapping” millions of SNPs throughout the human genome10, 11 thus enabling genome-wide association scans (GWAS) to localize genetic alterations that are likely to be involved in disease pathogenesis.
Recent GWAS studies confirm previous findings that the strongest genetic association for psoriasis lies within the HLA-C region.12 HLA-Cw6 was long ago reported to be associated with what is known as “type I psoriasis”, characterized by early age of onset (<40 years), being more likely to be familial, and with a more severe clinical course.13 Yet, the precise role of HLA-C in psoriasis is still unclear.
Interestingly, significant associations have also been found in gene regions involving specific inflammatory pathways, namely, (1) IL-23 signaling (IL23A, IL12B and IL23R), (2) modulation of Th2 immune responses (IL4, IL13), and (2) NFκB signaling.14–16 Other associations include epidermal defense genes that are highly overexpressed in psoriasis: DEFB417 and late cornified envelop proteins 3B and 3C (LCE3C/3D).18 Interestingly, some of these newly found genetic loci were found to overlap with the risk of developing other IMIDs, most notably Crohn’s disease.19
The IL-23/Th17 pathway
IL-23 is a heterodimeric cytokine composed of p19 (encoded by IL23A), and p40 (shared with IL-12 and encoded by IL12B) sub-units, and binds to a receptor complex encoded by IL23R and IL12RB1. IL-23 is produced by dendritic cells and macrophages,20 and is required for the growth, survival, and effector functions of Th17 cells.21
Th17 cells are CD4+ effector T helper cells that are developmentally and functionally distinct from the classic Th1 and Th2 lineages.22 Defined by the ability to produce IL-17, Th17 cells have also been shown to secrete other cytokines including IL-22.23 Similar to Th1 and Th2 cells, Th17 cells are thought to have evolved to provide adaptive immunity against pathogens. Organisms that can trigger a Th17 response include gram-positive bacteria Propionibacterium acnes; gram-negative bacteria Citrobacter rodentium, Klebsiella penumoniae and Bacteroides; Borrelia; Mycobacterium tuberculosis; and fungi Candida albicans.24–28 If Th17 cell differentiation is impaired, as in hyper IgE syndrome, recurrent C. albicans and Staph infections are observed.29
Three psoriasis-associated gene signals, IL23A, IL12B and IL23R, involve components of the IL-23/IL-23R ligand-receptor complex prompting speculation that inappropriate immune responses in psoriasis might center on aberrations in IL-23 signaling.30 Indeed, IL-23 and Th17 cells were found to be markedly abundant in psoriasis lesions,20,31 perhaps as a direct effect of genetic variations in regulatory regions of the above-mentioned genes.
The over-expression of the IL-23/Th17 pathway in psoriasis can explain the overproduction of psoriasin (S100A7) and other innate-defense molecules that typify psoriasis.32 Th17 associated cytokines, IL-17 and IL-22, have been shown to induce keratinocyte expression of anti-microbials β-defensin 2, β-defensin 3, lipocalin and S100 proteins.33,34 Alternatively, genetic polymorphisms in DEFB4 that encodes β-defensin 2 may also contribute to anti-microbial resistance. The expression of another anti-microbial peptide, cathelicidin, can also be enhanced by IL-17 in the presence of vitamin D3.35 These proteins may function as key inflammation inducers as discussed later, and also to decrease skin infections under conditions of a dysfunctional epidermal barrier.
IL-17 may also function as a potent pro-inflammatory cytokine that stimulates keratinocytes to produce neutrophil-attracting CXC chemokines (such as CXCL1, CXCL5 and CXCL8/IL-8), as well as CCL20 that draws CCR6+ cells into sites of inflammation.34,36 CCR6+ cells relevant to the inflammation in psoriasis include myeloid dendritic cells (mDCs) as well as Th17 cells.34,37 Finally, IL-17 can induce fibroblasts to produce IL-6,38 a cytokine that commits naïve T cells to the Th17 lineage, potentially activating a positive feedback loop that perpetuates Th17 inflammation.
