Abstract
Lung cancer is the leading cause of cancer death in the United States. About 85% of all lung cancers are linked to tobacco smoke, in which more than 50 lung carcinogens have been identified and one of the most abundant is 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone (NNK). The human lung epithelium constitutes the first line of defense against tobacco specific carcinogens, in which apically-localized receptors, transporters, and ion channels in the airway may play a critical role in this native defense against tobacco smoke. Here we showed that multidrug resistance protein-2 (MRP2) and cystic fibrosis transmembrane conductance regulator (CFTR), two ATP-binding cassette (ABC) transporters, are localized to the apical surfaces of plasma membrane in polarized lung epithelial cells. We observed that there is a functional coupling between CFTR and MRP2 that may be mediated by PDZ proteins. We also observed the existence of a macromolecular complex containing CFTR, MRP2, and PDZ proteins, which might form the basis for the regulatory cooperation between these two ABC transporters. Our results have important implications for cigarette smoke-associated lung diseases (such as smoke-related emphysema, chronic obstructive pulmonary disease, and lung cancer).
Keywords: Lung cancer, Tobacco-specific carcinogen, NNK, MRP2, CFTR, Protein interaction
1. Introduction
In the United States, about 26 % men and 21% women are smokers. Cigarette smoking, the single most alterable risk factor contributing to premature morbidity and mortality in the United States, accounts for approximately 430,000 deaths annually [1,2]. In fact, one in every five deaths in the United States is smoking related. Men who smoke increase their risk of death from lung cancer by more than 22 times and from bronchitis and emphysema by nearly 10 times. Women who smoke increase their risk of dying from lung cancer by nearly 12 times and the risk of dying from bronchitis and emphysema by more than 10 times [3]. Cigarette smoke contains more than 4000 components with more than 50 known as being carcinogenic which induce lung cancers in laboratory animals [4, 5]. Among those tobacco-specific carcinogens (TSCs), the nicotine-derived 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone (NNK) is one of the most abundant nitrosamines in the human environment and the most potent and specific carcinogen for lung tissues [4].
The human lung epithelium constitutes the first line of defense against TSCs, in which apically-localized receptors, transporters, and ion channels in the airway may play a critical role in this native defense against tobacco smoke. Multidrug resistance protein-2 (MRP2), originally known as the canalicular multispecific organic anion transporter (cMOAT), is one of the ATP-binding cassette (ABC) transporters, the largest family of transmembrane proteins that bind ATP and use the energy to drive the transport of various substrates across extra- and intracellular membranes [6, 7]. Genetic variations in these ABC transporter genes can cause or contribute to a wide variety of human disorders, including Dubin-Johnson syndrome, cystic fibrosis, neurological disease, retinal degeneration, cholesterol and bile transport defects, anemia, and abnormal drug response [6, 7]. MRP2 is expressed on the apical domain of hepatocytes, enterocytes, and proximal renal tubular cells, as well as in brain, placenta, and lung [8]. Although MRP2 transporter is known principally for its role in drug resistance, recent studies have shown that MRP2 can also transport certain TSCs, such as NNK and its metabolites 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and NNK-N-oxide [9,10].
Recently, MRP2 has been shown to physically interact with PDZ domain-containing proteins [11, 12]. PDZ domains are modular protein-interaction domains that fold to form peptide-binding clefts and typically mediate interactions with the carboxyl termini of proteins that terminate in consensus PDZ-binding sequences [13-15]. PDZ proteins that preferentially accumulate at the apical epithelial surface include NHERF1/EBP50, NHERF2/E3KARP, and PDZK1/CAP70, etc. NHERF1/EBP50 and NHERF2/E3KARP contain two PDZ domains and a carboxy-terminal domain that mediates association with MERM proteins (merlin, ezrin, radixin, moesin), while PDZK1 contains four tandem PDZ domains [13-15]. Many studies have reported the association of one or more of these PDZ proteins with an increasing number of apical membrane receptors, ion channels, and transporters, suggesting that apical membrane PDZ proteins nucleate the formation of multiprotein complexes that modulate trafficking, transport, and signaling in polarized cells [16-19].
Cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-activated Cl- channel lining the luminal/apical surfaces of epithelial cells in airway, gut, and exocrine glands [20]. CFTR Cl- channel can be either absorptive or secretory and plays a major role in water and salt transport in these epithelial cells [20]. MRP2 and CFTR, both of which belong to the ABCC subfamily [6], are PDZ motif-containing ABC transporters [11]. Studies from our lab and other groups showed that CFTR interacts with PDZ proteins through its PDZ motif [17, 18, 21-23]. Recently, it has been reported that Cl- channels can be modulated by MRP2 in isolated hepatocytes [24]. Given the similar localization in the lung and PDZ-domain binding properties of both CFTR and MRP2, it is possible that these two ABC transporters may have certain physical and functional interaction with each other. In the present study, we sought to explore this possibility and we found that there is a functional coupling between CFTR and MRP2 that may be mediated by PDZ proteins in lung epithelial cells. We also observed the existence of a macromolecular complex containing CFTR, PDZ proteins, and MRP2, which might form the basis for the regulatory cooperation between these two ABC transporters. Our results have important implications for cigarette smoke-associated lung diseases (such as smoke-related emphysema, chronic obstructive pulmonary disease, and lung cancer).
