Abstract
A longitudinal, prospective design was used to investigate a moderation effect in the association between a genetic vulnerability factor, a variable nucleotide repeat polymorphism in the promoter region of 5HTT (5-HTTLPR) and increases in youths’ substance use. The primary study hypothesis predicted that involved-supportive parenting would attenuate the link between the 5-HTTLPR polymorphism and longitudinal increases in substance use. African American youths residing in rural Georgia (N = 253; M age = 11.5 years) provided four waves of data on their own substance use; the youths’ mothers provided data on their own parenting practices. Genetic data were obtained from youths via saliva samples. Latent growth curve modeling indicated that 5-HTTLPR status (presence of 1 or 2 copies of the s allele) was linked with increases in substance use over time; however, this association was greatly reduced when youths received high levels of involved-supportive parenting. This study demonstrates that parenting processes have the potential to ameliorate genetic risk.
Parenting Moderates the Effects of 5-HTTLPR Status on Longitudinal Increases in Youths’ Substance Use
The increase in substance use that some young people experience across late childhood and early adolescence contributes to a nonnormative developmental trajectory that includes affiliation with unconventional peers, a lack of motivation, poor academic performance, and school dropout (Cairns, Cairns, & Neckerman, 1989). In addition, use of illicit drugs before age 14 has prognostic significance for substance use and mental health problems at later ages (Christie et al., 1988; Hawkins et al., 1997). Research on the etiology of substance use in early adolescence has focused on a range of contextual (e.g., parenting, peers, neighborhood) and intra-individual (e.g., temperament, self-regulation, psychological symptomatology) factors (Pandina & Johnson, 1999; Weinberg, Rahdert, Colliver, & Glanz, 1998). This research has begun to move beyond main effects models to address transactions between individual characteristics and environmental contexts that increase or decrease youths’ probability of initiating or avoiding regular substance use. For example, young adolescents with temperamental characteristics, such as high activity levels and emotional intensity, that render them vulnerable to substance use become less likely to use if their family environments provide instrumental and emotional support (Brody, Flor, Hollett-Wright, & McCoy, 1998). Conspicuously absent from transactional analyses to date, though, is a consideration of genetic factors that operate in conjunction with environmental factors to forecast substance use trajectories in early adolescence. The present research was designed to help fill the need for such studies.
Using a longitudinal, prospective research design, we investigated a moderation effect in the association between a genetic vulnerability factor, a variable nucleotide repeat polymorphism in the promoter region of 5HTT (5-HTTLPR), and increases in substance use among African American youths during early adolescence, 11 to 14 years of age. This developmental period is characterized by a precipitous increase in substance use (Johnston, O’Malley, & Bachman, 2000). It is the rate of this increase across early adolescence, and not use at a particular age, that has prognostic significance for adolescent and young adult substance use and abuse, psychosocial functioning, and mental health outcomes (Centers for Disease Control and Prevention, 2000). Because the study hypotheses were tested with four waves of data, the likelihood of a spurious finding is less than it would be if one wave of data were used (Singer & Willett, 2003). We predicted that involved-supportive parenting would attenuate or eliminate the link between the 5-HTTLPR polymorphism and longitudinal increases in substance use across early adolescence. Among African American parents, involved-supportive parenting is characterized by high levels of emotional support, instrumental assistance, and communication (Brody, Chen, et al., 2006). Research has consistently indicated that involved-supportive parenting has protective moderation effects for African American youths during early adolescence, reducing the impact of risk factors on youths’ substance use (Brody et al., 2004; DiClemente et al., 2001; K. S. Miller, Kotchick, Dorsey, Forehand, & Ham, 1998; Wills, Gibbons, Gerrard, Murry, & Brody, 2003; Wills, Murry, et al., 2007). These parenting processes will be described in more detail later. In the following sections, we first discuss gene × environment interactions, a central construct around which this study is organized. We then describe the theoretical and empirical bases for the study hypotheses.
Ecological (Bronfenbrenner & Ceci, 1994), systems (Lerner, 1991), and resilience (Luthar, 2006) approaches to lifespan development share the tenet that biological predispositions transact with contextual processes to create variations in phenotypes over time. Such transactions are termed gene × environment (G × E) interactions, which occur when genetic variation alters an organism’s sensitivity to specific environmental effects or when environmental features exert differential control over genetic effects (Kendler & Eaves, 1986). In this report, we focused on the role of the environment, and, more specifically, parenting, as a moderator of genetic vulnerability for substance use rather than the reverse. This decision was based upon findings from research programs with rural and urban African American families. This research detected powerful protective factors against substance use among children and adolescents that originated in the family environment, particularly in parents’ caregiving practices (Brody et al., 2004). The finding that African Americans’ parenting practices could attenuate or eliminate associations between genetic risk and substance use would constitute a powerful demonstration of those practices’ protective capacities and have considerable heuristic value for the development of preventive intervention programs.
We hypothesized that a functional polymorphism in the promoter region of the serotonin transporter gene, 5HTT, would be associated with longitudinal increases in youths’ substance use. The 5HTT is a key regulator of serotonergic neurotransmission, localized to 17p13 and consisting of 14 exons and a single promoter. The common polymorphism in the promoter region results in two variants, a short and a long allele, with the short allele resulting in lower serotonin transporter availability. The short variant contains 12 copies of a 22bp repeat element and the long variant has 14 copies of the repeat element. Youths with one or two copies of the 5HTT “short” allele are hypothesized to display greater increases in substance use across time than youths with two copies of the “long” allele. Findings from different literatures converge to support this hypothesis. Results of two recent meta-analyses indicated that the short allele variant of 5-HTTLPR significantly, albeit modestly, predicts alcohol dependence among adults (Feinn, Nellissery, & Kranzler, 2005; Lichtermann et al., 2000) and another synthesis of the literature suggested a link between the short allele variant of 5-HTTLPR and opioid dependence among adults (Kreek, Nielsen, Butelman, & LaForge, 2005). These literatures, however, are not entirely consistent; in some studies, no associations between the short allele variant of 5-HTTLPR and adult alcohol dependence were detected (e.g., Dick et al., 2007; Gelernter, Kranzler, & Cubells, 1997; Gorwood, Batel, Hamon, & Boni, 2000), whereas in others the long, rather than the short, allele variant was found to be associated with alcohol dependence among Korean male alcoholics (Kweon, Lee, & Lee, 2005) and obsessive cravings among alcohol-dependent adults (Bleich et al., 2007).
Although the literature addressing the association between the short allele variant of 5-HTTLPR and alcohol dependence among adults has yielded some mixed results, recent studies with non-alcohol-dependent populations apparently provide more consistent findings. The short allele variant of 5-HTTLPR has been found to be associated with alcohol consumption among college students (Herman, Philbeck, Vassilopoulos, & Depetrillo, 2003), maltreated youths (Kaufman et al., 2007), and a large, representative population in the United Kingdom (Munafò, Lingford-Hughes, Johnstone, & Walton, 2005). Taken together, these studies suggest that the presence of the short allele variant of 5-HTTLPR may be a risk factor for increases in substance use across early adolescence.