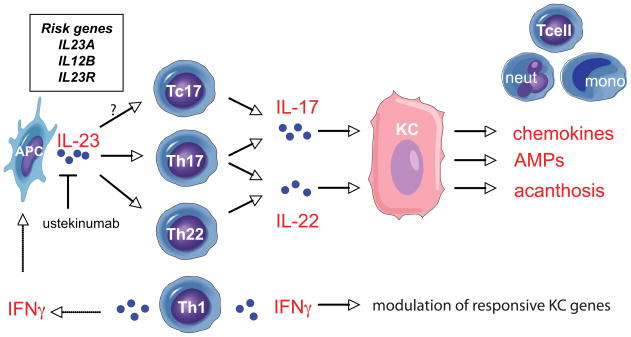
Recently, CD8+ T cells (Tc17) that produce IL-17 have been identified within the psoriatic epidermis.39 These cells may have an important role in promoting psoriatic epidermal response as their contributions obviate the need for cytokines to diffuse from the dermis.30 It is still unclear whether human Tc17 cells are influenced by the same conditions as Th17 cells, although murine models suggest that they might also be driven by IL-23.40 Ustekinumab, a recently FDA-approved monoclonal antibody that binds to the p40 sub-unit, has been shown to be highly effective for the treatment of psoriasis,41 thus further supporting the fundamental role of the IL-23/Th17 pathway in the pathogenesis of psoriasis. (Figure 2)
Figure 2. Model of immune interactions in the psoriatic lesion.
Antigen-presenting cells (APC) produce IL-23 and stimulate Th17 and Th22 cells, and possibly Tc17 cells, to release IL-17 and IL-22. Keratinocytes (KC), in response to IL-17, upregulate pro-inflammatory chemokines that attract T cells, neutrophils (neut) and mononuclear cells (mono) into the lesion. IL-22 promotes epidermal acanthosis, while both cytokines trigger anti-microbial protein (AMP) production. IFNγ, from Th1 cells, modulates numerous KC responsive genes, and stimulates APCs to release IL-23. Ustekinumab, an FDA-approved monoclonal antibody, blocks the p40 sub-unit of IL-23. Recently identified genes associated with psoriasis (box) include IL23A, IL12B and IL23R.
IL-22 function and regulation
IL-23 also stimulates the production of IL-22, an IL-10 family member cytokine that acts mainly on epithelial cells lining the digestive, respiratory and integumentary systems. IL-22 promotes epithelial resistance to injury after microbial infections of the lungs and gut, and may be involved in homeostasis and first-line defense against pathogens.23
In psoriasis, IL-22 is remarkably over-expressed most probably as a result of upregulated IL-23 and IL-6 levels.42,43 As noted above, IL-22 works synergistically with IL-17 to enhance the expression of anti-microbial peptides that are elevated in psoriasis.33 More significantly, it mediates epidermal acanthosis and abnormal differentiation of keratinocytes that are key pathologic findings in psoriasis.33,34,44 (Figure 2)
IL-22 production is commonly attributed to Th17 cells based on early studies utilizing murine models.33,43 Accordingly, we found that ~40% of IL-22-producing T helper cells in psoriasis are Th17 cells.45 However, we have also consistently observed very little overlap between T cells expressing IL-17, and those expressing IL-22, in normal or psoriatic skin.34,45 This has been affirmed by other groups who have also found that majority of IL-22+ cells are single producers that do not co-express IL-17 or the Th1 cytokine, IFNγ.46
These IL-22 producing T helper cells, Th22, co-express CCR6 and skin homing receptors CCR4 and CCR10,47,48 thus may presumably respond to the elevated CCL20 levels in psoriatic skin. IL-6 and TNF, both upregulated in psoriasis, have been shown to enhance Th22 differentiation, while the addition of IL-1β to this mix may promote differentiation of Th17 cells that produce both IL-17 and IL-22.48 Potentially, different DC subsets in psoriasis lesions might regulate Th17 vs. Th22 activation. CD11c+ dermal DCs have been shown to stimulate Th17 cells, while epidermal langerhans cells (LCs) have been shown to stimulate Th22 responses.49
Th1-Th2-Th17 imbalance
Psoriasis lesions contain an excess of Th1 T cells that are activated and produce IFNγ. We have previously demonstrated that IFNγ induces numerous inflammatory molecules in keratinocytes and contributes to inflammation in psoriasis.34 Much of this biology is described in past reports,50 so it will not be furthered discussed here. Recently, IFNγ has been shown to stimulate DCs to produce IL-1 and IL-23 that are Th17 and Th22 promoting cytokines.39 (Figure 2)
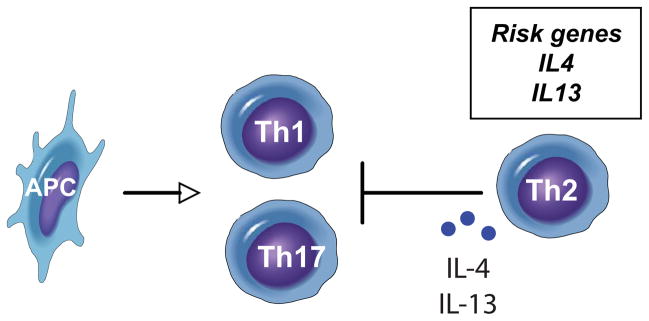
IL-4 and IL-13 are cytokines produced by T cells committed to the Th2 lineage. These cytokines have been shown to negatively regulate pathways induced by TNF, as well as the Th1 cytokine IFNγ, in keratinocytes via the activation of STAT6, SOCS1 and SOCS3.51 Significant clinical improvement was observed with IL-4 treatment for psoriasis,52 that might be attributed to the reduced expression of IL-12 and subsequently Th1 cells.53 IL-4 and IL-13 have been shown to inhibit development of Th17 cells from naïve T cells.54,55 As Th2 T cells, and consequently IL-4 and IL-13 expression, are decreased in psoriasis lesions, this suppression of Th1 and Th17 T cell activity is likely absent. Thus, genetic signals from the IL4/IL13 locus that promote an imbalance in effector T cell subsets might be a determinant for psoriasis. (Figure 3)
Figure 3. Model of Th1-Th2-Th17 interactions.