2. Materials and methods
2.1. Cell culture, antibodies, constructs, and reagents
MDCK (Madin-Darby canine kidney) cells, and MDCK cells stably overexpressing MRP2 (MDCK-MRP2) were grown in Eagle's Minimum Essential Medium (Invitrogen) supplemented with 10% FBS. Calu-3 (airway epithelial cells) and BHK (baby hamster kidney cells) were cultured as described [25]. For Ussing chamber experiments, Calu-3 cells were grown on permeable filters (6.5-mm diameter) for 7-10 days until they form tight junctions. IB3-1 Cystic Fibrosis bronchial epithelial cells were cultured as described previously [26]. CFTR monoclonal antibodies R1104 and GA-1 have been previously described [27]. An MRP2-specific antibody MAB4148 was procured from CHEMICON International, Inc (Temecula, CA). This antibody reacts with an internal epitope of MRP2, and does not cross-react with the human MDR1, MRP1, MRP3, or MRP5 gene products. We also generated our own anti-MRP2 antibody (rabbit-2825, against the last 12 amino acids of MRP2, i.e., a.a. 1534-1545) (Genemed Synthesis, CA), which was affinity-purified using Protein-A column. Maltose binding protein (MBP)-fusion proteins for C-terminal tails of CFTR (a.a. 1431 to 1480) was generated using pMAL vectors (New England Biolabs; Beverly, MA). GST-fusion protein for full-length PDZ proteins (NHERF1, NHERF2, and PDZK1) were generated using pGEX vector according to manufacturer's instruction (Amersham Pharmacia). His-S fusion proteins for C-terminal tails of human MRP1 and MRP2 were cloned into pTriEx-4-LIC vector (Novagen, Gibbstown, NJ). cpt-cAMP was obtained from Sigma Chemical Co. (St. Louis, MO). Monochlorobibimane (mBCl) was obtained from Invitrogen (Molecular Probes). NNK was procured from Toronto Research Chemicals (Toronto, ON, Canada). Other reagents have been described previously [22,23,25].
2.2. Immunofluorescence Microscopy
Calu-3 cell monolayers grown on permeable support (Transwell, 6.5-mm diameter) were washed in PBS and fixed in acetone/methanol (1:1) at 0 °C for 5 min. Cell monolayers were blocked in 3% nonfat milk in TBST (20 mM Tris, pH 7.2, and 150 mM NaCl) and incubated for one hour with primary antibodies, mouse anti-MRP2 monoclonal and mouse anti-CFTR monoclonal antibodies, followed by incubation for one hour with secondary antibody, AlexaFluor 568-conjugated goat anti-mouse IgG (Molecular Probes, Invitrogen). The fluorescence was examined by a confocal laser scanning microscopy (Carl Zeiss LSM 5 PASCAL), and images from Z-series sections (1 μm) were collected. Images were stacked using the software, Confocal Assistant 4.02, and processed by Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
2.3. Crude Membrane Preparation and Immunoblotting
Calu-3 cells were harvested in ice-cold PBS-calcium/magnesium, and cell pellet was pooled and transferred into homogenization buffer (250 mM Sucrose, 1 mM EDTA, 20 mM HEPES, pH 7.4) supplemented with protease inhibitor cocktail (Pierce) and subjected to homogenization for 20 strokes. The homogenized cells were spun at 800 g for 10 min, and the post-nuclear supernatant (PNS) was collected. Thereafter, the PNS was subjected to ultracentrifugation at 200,000 g for 60 min in Beckman Ultracentrifuge with 70.1 Ti Rotor. The resultant supernatant was collected as cytosol fraction, and the pellet (crude membrane) was resuspended with homogenization buffer. Protein concentration was determined by the method of Bradford (Bio-Rad). Both the cytosol fraction and crude membrane were subjected to SDS-PAGE in 4-15% gel, transferred to PVDF membranes, and immunoblotted with anti-MRP2 specific antibody.