Other studies provide a different kind of support for this link. Rather than examining the direct association between 5-HTTLPR and substance use, these researchers examined associations between 5-HTTLPR and indicators of low self-control, a risk factor for substance use (W. R. Miller & Brown, 1991; Rutter et al., 1997). This perspective has been supported by data showing that indicators of low behavioral and emotional self-control forecast the initiation and escalation of substance use in early adolescence (Brody & Ge, 2001; Mezzich et al., 1997; Wills et al., 2003). The 5-HTTLPR gene has been found to be associated with indicators of low self-control in both children (high activity, low attention, high levels of negative affect; Auerbach, Favoy, Ebstein, Kahana, & Levine, 2001; Propper & Moore, 2006; Suomi, 2004) and adults (disregard for rules, impulsivity, high levels of negative affect; Burt, 2006; Headley & Wearing, 1989; Kendler, Gardner, & Prescott, 2003; Kreek, Nielson, & LaForge, 2004). These studies converge to support consideration of 5HTT as a plausible predictor of increases in alcohol use during early adolescence.
Studies that include assessments of 5HTT and psychosocial experiences suggest a focus on moderation. A noteworthy example is a study by Caspi et al. (2003), in which a sample drawn from the Dunedin Longitudinal Study was used to test the hypothesis that the level and severity of depressive symptoms in early adulthood would be a product of maltreatment during childhood and the presence of one or two copies of the short-allele variant at the 5-HTTLPR locus. A significant and substantial G × E effect confirmed the hypothesis. This prospective finding is notable in theoretical terms because it demonstrated that genetic variability at 5HTT altered individuals’ reactivity to the psychosocial experience of childhood maltreatment. Several attempts have been made to replicate these findings, with the preponderance evincing similar results. As in Caspi and colleagues’ study, the criterion variable in the replication studies was the severity of depressive symptomatology and the studies were designed to test moderation effects between the short-allele variant of 5-HTTLPR and indicators of psychosocial risk that indexed negative life events (Dick et al., 2007; Eley et al., 2004; Gillespie, Whitfield, Williams, Heath, & Martin, 2005; Grabe et al., 2005; Kaufman et al., 2004; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005; Taylor et al., 2006; Surtees et al., 2006; Wilhelm et al., 2006; Zalsman et al., 2006) and low SES (Manuck, Flory, Ferrell, & Muldoon, 2004). All but the studies by Gillespie et al. (2005) and Surtees et al. (2006) replicated Caspi and associates’ findings that the short allele variant of 5-HTTLPR increased reactivity to psychosocial adversity, resulting in elevated depressive symptomatology. Although Dick and associates did not find a G × E interaction between the short allele variant of 5-HTTLPR and stress for alcohol dependence among adults, they replicated Caspi et al.’s (2003) findings by detecting such an interaction for lifetime depression. Fox et al. (2005) tested moderation effects between the short allele and psychosocial risk, as indexed by low family support, in children’s development of behavioral inhibition. As in the aforementioned studies, variation at the 5-HTTLPR locus moderated the association between low family support and the development of behavioral inhibition.
Although these studies have shown with remarkable consistency that the short allele form of 5-HTTLPR heightens reactivity to psychosocial adversity, they did not address an equally important question: Can psychosocial processes ameliorate the risk that 5HTT confers? Although a credible basis exists for hypothesizing protective moderation effects, little, if any, research has explored the possibility that favorable psychosocial conditions can moderate genetic risk, as resilience theorists suggest (Luthar, 2006). The present research addressed this question by examining the protective capacity of involved-supportive parenting.
Research has consistently indicated that involved-supportive parenting protects African American adolescents against substance use (Brody, Murry, et al., 2006). African American youths who receive such parenting are likely to develop behavioral and emotional self-regulation (Brody & Ge, 2001; Wills, Ainette, Mendoza, Gibbons, & Brody, 2007), internalize their parents’ norms for substance use (Brody, Ge, Katz, & Arias, 2000), and refrain from initiating use (DiClemente et al., 2001; K. S. Miller et al., 1998; Perrino, González-Soldevilla, Pantin, & Szapocznik, 2000; Wills et al., 2003). Involved-supportive parenting has also been found to promote a positive, resilient sense of self and to provide an “arena of comfort” when youths are confronted with stress (Luthar, 2006). Another reason to expect involved-supportive parenting to demonstrate protective properties is its propensity for fostering close attachments to parents, which renders youths less likely to use substances because they want to avoid creating difficulties in their relationships with their parents (Jessor & Jessor, 1977; Stacy & Newcombe, 1999). Thus, involved-supportive parenting is conjectured to offset the risks for substance use that the short allele variant of 5-HTTLPR is hypothesized to confer.
Summary of the Present Research
This study was conducted with rural African American youths and their mothers using procedures that have been shown to yield reliable data from substance use research; these include computer-based interviewing, ethnic matching of interviewers and participants, and extensive reassurances concerning confidentiality of the data (Murry & Brody, 2004; Patrick et al., 1994). Youths provided data on their substance use and mothers provided data on their parenting practices. Genetic data were obtained from youths using procedures developed in partnership with rural African American community members (Brody & Beach, 2007). We predicted that, among the overall sample, the presence of one or two copies of the short-allele variant of 5-HTTLPR would be associated with longitudinal increases in substance use and that this association would be attenuated among youths receiving high levels of involved-supportive parenting. We used latent growth curve modeling (Singer & Willett, 2003) with relevant demographic characteristics controlled to test the study hypotheses.
Method
Data were collected as part of a family-based preventive intervention study (see Brody et al., 2004). The data used in the present paper were collected from the families randomly assigned to the control condition, who did not take part in the intervention. Substance use data were obtained when the youths were 11, 12, 13, and 14 years old. Parenting data for the moderation analyses were obtained during the first data collection session and the genetic data were obtained 2 years after the last data collection.
Participants
Participants in the present study included 298 African American families residing in rural Georgia. From each family, a youth who was 11 years old when recruited (58% girls) and one parent provided data. In all cases, the parent who provided the data was the youth’s mother. Although fathers and other caregivers (e.g., grandmothers) were invited to participate in the study, very few were able to do so because of work schedules and child care commitments. At the first data collection session, youths’ mean age was 11.5 years (SD = .45) and mothers’ mean age was 37.8 years (SD = 7.45). Mothers’ mean educational level on a rating scale ranging from 1 to 6 was 4.58 (SD = 1.23), which indicated completion of high school. Of the mothers, 39.9% were married, 2.3% were married but separated, 5.7% were cohabiting with a significant other, 20.1% were in significant relationships but not cohabiting, and 31.9% were not in significant relationships. Mean household monthly income was $2,355.00 (SD = $1,693.34) and mean per capita monthly income was $559.80 (SD = $411.17). Although 80% of the mothers were employed outside the home and worked an average of 39 hours per week, 21% of the families lived below federal poverty standards and another 26% lived within 150% of the poverty threshold; they could be described as working poor (Boatright & Bachtel, 1999).