Effector T cells subsets stimulated by antigen-presenting cells (APC) in psoriasis include Th1 and Th17 cells. Th2 cells and associated cytokines, IL-4 and IL-13, that can suppress Th1 and Th17 activity, are decreased in psoriasis. Genes that confer risk of having psoriasis include IL4 and IL13 (box).
Dysregulated NFκB signaling
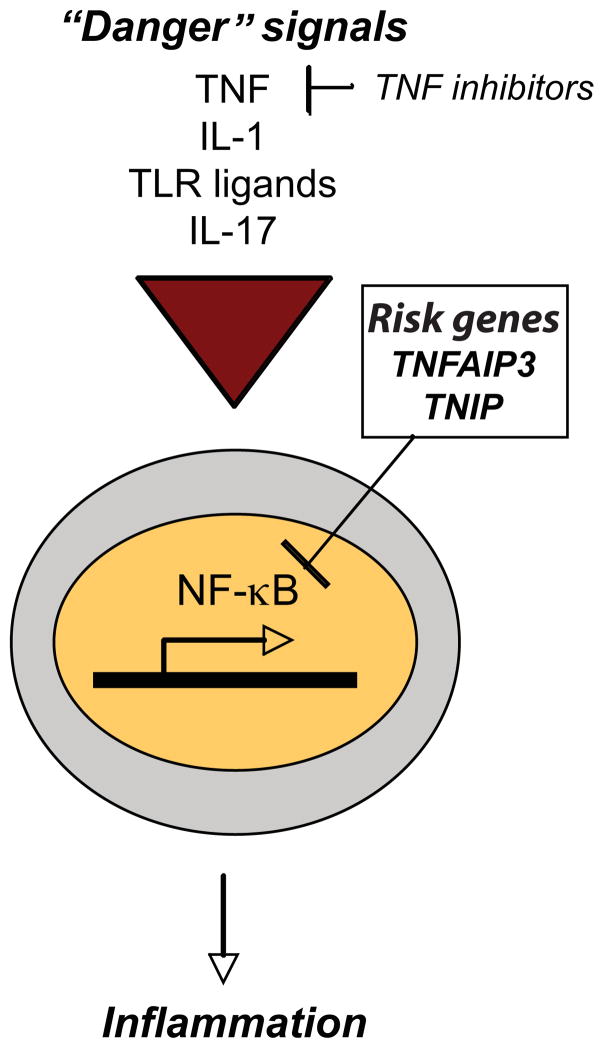
Nuclear factor-κB (NFκB) is a major transcription factor that plays a crucial role in immunology. In resting cells, NFκB is kept inactive by inhibitor of kB (IkB) proteins. Innate “danger” signals, i.e. TNF, IL-1 and toll-like receptor (TLR) signaling, trigger a cascade that phosphorylates, ubiquitinates and ultimately degrades IkB, releasing NFκB which translocates inside the nucleus to promote the transcription of responsive inflammatory genes.56 When unrestrained, chronic NFκB activation is associated with multiple autoimmune diseases.57 It is, thus, important to have negative feedback mechanisms in place to regulate the NFκB pathway.
One of these regulators is the ubiquitin-editing protein A20, encoded by TNFAIP3.56 Mice that are deficient in A20 expire from massive inflammation and tissue damage caused by sustained NFκB activation and enhanced cytokine production.58 This indicates that A20 is crucial for the termination of innate immune responses, and that genetic variations in TNFAIP3 may result in sustained inflammation. This could be relevant for psoriasis pathogenesis as TNF is over-expressed, in part from TNF and iNOS-expressing dendritic cells (TIP-DCs) that are abundant psoriatic dermis.59 In addition to TNF and other innate defense molecules, IL-17 has recently been shown to activate the classical NFκB pathway.60 (Figure 4)
Figure 4. NFκB pathway in psoriasis.