2.4. Glutathion-S-bimane (GS-B) Transport Assay
In brief, cells were incubated with 100 μM monochlorobimane (mBCl) in Hanks' balanced salt solution (HBSS) for 1 h at 4°C. The metabolic products of mBCl that pass through the plasma membrane into the cytosol, glutathione-S-bimane (GS-B), are fluorescent organic anions synthesized within the cell cytosol and are excreted specifically through MRP2 transporter [28, 29]. Thereafter, cells were washed with HBSS and incubated with mBCl-free medium at 4°C or 37°C. 300-μl aliquots were collected at different time points (every 4-5 min) and used for fluorescence measurement. Finally, cells were lysed with 0.5% TX-100 in HBSS, and fluorescence of cell lysates was measured. The excretion rate of GS-B from the cytosol of the intact cells into the extracellular medium was defined as the ratio of the excreted amount of the fluorescent glutathione conjugates divided by the amount remaining in the cells at 0 min. The total amount of cell proteins was determined by the method of Bradford (Bio-Rad) using bovine serum albumin as a standard.
2.5. Iodide efflux assay
Calu-3 cells were grown on 60-mm dishes, and iodide efflux was assayed as described [30]. Briefly, cells were loaded for 1 h at room temperature with loading buffer (136 mM NaI, 3 mM KNO3, 2 mM Ca(NO3)2, 11 mM glucose, and 20 mM HEPES, pH 7.4). Extracellular NaI was washed away thoroughly (6 ×) with efflux buffer (136 mM NaNO3 replacing 136 mM NaI in the loading buffer) and cells were equilibrated for 1 min in a final 1-ml aliquot. NNK (50-500 μM) was added at time 0 and included in the efflux buffer for the whole assay process. CFTR agonist cpt-cAMP (a cell permeable cAMP analog) was then added to the efflux buffer at time point of 4-min and thereafter. The first four aliquots were used to establish a stable base line in efflux buffer alone with or without NNK. The amount of iodide in each aliquot (1 ml) was determined by using an iodide specific Orion electrode (Thermo Scientific, Waltham, MA).
2.6. Short-circuit Current (Isc) Measurements
Polarized airway epithelial (Calu-3) cell monolayers were grown to confluency for 7-10 days on Costar Transwell permeable supports (filter area is 0.33 cm2). Filters were mounted in a Ussing chamber setup, and short-circuit currents (Isc) mediated through CFTR Cl- channel were measured as described [22,23]. The cells were bathed in Ringer's solution (mM) (basolateral: 140 NaCl, 5 KCl, 0.36 K2HPO4, 0.44 KH2PO4, 1.3 CaCl2, 0.5 MgCl2, 4.2 NaHCO3, 10 Hepes, 10 glucose, pH 7.2), and low Cl- Ringer's solution (mM) (apical: 133.3 Na-gluconate, 5 K-gluconate, 2.5 NaCl, 0.36 K2HPO4, 0.44 KH2PO4, 5.7 CaCl2, 0.5 MgCl2, 4.2 NaHCO3, 10 Hepes, 10 mannitol, pH 7.2) at 37 °C, gassed with 95% O2 and 5% CO2. NNK (50-500 μM) was added into the apical and/or basolateral sides of the cell monolayers for 20 min before cpt-cAMP was added into the apical side to elicit a CFTR-dependent Cl- current response. CFTR Cl- channel inhibitors (DPC: 500 μM; or CFTRinh-172: 1-10 μM) were added into the apical side to inhibit the Cl- currents toward the end of the experiment. The epithelial integrity was monitored by passing a 3-mV pulse across the epithelia every 1 min during the whole experiment.
2.7. Pull-down Assay
Pull-down assay was performed as described before [22,23,25]. Briefly, MDCK-MRP2 cells were lysed in lysis buffer (PBS-0.2% Triton X-100 + protease inhibitors). Cell lysates were mixed at 4°C for 15 min followed by centrifugation at 16,000 g for 10 min at 4°C. GST or GST-PDZ fusion proteins (NHERF1, NHERF2, and PDZK1; 0.3 μM of each) were added and mixed at 4°C with the clear supernatant for 60 min. After incubation, glutathione Sepharose beads (20 μl) were then added and mixed for another 2 h. Thereafter, the mixtures were spun at 800 g for 2 min, and the beads were washed three times with the same lysis buffer before the proteins were eluted from beads with Laemmli sample buffer (containing 2.5% β-mercaptoethanol). Eluates were separated on 4-15% gel and immunoblotted with anti-MRP2 antibody as described above. BHK cells were transiently transfected (Lipofectamine 2000, Invitrogen) with S-tagged MRP1 C-tail or S-tagged MRP2 C-tail (the C-terminal tails of MRP1 or MRP2 were cloned into pTriEx-4-LIC, Novagen). After 48-72 hrs post-transfection, the cells were lysed with PBS-0.2% TX100 and subjected to pull-down using 0.35 μM GST or GST-NHERF1 fusion protein, and immunoblotted with S-protein HRP conjugate (Novagen) which detects S-tag in the fusion proteins on Western blot according to the manufacturer's instructions.