Schools in four rural Georgia counties provided lists of 11-year-old students, from which youth participants were selected randomly (see Brody et al., 2004). Families were contacted and enrolled in the study by community liaisons who resided in the counties where the participants lived. The community liaisons were African American community members, selected on the basis of their social contacts and standing in the community, who worked with the researchers on participant recruitment and retention. The liaisons sent letters to the families and followed up on the letters with phone calls to the mothers. During the phone conversations, the community liaisons answered any questions that the mothers asked. Families who were willing to participate in the pretest were told that a research staff member would contact them to schedule the administration of the assessment in the families’ homes. Parents gave written consent to their own and the youths’ participation, and youths gave written assent to their own participation. Each family was paid $100 after each of the four assessments.
Of the 298 families who provided data at the first assessment, 289 provided data at all three subsequent assessments, an overall retention rate of 97%. Two years after the fourth data collection, these families were contacted regarding collection of DNA from the youths; 253 (85%) agreed to participate. Families who provided genetic data were compared with those who did not via t-tests conducted on per capita income, maternal education, and the study variables measured at the first assessment, and χ2 tests performed on maternal marital status and youth gender. No differences emerged.
Preparation for the Collection of Genetic Data
Several steps were taken to prepare for DNA data collection. The researchers met regularly over a 2-year period to review the genetic and family process literature. These researchers included a psychiatrist who specialized in human genetics; a biostatistician who specialized in the analysis of genetic data; and a developmental psychologist, a clinical psychologist, and a family scientist, all of whom specialized in family processes. The meetings they held resulted in the formulation of the hypotheses tested in this report. In addition, two focus groups of rural African Americans were formed, one for parents and one for adolescents. Each group included 10 persons who met for 2 hours. These groups were formed to help the investigators understand any concerns that might arise about the collection of DNA and to develop procedures for dealing with these concerns. Several concerns arose that involved procedural clarity, detection, and potential benefits. Many focus group members wanted a clear explanation of the procedures for obtaining the DNA and they wanted to know how DNA collection would advance knowledge about the development of African American youths. This feedback was incorporated into the development of a brochure, available from the first author, that included answers to frequently asked questions. The answers addressed in a straightforward manner the issues the focus groups raised. A copy of the brochure was given to each participating family to provide them with written information that they could consult in addition to the verbal description of the protocol given during DNA collection.
A pilot study was conducted to assess the viability of DNA collection from saliva versus whole blood (Philibert, Zadorozhnyaya, Beach, & Brody, in press). As predicted, concentrations of DNA were higher in blood than in saliva; the saliva samples, however, contained adequate amounts of DNA to permit genotyping. We concluded that the ease and economy of DNA collection from saliva made it appropriate for the research questions we planned to address.
Procedure
Trained African American field researchers conducted computer-based interviews in participants’ homes to gather data on demographics, parenting, and youth substance use. Mothers and youths were interviewed individually and privately; they were told that their answers were strictly confidential and would not be disclosed to anyone within or outside the family. Mothers provided informed consent for their own and the youths’ provision of interview data and the collection of DNA from the youths; youths assented to their own participation.
Measures
The measures were selected for their relevance to the evaluation of the preventive intervention program. They were derived from previous research, which included focus group meetings and pilot testing followed by construct validation of the instruments (Brody et al., 1994; Brody et al., 2004). Parenting data were obtained from mothers and substance use data were obtained from youths.
Demographics
Youth age and gender, and maternal age, employment status, and monthly income, were recorded. Each mother reported her education level on a 10-point scale that was collapsed, to eliminate small cell sizes, into a 6-point measure ranging from grade school to graduate school completion. Each mother reported the numbers of children and adults living in her home, her marital status, and her relationship status.
Involved-supportive parenting
Involved-supportive parenting was operationalized to include high levels of emotional support, instrumental assistance, and communication. Such parenting is positively associated with self-regulation, academic competence, and social competence, and negatively associated with substance use increases, among African American youths across early adolescence (Brody & Ge, 2001; Brody, Kim, Murry, & Brown., 2005; Luthar, 2006). Involved-supportive parenting was measured using mothers’ responses to the Interaction Behavior Questionnaire (Prinz, Foster, Kent, & O’Leary, 1979), which assesses the level of support, involvement, and communication in the parent-child relationship. The 20 true-false items include statements such as, “You listen when your child needs somebody to talk to,” “You understand your child, you know where she/he is coming from,” “You enjoy spending time with your child,” and “You think you and your child get along well.” Cronbach’s alpha for the study sample was .84.
Substance use
Youths reported their past-year cigarette smoking, alcohol use, and marijuana use (Johnston et al., 2000). Past-month use was not included in the analysis because too few youths in this sample, who were 11 years old at the first assessment and 14 years old at the last assessment, reported having used substances during the past month (see Johnston et al., 2000). The items regarding past-year substance use were, “During the past 12 months, how many times have you: smoked cigarettes; drunk beer, wine, wine coolers, whiskey, gin, or other liquor; had three or more drinks of alcohol at one time; smoked marijuana?” These four items were rated on a five-point scale ranging from 0 to 12 times or more; responses were summed to form a past-year substance use index. This composite score for substance use involvement was used in the analyses, a procedure that is consistent with prior research (Brody & Ge, 2001; Hays, Widaman, DiMatteo, & Stacy, 1987; Needle, Su, & LaVie, 1989; Newcomb & Bentler, 1988; Wills, Murry, et al., 2007).
Genotyping
DNA was obtained from youths using Oragene™ DNA kits (Genetek; Calgary, Alberta, Canada). Youths rinsed their mouths with tap water, then deposited 4 ml of saliva in the Oragene sample vial. The vial was sealed, inverted, and shipped via courier to a central laboratory in Iowa City, where samples were prepared according to the manufacturer’s specifications. These data collection procedures were approved by the University of Georgia’s Institutional Review Board. Genotype at 5-HTTLPR was determined for each sample as previously described (Bradley, Dodelzon, Sandhu, & Philibert, 2005). Of the sample, 7.0% were homozygous for the short allele (ss), 36.3% were heterozygous (sl), and 57.7% were homozygous for the long allele (ll). Consistent with prior research (Hariri et al., 2005), genotyping results were used to form two groups of participants: those homozygous for the long allele and those with either 1 or 2 copies of the short allele. Among the participants, 4.4% had a “very long” variant of 5-HTTLPR. Because the activity of this variant on the hypothesized associations has not been well characterized, these youths were excluded from the data analyses.