Multiple “danger” signals, including TNF, IL-1, toll-like receptor (TLR) ligands and IL-17, may stimulate the transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), to translocate into the nucleus and promote the transcription of inflammatory genes. Gene polymorphisms that promote unregulated NFkB activity may contribute to psoriasis susceptibility. Genome-wide associated studies (GWAS) have identified polymorphisms in TNFAIP3 and TNIP1, both negative regulators of the NFκB pathway (box).
TNIP1 is another negative regulator that binds to A20 to inhibit NFκB activation.56 (Figure 4) Counter-intuitively, TNIP1 was found to be upregulated in the skin of psoriasis patients versus controls.15 This might imply that defective protein may be produced by gene variations in TNIP1. An alternative explanation could be that excessive TNIP1 inhibits RARα61 potentially disrupting the Th17/Treg balance in psoriasis.62
Consolidating the immunologic pathways
We now have compelling scientific evidence that points to dysregulated immunologic circuits as the core of psoriasis inflammation. But what triggers the inflammatory cascade?
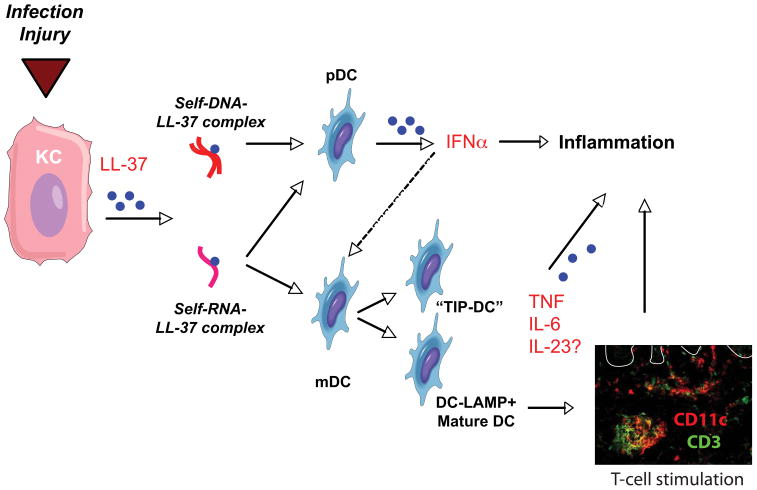
Infections or injury to the skin can promote lesion formation in susceptible individuals. These triggers have recently been shown to stimulate keratinocyte production of the anti-microbial cathelicidin (LL-37)63 that, when complexed with self-DNA, binds to TLR9 on plasmacytoid DCs (pDCs).64 These pDCs produce massive amounts of IFNα and are implicated in the initiation of psoriasis lesions.65 (Figure 5) Accordingly, patients treated with a topical pDC agonist, imiquimod, upregulate IFNα and experience exacerbations in psoriasis.66
Figure 5. Potential initiators of the inflammatory cascade in psoriasis.
Infection and injury stimulate keratinocytes (KC) to release the anti-microbial, cathelicidin (LL-37). LL-37 forms complexes with self-DNA from damaged cells, and stimulates plasmacytoid dendritic cells (pDC) to release IFNα that activates myeloid dendritic cells (mDC). Simultaneously, LL-37 might form complexes with self-RNA to stimulate pDCs, as well as mDCs triggering the release of inflammatory cytokines TNF, IL-6 and, possibly, IL-23. Activation of mDCs by self-RNA-LL37 complexes promotes maturation of dendritic cells (DC-LAMP+ mature DCs) that enhances antigen-presenting capabilities to T cells. Double-label immunofluorescence demonstrates proximity of dendritic cells (CD11c, red) and T cells (CD3, green) in psoriatic dermis. White line delineates dermo-epidermal junction.