2.8. Macromolecular Complex Assembly
This in vitro complex formation [22,23] was performed by mixing MBP-CFTR C terminal 50 a.a. fusion protein (0.6 μM) with GST-PDZ fusion proteins (NHERF1, NHERF2, or PDZK1) at room temperature for 1 h, followed by mixing with glutathione beads (20 μl) for another 1 h. This step, which is called pairwise binding [27], was done in 200 μl of lysis buffer (PBS-0.2% Triton-X 100 + protease inhibitors). The complex was washed twice with the same buffer and allowed to incubate further with cleared lysates (16,000 g for 10 min as described above) from MDCK-MRP2 cells at 4 °C for 3 h with constant mixing. The complex was washed extensively with lysis buffer, eluted with sample buffer, and immunoblotted with anti-MRP2 antibody as described above.
2.9. Statistics
Data are expressed as mean ± SEM of at least three independent experiments. Statistical significance of the experimental data was determined by Student's t-test and p-values less than 0.05 were considered to be significant.
3. Results
3.1. MRP2 is expressed in airway epithelial cells and localized primarily at the apical site of plasma membrane
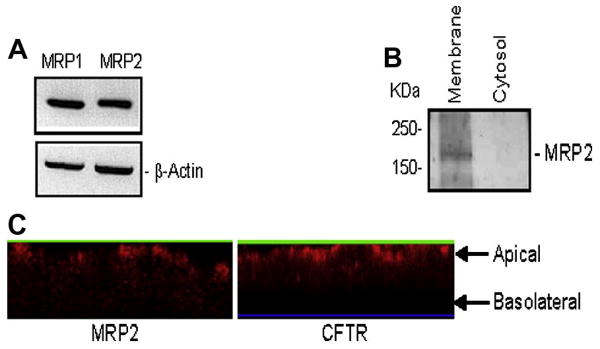
First we sought to establish the presence of MRP2 in airway epithelial cells by using three different methods: RT-PCR, immunoblotting, and immunofluorescence microscopy. Using human sequence specific oligos for MRP2 and MRP1, we demonstrated that Calu-3 (airway epithelial cells; these cells express a large amount of CFTR, which makes it relatively easy to monitor CFTR activity using iodide efflux assay) express both of the transcripts (Fig. 1A). β-actin was used as an internal control (Fig. 1A).
Figure 1. MRP2 is expressed at the apical plasma membrane in lung epithelial cells.
(A) RT-PCR detection of MRP1 and MRP2 in Calu-3 cells. β-actin was used as an internal control. (B) MRP2 is detected in crude membrane, but not in cytosol prepared from Calu-3 cells. (C) MRP2 (left) is expressed in part at the apical cell surfaces in Calu-3 cells, as for CFTR (right).
Using an MRP2-specific antibody, we observed the expression of MRP2 that appears as a major immunoreactive band of approximately 190 kD in crude membrane prepared from Calu-3 cells, but not in the cytosol (Fig. 1B).
Unlike MRP1 and MRP3, which are localized to basolateral membranes of various cells, MRP2 was reported to be localized at the apical membrane of many cells as in liver, gut, kidney, etc [31]. Here, fluorescence microscopy was also performed to confirm the subcellular localization of MRP2 in lung epithelial cells. Figure 1C shows the immunofluorescent localization of MRP2 (left) and CFTR (right) in polarized Calu-3 monolayers grown on a permeable support. The monolayers were fixed and stained after 7-10 days in culture [32]. Confocal fluorescence images were collected, and the z-sections of these images show that MRP2 is expressed in part at or near the apical cell surface, but not in basolateral membrane (Fig. 1C, left). CFTR staining was also performed and demonstrated the apical localization (Fig. 1C, right). Nonimmune IgG was used as negative control (data not shown).
3.2. MRP2 functions as a transporter for TSCs in lung epithelial cells
It has been demonstrated that MRP2 plays an important role in transport of TSCs in human embryonic kidney cells [9,10]. Here we confirmed that observation in airway epithelial cells. A fluorescence assay for MRP2 transporter activity has been standardized by examining MRP2-mediated excretion of a fluorescent substrate, glutathione-S-bimane (GS-B) [28]. GS-B is a fluorescent glutathione conjugate synthesized within the cells from mBCl, and the excreted GS-B into the assay buffer was quantitated every 4 or 5 minutes. As shown in Fig. 2A, MDCK cells over-expressing MRP2 (MDCK-MRP2) demonstrated an increased time-dependent excretion of GS-B compared to MDCK cells at 37°C. MDCK cells also showed a time-dependent excretion of the fluorescent substrate due to the presence of endogenous MRP2 (Fig. 2A). Using this assay, we demonstrated that Calu-3 cells also show MRP2-like function (Fig. 2B).