Results
Descriptive Statistics
As expected, prevalence rates for lifetime use at the first assessment, when the youths were 11 years old, were low: 5.4% for cigarette smoking, 12.4% for alcohol use, 0.7% for heavy drinking, and 0.0% for marijuana use. The mean composite substance use score when the youths were 11 was 0.55, SD = 1.31. Prevalence rates increased over time; at the last assessment, when the youths were 14 years old, lifetime use rates were 21.0% for cigarette smoking, 42.0% for alcohol use, 5.0% for heavy drinking, and 5.0% for marijuana use. The mean composite substance use score was 1.51, SD = 2.19. These rates for African American youths are consistent with data from other studies (Johnston et al., 2000). Descriptive statistics for the parenting variable indicated relatively high levels of involved-supportive parenting (M = 16.73, SD = 3.76). Table 1 presents the means and standard deviations for the study variables.
Table 1.
Means, Standard Deviations, Medians, and Interquartile Ranges (IQR) for Study Variables
| Variables | M | SD | median | IQR |
|---|---|---|---|---|
| 1. Involved-supportive parenting | 16.58 | 3.85 | ||
| 2. Economic distress | 20.86 | 3.78 | ||
| 3. Parental education | 5.00 | 1.25 | ||
| 4. Single parenthood | 1.00 | 1.00 | ||
| 5. Per capita income | 557.20 | 406.40 | ||
| 6. Youth gender | 2.00 | 1.00 | ||
| 7. Substance use, wave 1 | 0.55 | 1.30 | ||
| 8. Substance use, wave 2 | 0.78 | 1.49 | ||
| 9. Substance use, wave 3 | 1.15 | 1.96 | ||
| 10. Substance use, wave 4 | 1.45 | 2.01 |
Plan of Analysis for the Study Hypotheses
Latent growth curve modeling (LGC; Singer & Willett, 2003) was used to test the study hypotheses. Previous studies in which G × E hypotheses about the associations between the short allele variant of 5-HTTLPR and substance use were tested included data collected at one or two time points. In these analyses, participants’ rank orders on a genetic risk factor were compared with their rank orders on an outcome of interest at one or more time points. Such autoregressive procedures examine change over time by using residual change scores obtained by calculating the extent to which substance use scores at one point in time differ from subsequent scores. Thus, change is assessed relative to predicted scores. When four waves of data are available, LGC modeling uses all of the data points to determine change in substance use within individuals, then allows an examination of the predictors of differences among individuals in their rates of change in substance use. As applied to this study, LGC fits an ordinary least squares regression line to the four data points for substance use for each youth; these lines describe change in substance use for each youth over the course of the study. The slope of each regression line indicates the rate at which substance use changes across the four waves of data collection. Thus, change in substance use over time is determined using actual data rather than residual scores.
LGC calculates the mean and variance for the slope and intercept of substance use. LGC treats the substance use intercept and slope as latent variables. Factor loadings for the intercept, or initial level, are fixed to 1. The latent construct corresponding to change in substance use varies depending on the mathematical form being tested (e.g., linear, quadratic, and so forth). In this study, we used a linear model. Because rates of substance use are low for 11-year-olds, and the rate of change across early adolescence has more prognostic significance than does the initial rate for later substance use, substance abuse, and psychological functioning, the study hypotheses focused on the slope of substance use rather than the initial (intercept) substance use level.
Because substance use data are inherently skewed, they pose a threat to LGC modeling’s assumption of joint multivariate normal distribution. Accordingly, we used bootstrapping to correct for skewness and decrease the likelihood of Type I errors in LGC, per the suggestions of Bollen and Stine (1993) and Yung and Bentler (1996). Bootstrapping procedures determine the degree of departure from normality in the data and adjust critical chi-square values, parameter estimates, and standard errors according to the degree of skewness in the data (Davison & Hinkley, 1997). In this study, bootstrapping was applied to the substance use data across the four waves of the study using procedures in Mplus (Muthén & Muthén, 1998–2007). The adjusted critical chi-square values, parameter estimates, and standard errors were used in all of the analyses reported below.
Testing the study hypotheses involved several steps. First, a univariate growth curve analysis was executed to test the measurement model for the latent growth construct, substance use. A second LGC model was evaluated to determine whether the hypothesized association between increases in youths’ substance use and the short allele variant of 5-HTTLPR was statistically significant and to ensure that the hypothesized model would be consistent with the data. A third LGC model was evaluated to determine whether the association between the short allele variant of 5-HTTLPR and the slope of substance use would change when involved-supportive parenting was added as an additional predictor. Finally, a fourth model was executed to estimate the main effects of 5-HTTLPR risk status and involved-supportive parenting, as well as the interaction of 5-HTTLPR risk status and parenting, in predicting substance use slopes. To test the interaction effect, the interaction term was inserted into the LGC model and its strength of association with the latent substance use slope parameter was assessed. In this context, a significant path indicates that the substance use–genetic risk relationship differs across levels of involved-supportive parenting, yielding a G × E interaction. For all of the models described above, the predictor variables were mean centered and maternal education, monthly per capita income, youth gender, and maternal marital status were systematically controlled in every equation when predicting growth in substance use. In addition, all error terms within the model were correlated.
Test of the Study Hypotheses Using LGC Modeling
We began by testing the measurement, or unconditional, model for the substance use latent growth construct by fitting a univariate growth curve for substance use. The model was based on reported levels of substance use at ages 11, 12, 13, and 14, with the intercept specified by setting the factor loading for substance use to 1. The slope for the four observed values of substance use reflected the number of years after the first assessment when each subsequent assessment was obtained. Table 2 presents the intercept, intercept variance, slope, slope variance, and model fit indices for the unconditional model as well as for the conditional models that addressed the study hypotheses. The coefficients presented in Table 1 are standardized. The linear growth curve for substance use across the four waves fit the data well. As the data in Table 2 reveal, the variances for both the intercept and the slope were statistically significant, indicating significant variability around the means and variances for the substance use intercept and slope.
Table 2.
Intercepts, Slopes, and Model Fit Indexes for the Unconditional and Conditional Models
| Model | Intercept (SE) | Slope (SE) | Model Fit Index |
|---|---|---|---|
| Unconditional Model | χ2 = 5.13, df = 5, p = .40; CFI = .92; RMSEA = .01 | ||
| Substance use | |||
| M | .32* (.06) | .20* (.05) | |
| Variance | .34* (.13) | .16* (.07) | |
| Conditional Models (including control variables) | |||
| Model 1 | χ2 = 19.83, df = 15, p = .18; CFI = .96; RMSEA = .05 | ||
| 5-HTTLPR status | −.05 (.10) | .21* (.09) | |
| Model 2 | χ2 = 21.55, df = 17, p = .20; CFI = .97; RMSEA = .04 | ||
| 5-HTTLPR status | −.07 (.11) | .19* (.09) | |
| Involved-supportive parenting | −.02 (.33) | −.02 (.02) | |
| Model 3 | χ2 = 23.55, df = 19, p = .21; CFI = .97; RMSEA = .04 | ||
| 5-HTTLPR status | −.07 (.11) | .19* (.09) | |
| Involved-supportive parenting | −.02 (.05) | −.02 (.03) | |
| 5-HTTLPR status × involved-supportive parenting | .01 (.05) | −.07* (.03) |
Note. Coefficients are standardized. SE: standard error.
p < .05.