In addition to stimulating pDCs, LL-37 has been shown to complex with self-RNA to trigger the activation of myeloid DCs (mDC) through TLR8.67 This stimulates mDC production of TNFα and IL-6, and promotes their differentiation into mature DCs.67 (Figure 5) Interestingly, the self-RNA complexes were found to co-localize with the clusters of DC-LAMP+ mDCs in psoriasis dermis67 previously described by Lowes et al in 2007.59 As myeloid dendritic cells in psoriasis have been shown to produce IL-2320, it is plausible that self-RNA complexes might potentially initiate the inflammatory cascade. (Figure 5)
Upon initiation of the inflammatory cascade, dysregulations in the IL-23 pathway may lead to expansion and activation of Th17 and Th22 T cells. Effects of their cytokine products, as well as TNF and INF-γ, on keratinocytes induce complex inflammatory circuits that stimulate keratinocyte proliferation, vascular proliferation and further leukocyte accumulation and activation in psoriasis lesions. In addition, genetic variations in the IL4/IL13 locus may cause downregulated Th2 responses and promote unregulated Th17/Th1 activity. Finally, decreased efficiency of negative NFκB regulators, TNFAIP3 and TNIP1, might sustain inflammation initiated by TNF, IL-1, TLR ligation, and IL-17, in susceptible individuals.
Recent advances in genetics and immunology have demonstrated the immune pathways relevant to psoriasis pathogenesis. The simultaneous expansion of our pharmacologic armamentarium for psoriasis have made it conceivable that we may eventually be able to stratify patients based on genetic risk factors and immunologic profiles, and tailor their individual treatment accordingly.
Acknowledgments
This publication was made possible by grant number 5UL1RR024143 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The authors do not have any financial interest related to this work.
Footnotes
Disclosure: No relevant conflicts of interest in relations to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Rongioletti F, Fiorucci C, Parodi A. Psoriasis induced or aggravated by drugs. J Rheumatol Suppl. 2009;83:59–61. doi: 10.3899/jrheum.090227. [DOI] [PubMed] [Google Scholar]
- 3.Gudjonsson J, Elder J. Psoriasis. In: Wolff K, Goldsmith L, Katz S, et al., editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill; 2007. [Google Scholar]
- 4.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 5.Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256(22):3110–3116. [PubMed] [Google Scholar]
- 6.Prinz J, Braun-Falco O, Meurer M, et al. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet. 1991;338(8762):320–321. doi: 10.1016/0140-6736(91)90464-z. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb SL, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1(5):442–447. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 8.Tobin AM, Kirby B. TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs. 2005;19(1):47–57. doi: 10.2165/00063030-200519010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85(3):311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 10.Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat Genet. 2003;33 (Suppl):228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- 11.A haplotype map of the human genome. Nature. 2005;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78(5):827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–456. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 14.Nair RP, Ruether A, Stuart PE, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128(7):1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollox EJ, Huffmeier U, Zeeuwen PL, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40(1):23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Cid R, Riveira-Munoz E, Zeeuwen PL, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41(2):211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf N, Quaranta M, Prescott NJ, et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet. 2008;45(2):114–116. doi: 10.1136/jmg.2007.053595. [DOI] [PubMed] [Google Scholar]
- 20.Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199(1):125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19(6):652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung DR, Kasper DL, Panzo RJ, et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170(4):1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 25.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 26.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165(11):6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 27.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41(2):79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 29.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder JT, Bruce AT, Gudjonsson JE, et al. Molecular Dissection of Psoriasis: Integrating Genetics and Biology. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 31.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 32.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32(6):982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 33.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peric M, Koglin S, Kim SM, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181(12):8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedrick MN, Lonsdorf AS, Shirakawa AK, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119(8):2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- 39.Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181(7):4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182(9):5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 41.Reich K, Yasothan U, Kirkpatrick P. Ustekinumab. Nat Rev Drug Discov. 2009;8(5):355–356. doi: 10.1038/nrd2878. [DOI] [PubMed] [Google Scholar]
- 42.Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150(3):407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 44.Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178(4):2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 45.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volpe E, Touzot M, Servant N, et al. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114(17):3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 47.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 48.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 49.Fujita H, Nograles KE, Kikuchi T, et al. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911472106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25(6):295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179(2):984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 52.Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9(1):40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 53.Guenova E, Volz T, Sauer K, et al. IL-4-mediated fine tuning of IL-12p70 production by human DC. Eur J Immunol. 2008;38(11):3138–3149. doi: 10.1002/eji.200838463. [DOI] [PubMed] [Google Scholar]
- 54.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Newcomb DC, Zhou W, Moore ML, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182(9):5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verstrepen L, Carpentier I, Verhelst K, Beyaert R. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol. 2009;78(2):105–114. doi: 10.1016/j.bcp.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82(7):434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 58.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102(52):19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurevich I, Aneskievich BJ. Liganded RARalpha and RARgamma interact with but are repressed by TNIP1. Biochem Biophys Res Commun. 2009;389(3):409–414. doi: 10.1016/j.bbrc.2009.08.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nolting J, Daniel C, Reuter S, et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206(10):2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 65.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilliet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140(12):1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 67.Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206(9):1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]