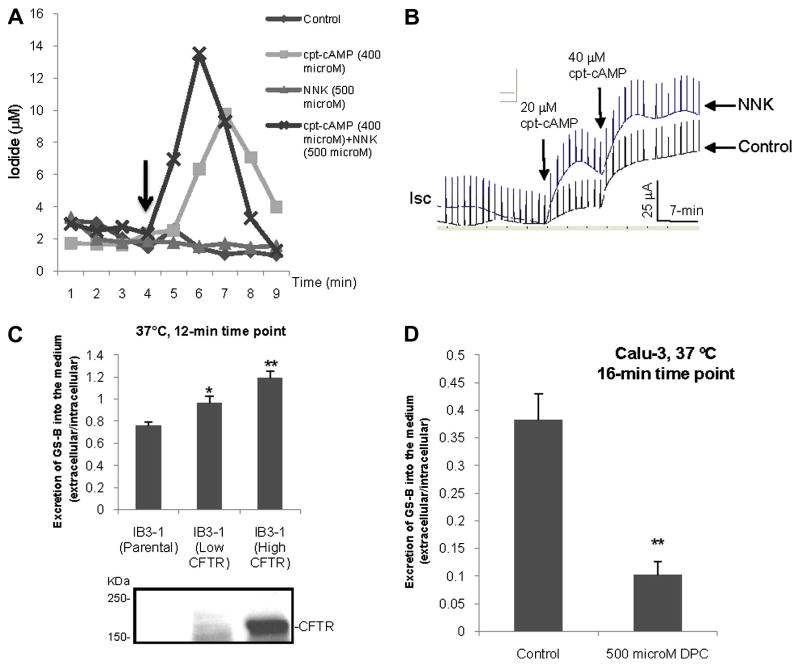
Figure 2. Tobacco-specific carcinogen, NNK, reduces the transport of MRP2 substrate.
(A) Excretion of fluorescent substrates, GS-B, from MDCK or MDCK-MRP2 cells at different time points at 4°C or 37°C. (B) Excretion of fluorescent substrates, GS-B, from Calu-3 cells at different time points at 4°C or 37°C. n = 4, *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant, compared to the 4°C group. (C) Excretion of fluorescent substrates, GS-B, from MDCK-MRP2 cells at different time points at 37°C in the presence or absence of NNK (500 μM). n = 3, *p < 0.05, **p < 0.01, n.s., not significant, compared to the control. (D) Excretion of fluorescent substrates, GS-B, from Calu-3 cells at 16-min time point at 37°C in the presence or absence of NNK (500 μM). n = 4, **p < 0.01, compared to the control.
We also tested the effect of one of the most prevalent and toxic TSCs, NNK, on MRP2-mediated transport of GS-B in this assay, and we observed a significant reduction of the excretion of the fluorescent substrate (GS-B) both in MDCK- MRP2 and Calu-3 cells by NNK (Fig. 2C and 2D), suggesting that NNK acts as a competitive substrate for MRP2. It is reported that NNK has immunosuppressive effect on respiratory epithelial cells [33] and alveolar macrophages [5, 34]. In those studies, various dosages of NNK (100 - 1000μM) were used, although 500 μM NNK was used most of the time. In our study, we used 50-500 μM NNK, and we also used 500 μM NNK most of the time.
3.3. Tobacco-specific carcinogens up-regulates CFTR activity
We investigated whether CFTR activity could be affected by TSCs, such as NNK. We monitored CFTR-mediated iodide efflux in response to CFTR agonist cpt-cAMP in the presence of NNK in the cultured monolayers of Calu-3 cells. Interestingly, NNK potentiates CFTR-mediated iodide efflux in Calu-3 monolayers (Fig. 3A). It is to be noted, however, that NNK by itself does not stimulate iodide efflux. In order to confirm this effect of NNK on CFTR function, polarized monolayers of Calu-3 cells were mounted in a Ussing chamber, and the effect of NNK on CFTR-mediated chloride short-circuit currents (Isc) was monitored. As shown in Fig. 3B, NNK significantly potentiates CFTR activity compared to control group in response to CFTR agonist. It has been argued by Schuller et al [35] that NNK is a β-adrenergic agonist. We have previously demonstrated that β2-AR agonist activates CFTR Cl- channel [21]. Given the observation that NNK alone has no effect on CFTR function, it is unlikely that NNK, at least in lung epithelial cells, acts on CFTR through the β2-AR pathway.
Figure 3. Tobacco-specific carcinogens up-regulates CFTR activity.
(A) CFTR-mediated iodide efflux in Calu-3 cell monolayers in the presence or absence of NNK (500 μM). Arrow indicates addition of cpt-cAMP (400 μM) at the time point of 4-min and thereafter. (B) Short-circuit currents (Isc) mediated through CFTR Cl- channel in response to cpt-cAMP in polarized Calu-3 cells mounted in a Ussing chamber in the absence or presence of NNK (100 μM, apical/basolateral) 30 min prior to cpt-cAMP addition. (C) Excretion of fluorescent substrates, GS-B, from CFTR gene corrected IB3-1 cells (top). CFTR protein level was also shown for respective IB3-1 cells (bottom). n = 3, *p < 0.05, **p < 0.01, n.s., not significant, compared to the parental IB3-1 cells (no CFTR gene correction). (D) Excretion of fluorescent substrates, GS-B, from Calu-3 cells at 16-min time point at 37°C in the presence of CFTR blocker DPC (500 μM).