In the models that tested the study hypotheses, 5-HTTLPR risk status was dummy coded. Participants with ss or sl alleles were combined into a genetic risk group and assigned a code of 1, participants with ll alleles were assigned a code of 0, and the genetic risk predictor was regressed on the substance use intercept and slope.
The first model demonstrated a good fit to the data. The results were consistent with the first hypothesis: Genetic risk was associated with a significantly higher rate of substance use across early adolescence, β = .21, p < .05. As expected, genetic risk was not significantly associated with the intercept for substance use at age 11. The second model also fit the data well. Genetic risk status continued to forecast substance use across early adolescence, β = .19, p < .05, when involved-supportive parenting was added to the model. Parenting was not associated with either the intercept or slope of substance use.
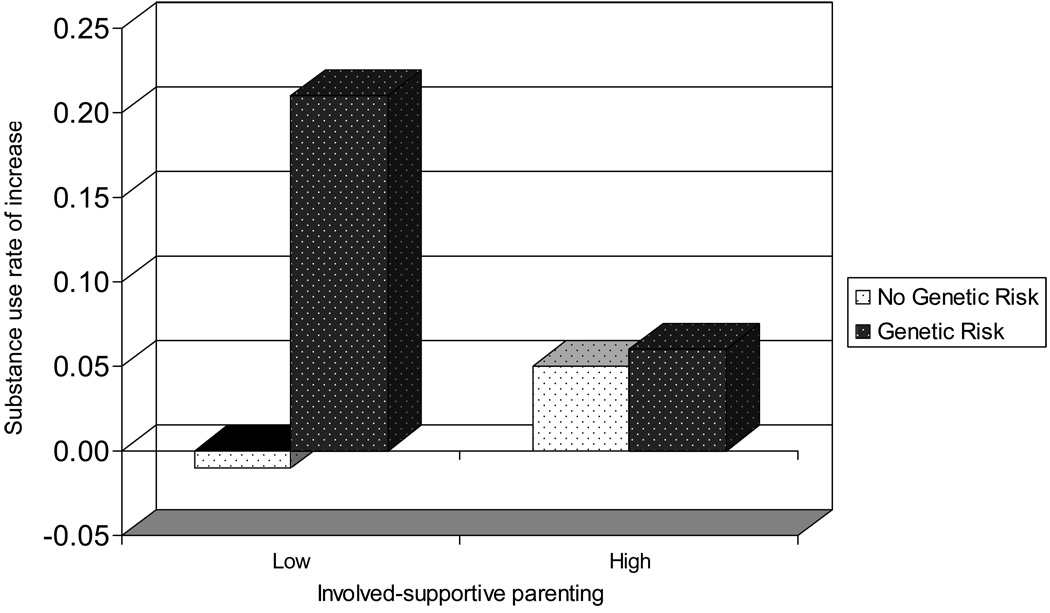
The third model tested the hypothesis that involved-supportive parenting would moderate genetic vulnerability to growth in substance use across early adolescence. Before conducting this test, we determined whether a gene-environment correlation existed between 5-HTTLPR and involved-supportive parenting by correlating genetic risk status and parenting assessed at the first data collection session. The correlation was −.13, ns, indicating that 5-HTTLPR variation was not associated with involved-supportive parenting. The third model also demonstrated a good fit to the data. Genetic risk status was significant in predicting increases in substance use across early adolescence, β = .19, p < .05. Again, involved-supportive parenting was not associated with the intercept or slope of youth substance use. The only other variable that yielded significant predictions beyond the previous variables in the model was the interaction between 5-HTTLPR risk status and involved-supportive parenting, β = −.07, p < .05. The link between 5-HTTLPR risk status and the slope of youths’ substance use decreased as provision of involved-supportive parenting increased. Thus, as hypothesized, involved-supportive parenting moderated the contribution of 5-HTTLPR risk status to increases in youths’ substance use. To illustrate this finding, Figure 1 presents the average slopes, or rates of increase in substance use, for each genetic risk × parenting combination. “Low” levels of involved-supportive parenting are those at or below the 30th percentile for the study sample; “high” levels are at or above the 70th percentile. The figure illustrates two notable findings. First, the average rate of increase of substance use across early adolescence for youth at genetic risk was three times less for youths receiving high (.06) versus low (.21) levels of involved-supportive parenting. Second, the difference in the average rates of increase in substance use for youths with and without genetic risk in the high involved-supportive parenting group was negligible, .06 for the genetic risk group and .05 for the non-risk group. The same comparison for youths with and without genetic risk in the low involved-supportive parenting group was more pronounced. The average increase in substance use was .21 for the genetic risk group and −.01 for the non-risk group. The effect size for the parenting moderation effect on the longitudinal link between 5-HTTLPR and increases in substance use, as calculated using Mplus, was .24. An effect size of this magnitude is typically considered small. Taken together, the data support the hypothesis that involved-supportive parenting has the potential to ameliorate the contribution of genetic risk to growth in youth substance use across early adolescence.1
Figure 1.
Mean rate of increase on the substance use index for genetic risk × parenting combinations. Groups classified as low on involved-supportive parenting include families at or below the 30th percentile for the study sample; groups classified as high include those at or above the 70th percentile.
Discussion
Using a longitudinal design, we tested an LGC model of the links between 5-HTTLPR and the development of substance use among African American youths across late childhood and early adolescence. The results indicated that (a) 5-HTTLPR status was linked positively with the development of substance use and (b) the association between 5-HTTLPR status and the development of substance use was ameliorated when youths received high levels of involved-supportive parenting. To our knowledge, this is the first longitudinal study that integrates data from the molecular-genetic level with assessments of parenting practices to forecast substance use development. The results extend findings from longitudinal analyses in which involved-supportive parenting among African Americans ameliorated the effects of contextual risk on substance use. Previous studies have focused on the protective capacities of parenting practices to ameliorate risk from economic disadvantage, neighborhood disorganization, and racial discrimination (Brody, Murry, Kim, & Brown, 2002; Brody, Chen, et al., 2006; Gibbons, Gerrard, Cleveland, Wills, & Brody, 2004). The present results indicate that the protective effects of involved-supportive parenting also extend to the vulnerability that 5-HTTLPR status confers. Moreover, the moderation effects were relatively strong. The risk conferred by 5-HTTLPR status among youths whose parents engaged in low levels of involved-supportive parenting was three times as large as the coefficient among those whose parents engaged in high levels of such parenting. The moderation effects’ magnitude is comparable to the impact of effective prevention programming (Brody et al., 2004).