3.4. CFTR up-regulates MRP2 function
Given that NNK can potentiate CFTR function, the question is, what happens to MRP2 function in the presence of CFTR? To test this, a human cystic fibrosis cell line (IB3-1 cells) has been used as it does not express functional CFTR [36], but does express MRP2 (comparable to the level in Calu-3; data not shown). We have corrected IB3-1 cells expressing varying amounts of exogenous wild-type CFTR. As shown in Fig. 3C, IB3-1 cells demonstrate increased MRP2 activity with increased amounts of functional CFTR at 37°C. The CFTR expression is also shown in the bottom panel. In addition, we also monitored MRP2 activity when CFTR function was attenuated by CFTR blockers (such as DPC). We observed a reduction in MRP2 transporter activity when CFTR function was compromised in Calu-3 cells (Fig. 3D).
3.5. MRP2 interact with PDZ proteins in a PDZ-motif dependent manner
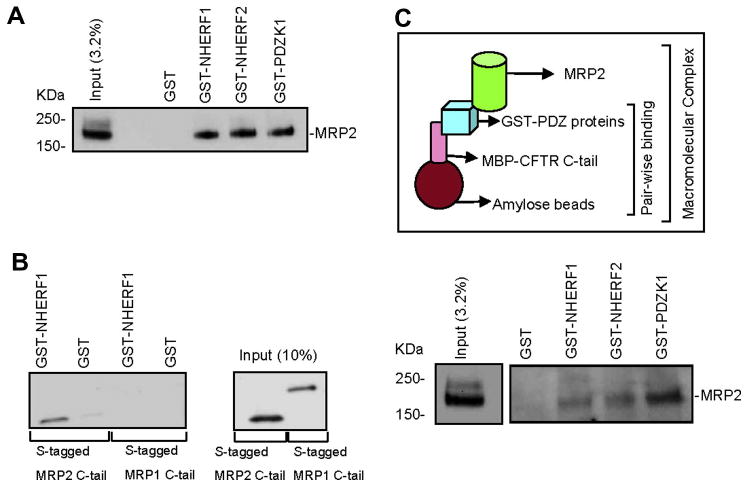
Given that we observed a functional coupling between MRP2 transporter activity and CFTR Cl- channel function, we next asked if a physical association between CFTR and MRP2 could be detected as well. Like CFTR, MRP2 also possesses a consensus PDZ motif at its C-terminus (-STKFCOOH) [11]. We therefore explored the binding affinity between MRP2 and several PDZ proteins that are reported to localize to the apical membrane of epithelial cells [13-15, 37]. Pull-down assays demonstrated that MRP2 binds to several apical membrane associated PDZ proteins (NHERF1, NHERF2, and PDZK1) with comparable affinity (Fig. 4A).
Figure 4. A macromolecular complex containing the transporter (MRP2), ion channel (CFTR), and the PDZ proteins is important for the cooperative regulation.
(A) MRP2 was pulled down by various GST-PDZ proteins. (B) MRP2 C-tail, rather than MRP1 C-tail, was pulled down by GST-NHERF1. (C) (Top panel) Pictorial representation of the in vitro macromolecular complex assembly. (Bottom panel) Macromolecular complex containing MRP2, PDZ proteins, and CFTR.
We further investigated whether the interaction between MRP2 and PDZ proteins is PDZ-motif dependent. BHK cells transiently transfected with S-tagged MRP1 C-tail or S-tagged MRP2 C-tail were subjected to pull-down with GST or GST-NHERF1 fusion protein, and immunoblotted with S-protein HRP conjugate which detects S-tag in the fusion proteins on Western blot. MRP2 C-tail, which contains the PDZ-binding motif, was shown to bind NHERF1, whereas MRP1 C-tail (which does not possess PDZ motif) did not bind NHERF1 (Fig. 4B), nor did it bind to the other two PDZ proteins NHERF2 or PDZK1 (data not shown).
3.6. A macromolecular complex of MRP2, CFTR, and PDZ proteins may be important for the cooperative regulation
MRP2 is shown to be expressed at the apical surface (Fig. 1C) of lung epithelial cells where CFTR is known to reside, and MRP2 can interact with PDZ proteins (Fig. 4A, 4B) as also reported by others [11,12]. The same is also true for CFTR as reported previously [17, 18, 21]. These findings suggest that there may exist a physical interaction between MRP2 transporter and CFTR chloride channel, which serves as an anatomical basis for the cooperative regulation of their function as observed above (Fig. 3A-3D). We hypothesized that the interaction between MRP2 and CFTR might be mediated by PDZ proteins. To address this, we assembled a macromolecular complex of CFTR C-tail, PDZ proteins, and MRP2 in vitro, which is represented schematically in Fig. 4C (top). A macromolecular complex was formed between CFTR C-terminal 50 amino acids, PDZ proteins, and MRP2 (Fig. 4C, bottom).
Although a macromolecular complex was observed with all three PDZ proteins, it is to be noted that PDZK1 seems to be the most favored. MBP-CFTR C-tail does not bind directly to MRP2 nor does the complex form in the presence of GST alone; and MBP was used as a negative control and does not form a complex, either (data not shown). These results suggest that a macromolecular complex consisting of MRP2-PDZ proteins-CFTR is likely to be present in the apical surface of airway epithelial cells, which forms the basis for the functional coupling of MRP2 transporter activity to CFTR channel function we observed in this study.
4. Discussion
The lungs represent the most important surface of contact with the external milieu. During normal breathing, the airways daily transport to the lung 10,000 liters of environmental air, which is frequently contaminated with a variety of pollutants, particles, bacteria, and viruses that are deposited in the airways. The respiratory epithelium forms a continuous lining to the airways and to the environment and it plays a unique role as a protective physical and functional barrier to external deleterious agents by elaboration of a series of defense mechanisms developed to protect the airways from the insults. Studies suggest that many transporters, ion channels, and receptors localized in the apical and/or basolateral surface membrane of airway epithelium not only participate but also cooperate in this natural defense mechanism against external stimuli.
In the present study, we observed that two apically-localized ABC transporters, MRP2 and CFTR, cooperate both functionally and physically in lung epithelia. In our study, we found that NNK, one of the most toxic tobacco specific carcinogens from cigarette smoke, can up-regulate CFTR chloride channel function in cultured lung epithelial cells.
However, Kreindler et al recently reported that cigarette smoke extract inhibited chloride secretion in human bronchial epithelial cells [38]. Another group also reported suppressed CFTR function in cigarette smokers and decreased CFTR expression and function in Calu-3 and T84 epithelial cells by cigarette smoke [39]. In the studies by these authors, the epithelial cells were exposed to the collective cigarette smoke which is a complex mixture of biologically active chemicals and contains more than 4000 functionally diverse components with more than 50 known carcinogens (including NNK). The overall effect of the cigarette smoke extract seems to elicit inhibition of CFTR function and chloride secretion [38, 39]. While in our present study, NNK, which is the most potent and specific carcinogen in cigarette smoke for lung tissues, potentiated the CFTR-mediated chloride currents in cultured airway epithelial cells. Our observation clearly demonstrated that different components within the cigarette smoke extract elicit different functional alterations of CFTR chloride channel, suggesting a functional/mechanistic diversity of the individual components of the cigarette smoke extract.
In our present study, we also found that MRP2 is expressed at the apical surface of airway epithelial cells and pumps out the tobacco specific carcinogen (such as NNK). We observed the existence of a macromolecular complex containing CFTR, PDZ proteins, and MRP2, which might form the basis for the regulatory cooperation between these two ABC transporters. This is a very interesting observation, which has important implications for both normal and path-physiological lung conditions. One interpretation of this observation to normal lung function would be that the response of the lung airway epithelia to cigarette smoke is possibly the activation of the ABC transporters. MRP2 will pump out the TSCs. In a coordinated fashion, CFTR pumps chloride out which leads to the sodium being pumped out (through sodium channels) followed by water through paracellular pathway. This can therefore help wash out the TSCs (which are already pumped out of the cells by MRP2 and are in the airway surface liquid) into the sputum. Probably, this is partially the mechanism of how airway epithelial cells act as a first line of defense against cigarette smoking. An intriguing hypothesis that is worthy of further investigation is “are patients suffering from smoke-related diseases (such as smoke-related emphysema and lung cancer) have non-functional or down-regulated ABC transporters (MRP2 and CFTR) compared to normal individuals”. If this is the case, then genetic carriers of the cystic fibrosis gene (the loss or dysfunction of the CFTR chloride channel activity) would be at a higher risk of developing smoke-related abnormalities as they have reduced levels of functional CFTR. Further studies aimed at elucidating the transduction pathway involved in the MRP2 signaling of CFTR chloride channels and vice versa may provide us a general idea as to how exactly these two ABC transporters can be regulated cooperatively, and how these transporters assist in fighting against tobacco-specific carcinogens from cigarette smoke.
Acknowledgments
We thank Dr. Koushik Roy for assistance with the MRP2 transporter fluorescent assay. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK058545 and DK074996 (to A.P.N.), American Lung Association (to A.P.N.), and American Heart Association (Greater Southeast Affiliate) Beginning-grant-in-aid #0765185B (to C.L.).