As stated previously, the growth model indicated that the slopes for substance use were significantly related to 5-HTTLPR status. This is the first longitudinal study to demonstrate this relation with an African American sample. Although some cross-sectional studies have demonstrated associations between 5-HTTLPR and dependence on alcohol and heroin during adulthood, and other studies have described links between 5-HTTLPR and alcohol use with non-alcohol-dependent samples, no studies have reported a prospective relation with the development of substance use in a community sample. The parenting moderation effects, however, suggest the limitations of main effect genetic models in understanding increases in substance use. Consistent with previous theorizing (e.g., Cadoret, Yates, Troughton, Woodworth, & Stewart, 1996), the current results suggest that gene-environment interactions may be better able than genetic main effects to identify the circumstances under which particular genetic diatheses are associated with substance use.
The finding that involved-supportive parenting buffered the link between 5-HTTLPR and development of substance use raises questions about the protective mechanisms through which it achieved this effect. Earlier we presented evidence that 5-HTTLPR has been associated with indicators of behavioral and emotional self-regulatory systems in adults and children. These self-regulatory systems have been shown to have prognostic significance for the initiation and escalation of substance use. The emotional or affect regulation system includes impulsiveness, impatience, irritability, and anger proneness (Block, Block, & Keyes, 1988; Wills, Sandy, & Yaeger, 2002; Wills, Ainette, et al., 2007). Indicators of the behavioral control system include planfulness, problem solving, and persistence (Rutter et al., 1997). Evidence now indicates clearly that involved-supportive parenting also contributes to the development of both the affective and behavioral self-regulatory systems in African American children and adolescents. For example, the provision of emotional support, instrumental assistance, and communication contribute to youths’ downregulation of negative emotions, such as anger proneness, irritability, and impulsivity, that forecast substance use (Simons, Chen, Stewart, & Brody, 2003). Similarly, these parenting practices facilitate the development of goal setting, planfulness, persistence, and a future time perspective among African American youths; these behavioral self-regulatory processes reduce the likelihood that youths will use substances particularly when they are in situations that give them the opportunity to do so (Brody et al., 2004). We hypothesize that involved-supportive parenting processes buffer youths against risks that the 5-HTTLPR confers on the development of the affect and behavioral self-regulatory systems. By facilitating youths’ ability to downregulate negative emotions, upregulate positive emotions, set goals, plan to attain them, and work persistently toward them, the provision of involved-supportive parenting may override the relation between 5-HTTLPR and the affective and behavioral self-regulatory systems, reducing youths’ risk for substance use. Future research is needed to test this hypothesis.
Two findings merit particular attention.. First, genetic risk status was not associated with substance use at the incept (wave 1) of the growth curve analysis, when the youths were 11. The most plausible explanation for this finding was the low levels of substance use in which the youths had engaged during the previous year, which made detection of an association difficult. This finding highlights a general limitation in the literature that addresses links between genetic risk status and the development of substance use; few, if any, studies examine longitudinal linkages between genetic vulnerabilities and substance use outcomes for youths at different developmental stages. Second, parenting was not associated with the slope of substance use in the presence of 5-HTTLPR; its contribution was its moderation of genetic risk. In prior research, longitudinal links of parenting with substance use and other related outcomes indicated that parenting processes have their greatest effects on youths at highest risk. Presumably, protective parenting processes deter substance use by facilitating youths’ development of inhibitory controls (see Rutter, 1985). Another plausible explanation for the pattern of parenting effects in this study comes from an emerging body of research indicating that individuals with one or two copies of the short allele variant of 5-HTTLPR are more sensitive to both positive and negative aspects of the environment. Youths in the present study with the high-risk genotype may have been more attuned to, and thus benefited more from, protective parenting than were youths with the low-risk genotype (Heinz et al., 2007).
Our ongoing partnerships with rural African American community members were essential in enabling us to gather the data needed for this study. Our prior research indicates that partnerships with community members result in culturally sensitive research procedures and raise engagement rates (Murry & Brody, 2004). In the present study, the focus group process that we developed for our longitudinal developmental and intervention research programs provided us with feedback regarding participants’ concerns about providing DNA samples and suggestions for allaying those concerns. Focus group feedback was incorporated in the development of the question-and-answer pamphlet that we gave to parents and youths when they were asked to provide DNA samples. The focus groups also critiqued the telephone scripts used to inform parents and youths about the DNA collection. Community members’ involvement in the development and implementation of a new research topic yields many benefits; in this case, our participation rates depended on it.
African American children grow up in diverse communities that vary in population density and economic status. The present study included youths from relatively small communities in rural Georgia. Small towns and rural areas are becoming more similar to urban environments as substance abuse increases rapidly outside the inner cities (Helge, 1990). Historically, residing in rural communities has protected African American adolescents from the high-risk behaviors prevalent in urban areas. Recent epidemiologic data, however, indicate that African American youths in rural areas are engaging in substance use at rates equal to or exceeding those in densely populated inner cities (Kogan, Berkel, Chen, Brody, & Murry, 2006). We expect involved-supportive parenting to buffer genetic risk for substance use regardless of families’ residential locations, although this remains an empirical question. Research also suggests that the provision of support, involvement, and communication by a variety of social agents will moderate genetic risk for African American children and adolescents. Reliance on family, extended kin, and community networks has supported African Americans through multiple social transitions (Sudarkasa, 1988). Extended family members’ and informal community mentors’ provision of emotional support, instrumental assistance, and communication should be included in future studies that address processes that compensate for genetic risk.
The results of this study point to the potential of preventive interventions to protect youths from genetic risks for substance use, behavior problems, and emotional difficulties. Longitudinal, prospective studies that identify protective processes against genetic diatheses should inform the design of preventive interventions. As these processes are identified for specific populations, they can be used as malleable targets of universal prevention programs.
Some aspects of the present research should be noted as possible limitations. Future research should assess support, assistance, involvement, and communication from grandparents, extended family members, and other important adults in the community. The provision of support from an array of social agents in addition to parents may ameliorate genetic risk. With older samples, it also may be appropriate to test moderation hypotheses in relation to diagnostic indices of substance abuse or dependence. These cautions notwithstanding, the present study demonstrated that involved-supportive parenting reduced the association between the 5-HTTLPR polymorphism and longitudinal increases in substance use.
Acknowledgments
The research reported in this article was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/ccp/
We investigated the rival hypothesis that the G×E interaction was due to a restriction in range of the substance use composite index for the no-genetic-risk group, which did not permit parenting to relate to substance use. We regressed parenting on the slope for substance use and, if an association emerged, determined whether genetic risk moderated it. Involved-supportive parenting was negatively linked to the slope of substance use. This association was not moderated by 5-HTTLPR status, indicating that the G×E finding obtained was not an artifact of restriction in range of substance use for the no-genetic-risk group.
Contributor Information
Gene H. Brody, Center for Family Research, University of Georgia
Steven R. H. Beach, Institute for Behavioral Research, University of Georgia
Robert A. Philibert, Department of Psychiatry, University of Iowa
Yi-fu Chen, Center for Family Research, University of Georgia.
Man-Kit Lei, Center for Family Research, University of Georgia.
Velma McBride Murry, Center for Family Research, University of Georgia.
Anita C. Brown, Center for Family Research, University of Georgia
References
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Bleich S, Bönsch D, Rauh J, Bayerlein K, Fiszer R, Frieling H, et al. Association of the long allele of the 5-HTTLPR polymorphism with compulsive craving in alcohol dependence. Alcohol and Alcoholism. 2007;42:509–512. doi: 10.1093/alcalc/agm068. [DOI] [PubMed] [Google Scholar]
- Block J, Block JH, Keyes S. Longitudinally foretelling drug usage in adolescence: Early childhood personality and environmental precursors. Child Development. 1988;59:336–355. doi: 10.1111/j.1467-8624.1988.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Boatright SR, Bachtel DC. The Georgia county guide. 18th ed. Athens: University of Georgia Cooperative Extension Service; 1999. [Google Scholar]
- Bollen KA, Stine RA. Bootstrapping goodness-of-fit measures in structural equation modeling. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 111–135. [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics: Part B. Neuropsychiatric Genetics. 2005;136:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH. Adding genetic data to longitudinal, developmental, and randomized prevention research designs: Getting going. 2007, May; Presentation given at the meeting of the Society for Prevention Research; Washington, DC. [Google Scholar]
- Brody GH, Chen Y-f, Murry VM, Ge X, Simons RL, Gibbons FX, et al. Perceived discrimination and the adjustment of African American youths: A five-year longitudinal analysis with contextual moderation effects. Child Development. 2006;77:1170–1189. doi: 10.1111/j.1467-8624.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Flor DL, Hollett-Wright N, McCoy JK. Children’s development of alcohol use norms: Contributions of parent and sibling norms, children’s temperaments, and parent-child discussions. Journal of Family Psychology. 1998;12:209–219. [Google Scholar]
- Brody GH, Ge X. Linking parenting processes and self-regulation to psychological functioning and alcohol use during early adolescence. Journal of Family Psychology. 2001;15:82–94. doi: 10.1037//0893-3200.15.1.82. [DOI] [PubMed] [Google Scholar]
- Brody GH, Ge X, Katz J, Arias I. A longitudinal analysis of internalization of parental alcohol use norms and adolescent alcohol use. Applied Developmental Science. 2000;4:71–79. [Google Scholar]
- Brody GH, Kim S, Murry VM, Brown AC. Longitudinal links among parenting, self-presentations to peers, and the development of externalizing and internalizing symptoms in African American siblings. Development and Psychopathology. 2005;17:185–205. doi: 10.1017/s0954579405050108. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair L, et al. The Strong African American Families Program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Kim S, Brown AC. Longitudinal pathways to competence and psychological adjustment among African American children living in rural single-parent households. Child Development. 2002;73:1505–1516. doi: 10.1111/1467-8624.00486. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Kogan SM, Gerrard M, Gibbons FX, Molgaard V, et al. The Strong African American Families Program: A cluster-randomized prevention trial of long-term effects and a mediational model. Journal of Consulting and Clinical Psychology. 2006;74:356–366. doi: 10.1037/0022-006X.74.2.356. [DOI] [PubMed] [Google Scholar]
- Brody GH, Stoneman Z, Flor D, McCrary C, Hastings L, Conyers O. Financial resources, parent psychological functioning, parent co-caregiving, and early adolescent competence in rural two-parent African-American families. Child Development. 1994;65:590–605. [PubMed] [Google Scholar]
- Burt A. Genes, rule-breaking, and popularity: Identification of an evocative rGE. 2006, July; Paper presented at the annual meeting of the Behavior Genetics Association; Storrs, CT. [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. An adoption study of drug abuse/dependency in females. Comprehensive Psychiatry. 1996;37:88–94. doi: 10.1016/s0010-440x(96)90567-2. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Cairns BD, Neckerman HJ. Early school dropout: Configurations and determinants. Child Development. 1989;60:1437–1452. doi: 10.1111/j.1467-8624.1989.tb04015.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth risk behavior surveillance—United States, 1999. Morbidity and Mortality Weekly Report. 2000;49 [PubMed] [Google Scholar]
- Christie KA, Burke JD, Jr, Regier DA, Rae DS, Boyd JH, Locke BZ. Epidemiologic evidence for early onset of mental disorders and higher risk of drug abuse in young adults. American Journal of Psychiatry. 1988;145:971–975. doi: 10.1176/ajp.145.8.971. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. New York: Cambridge University Press; 1997. [Google Scholar]
- Dick DM, Plunkett J, Hamlin D, Nurnberger J, Kuperman S, Schuckit M, et al. Association analyses of the serotonin transporter gene with lifetime depression and alcohol dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Psychiatric Genetics. 2007;17:35–38. doi: 10.1097/YPG.0b013e328011188b. [DOI] [PubMed] [Google Scholar]
- DiClemente RJ, Wingood GM, Crosby R, Sionean C, Cobb BK, Harrington K, et al. Parental monitoring: Association with adolescents’ risk behaviors. Pediatrics. 2001;107:1363–1368. doi: 10.1542/peds.107.6.1363. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. American Journal of Medical Genetics. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Fox N, Nichols K, Henderson H, Rubin K, Schmidt C, Hamer D, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells J. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody G. Perceived discrimination and substance use in African American parents and their children: A panel study. Journal of Personality and Social Psychology. 2004;86:517–529. doi: 10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Morton N. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychological Medicine. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Batel A, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biological Psychiatry. 2000;48:259–264. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Völzke H, Lucht M, Freyberger HJ, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Molecular Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Widaman KF, DiMatteo MR, Stacy AW. Structural equation models of current drug use: Are appropriate models so simple(x)? Journal of Personality and Social Psychology. 1987;52:134–144. doi: 10.1037//0022-3514.52.1.134. [DOI] [PubMed] [Google Scholar]
- Headley B, Wearing A. Personality, life events, and subjective well-being: Toward a dynamic equilibrium model. Journal of Personality and Social Psychology. 1989;57:731–739. [Google Scholar]
- Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, et al. Serotonin transporter genotype (5-HTTLPR): Effects of neutral and undefined conditions on amygdala activation. Biological Psychiatry. 2007;61:1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Helge D. A national study regarding at-risk students. Washington, DC: National Rural Development Institute; 1990. May, [Google Scholar]
- Herman A, Philbeck J, Vassilopoulos N, Depetrillo P. Serotonin transporter promoter polymorphism and differences in alcohol consumption behavior in a college student population. Alcohol. 2003;38:446–449. doi: 10.1093/alcalc/agg110. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. New York: Academic Press; 1977. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the Future national survey results on drug use, 1975–1999. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2000. NIH Publication No. 00-4802. [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, et al. Genetic and environmental predictors of early alcohol use. Biological Psychiatry. 2007;61:1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the USA. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Eaves L. Models for the joint effect of genotype and environment on liability to psychiatric illness. American Journal of Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Personality and the experience of environmental adversity. Psychological Medicine. 2003;33:1193–1202. doi: 10.1017/s0033291703008298. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kogan SM, Berkel C, Chen Y-f, Brody GH, Murry VM. Metro status and African-American adolescents’ risk for substance use. Journal of Adolescent Health. 2006;38:454–457. doi: 10.1016/j.jadohealth.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: Alcoholism, opiate, and cocaine addiction. Neuromolecular Medicine. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- Kweon YF, Lee HK, Lee CT. Association of the serotonin transporter gene polymorphism with Korean male alcoholics. Journal of Psychiatric Research. 2005;39:371–376. doi: 10.1016/j.jpsychires.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Lerner RM. Changing organism-context relations as the basic process of development: A developmental context and perspective. Developmental Psychology. 1991;27:27–32. [Google Scholar]
- Lichtermann D, Hranilović D, Trixler M, Franke P, Jernej B, Delmo CD, et al. Support for allelic association of a polymorphic site in the promoter region of the serotonin transporter gene with risk for alcohol dependence. American Journal of Psychiatry. 2000;157:2045–2047. doi: 10.1176/appi.ajp.157.12.2045. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 3. Risk, disorder, and adaptation. 2nd ed. Vol. 3. Hoboken, NJ: Wiley; 2006. pp. 739–795. [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;5:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Mezzich AC, Tarter RE, Giancola PR, Lu S, Kirisci L, Parks S. Substance use and risky behavior in female adolescents. Drug and Alcohol Dependence. 1997;44:157–166. doi: 10.1016/s0376-8716(96)01333-6. [DOI] [PubMed] [Google Scholar]
- Miller KS, Kotchick BA, Dorsey S, Forehand R, Ham AY. Family communication about sex: What are parents saying and are their adolescents listening? Family Planning Perspectives. 1998;30:218–235. [PubMed] [Google Scholar]
- Miller WR, Brown JM. Self-regulation as a conceptual basis for the prevention of addictive behaviors. In: Heather N, Miller WR, Greely J, editors. Self-control and the addictive behaviors. Sydney, Australia: Maxwell MacMillan; 1991. pp. 3–79. [Google Scholar]
- Munafò M, Lingford-Hughes AR, Johnstone EC, Walton RT. Association between the serotonin transporter gene and alcohol consumption in social drinkers. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2005;135B:10–14. doi: 10.1002/ajmg.b.30162. [DOI] [PubMed] [Google Scholar]
- Murry VM, Brody GH. Partnering with community stakeholders: Engaging rural African American families in basic research and the Strong African American Families preventive intervention program. Journal of Marital and Family Therapy. 2004;30:271–283. doi: 10.1111/j.1752-0606.2004.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5th ed. Los Angeles, CA: Authors; 1998–2007. [Google Scholar]
- Needle RH, Su SS, LaVie Y. A comparison of the empirical utility of three composite measures of adolescent drug involvement. Addictive Behaviors. 1989;14:429–441. doi: 10.1016/0306-4603(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, Bentler PM. Impact of adolescent drug use and social support on problems of young adults: A longitudinal study. Journal of Abnormal Psychology. 1988;97:64–75. doi: 10.1037//0021-843x.97.1.64. [DOI] [PubMed] [Google Scholar]
- Pandina RJ, Johnson VL. Why people use, abuse, and become dependent on drugs: Toward a heuristic model. In: Glanz MD, Hartel CR, editors. Drug abuse: Origins and interventions. Washington, DC: American Psychological Association; 1999. pp. 119–147. [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino T, González-Soldevilla A, Pantin H, Szapocznik J. The role of families in adolescent HIV prevention: A review. Clinical Child and Family Psychology Review. 2000;3:81–96. doi: 10.1023/a:1009571518900. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Zadorozhnyaya O, Beach SRH, Brody GH. A comparison of the genotyping results using DNA obtained from blood and saliva. Psychiatric Genetics. doi: 10.1097/YPG.0b013e3283060f81. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O'Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavior Analysis. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multilevel psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Rutter M. Resilience in the face of adversity: Protective factors and resistance to psychiatric disturbance. British Journal of Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- Rutter M, Dunn J, Plomin R, Simonoff E, Pickles A, Maughan B, et al. Integrating nature and nurture: Implications of person-environment correlations and interactions for developmental psychopathology. Development and Psychopathology. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Simons RL, Chen Y-f, Stewart EA, Brody GH. Incidents of discrimination and risk for delinquency: A longitudinal test of strain theory with an African American sample. Justice Quarterly. 2003;20:827–854. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Stacy AW, Newcomb MD. Adolescent drug use and adult drug problems in women: Direct, interactive, and mediational effects. Experimental and Clinical Psychopharmacology. 1999;7:160–173. doi: 10.1037//1064-1297.7.2.160. [DOI] [PubMed] [Google Scholar]
- Sudarkasa N. Interpreting the African heritage in Afro-American family organization. In: McAdoo HP, editor. Black families. 2nd ed. Beverly Hills, CA: Sage; 1988. pp. 27–43. [Google Scholar]
- Suomi SJ. How gene-environment interactions shape biobehavioral development: Lessons from the study of rhesus monkeys. Research in Human Development. 2004;1:205–222. [Google Scholar]
- Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biological Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Weinberg NZ, Rahdert E, Colliver JD, Glanz MD. Adolescent substance abuse: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:252–261. doi: 10.1097/00004583-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, et al. Life events, first depression onset and the serotonin transporter gene. British Journal of Psychiatry. 2006;188:210–215. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- Wills TA, Ainette MG, Mendoza D, Gibbons FX, Brody GH. Self-control, symptomatology, and substance use precursors: Test of a theoretical model in a community sample of 9-year-old children. Psychology of Addictive Behavior. 2007;21:205–215. doi: 10.1037/0893-164X.21.2.205. [DOI] [PubMed] [Google Scholar]
- Wills TA, Gibbons FX, Gerrard M, Murry VM, Brody GH. Family communication and religiosity related to substance use and sexual behavior in early adolescence: A test for pathways through self-control and prototype perceptions. Psychology of Addictive Behaviors. 2003;17:312–323. doi: 10.1037/0893-164X.17.4.312. [DOI] [PubMed] [Google Scholar]
- Wills TA, Murry VM, Brody GH, Gibbons FX, Gerrard M, Walker C, et al. Ethnic pride and self-control related to protective and risk factors: Test of the theoretical model for the Strong African American Families program. Health Psychology. 2007;26:50–59. doi: 10.1037/0278-6133.26.1.50. [DOI] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM. Moderators of the relation between substance use level and problems: Test of a self-regulation model in middle adolescence. Journal of Abnormal Psychology. 2002;111:3–21. [PubMed] [Google Scholar]
- Yung Y-F, Bentler PM. Bootstrapping techniques in analysis of mean and covariance structures. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 195–226. [Google Scholar]
- Zalsman G, Huang Y-y, Oquendo MA, Burke AK, Hu X-z, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]