Footnotes
Conflict of Interest Statement: We confirm that all authors fulfill all conditions required for authorship. All authors have read and approved the manuscript. The authors of this study have no conflict of interest or any financial disclosures to make.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson DE, Kirkendall RS, Lawton RL, Chrismon JH, Merritt RK, Arday DA, Giovino GA. Surveillance for smoking–attributable mortality and years of potential life lost; by state—United States. MMWR CDC. 1994;43:1–8. [PubMed] [Google Scholar]
- 2.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. Circulation. 1997;96:3243–3247. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Smoking-attributable mortality and years of potential life lost — United States. Morbidity and Mortality Weekly Report. 1993;42:645–648. [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 5.Proulx LI, Castonguay A, Bissonnette EY. Cytokine production by alveolar macrophages is down regulated by the a-methylhydroxylation pathway of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 2004;25:997–1003. doi: 10.1093/carcin/bgh103. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Rzhetsky A, Allikmets R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 7.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 8.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302:407–415. doi: 10.1124/jpet.102.035014. [DOI] [PubMed] [Google Scholar]
- 9.Leslie EM, Ito KI, Upadhyaya P, Hecht SS, Deeley RG, Cole SPC. Transport of the β-O-glucuronide conjugate of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by the multidrug resistance protein 1 (MRP1) J Biol Chem. 2001;276:27846–27854. doi: 10.1074/jbc.M102453200. [DOI] [PubMed] [Google Scholar]
- 10.Leslie EM, Ghibellini G, Nezasa K, Brouwer KL. Biotransformation and transport of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in bile duct-cannulated wild-type and Mrp2/Abcc2-deficient (TR) Wistar rats. Carcinogenesis. 2007;28:2650–2656. doi: 10.1093/carcin/bgm187. [DOI] [PubMed] [Google Scholar]
- 11.Hegedus T, Sessler T, Scott R, Thelin W, Bakos E, Varadi A, Szabo K, Homolya L, Milgram SL, Sarkadi B. C-terminal phosphorylation of MRP2 modulates its interaction with PDZ proteins. Biochem Biophys Res Commun. 2003;302:454–461. doi: 10.1016/s0006-291x(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 12.Kocher O, Comella N, Gilchrist A, Pal R, Tognazzi K, Brown LF, Knoll JH. PDZK1, a novel PDZ domain-containing protein up-regulated in carcinomas and mapped to chromosome 1q21, interacts with cMOAT (MRP2), the multidrug resistance-associated protein. Lab Invest. 1999;79:1161–1170. [PubMed] [Google Scholar]
- 13.Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 15.Bezprozvanny I, Maximov A. PDZ domains: more than just a glue. Proc Natl Acad Sci USA. 2001;98:787–789. doi: 10.1073/pnas.98.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschuler Y, Hodson C, Milgram SL. The apical compartment: trafficking pathways, regulators and scaffolding proteins. Curr Opin Cell Biol. 2003;15:423–429. doi: 10.1016/s0955-0674(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Yue H, Derin RB, Guggino WB, Li M. Accessory protein facilitated CFTR–CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 2000;103:169–179. doi: 10.1016/s0092-8674(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 18.Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 20.Welsh MJ, Tsui LC, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver C, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited diseases: Membrane transport systems. McGraw-Hill; New York: 1995. pp. 3799–3876. [Google Scholar]
- 21.Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, Clancy JP. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA. 2003;100:342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Weinman SA. Mrp2 modulates the activity of chloride channels in isolated hepatocytes. Hepatology. 2002;36:65–71. doi: 10.1053/jhep.2002.33998. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Roy K, Dandridge K, Naren AP. Molecular assembly of cystic fibrosis transmembrane conductance regulator in plasma membrane. J Biol Chem. 2004;279:24673–24684. doi: 10.1074/jbc.M400688200. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of ΔF508-CFTR. Am J Physiol Cell Physiol. 2000;278:C259–C267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 27.Naren AP. Methods for the study of intermolecular and intramolecular interactions regulating CFTR function. Methods Mol Med. 2002;70:175–186. doi: 10.1385/1-59259-187-6:175. [DOI] [PubMed] [Google Scholar]
- 28.Ryu S, Kawabe T, Nada S, Yamaguchi A. Identification of basic residues involved in drug export function of human multidrug resistance-associated protein 2. J Biol Chem. 2000;275:39617–39624. doi: 10.1074/jbc.M005149200. [DOI] [PubMed] [Google Scholar]
- 29.Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci. 1998;111:1137–1145. doi: 10.1242/jcs.111.8.1137. [DOI] [PubMed] [Google Scholar]
- 30.Chang XB, Tabcharani JA, Hou YX, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- 31.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 32.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol in oxidative stress-induced disruption of tight junction. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 33.Proulx LI, Gaudreault M, Turmel V, Augusto LA, Castonguay A, Bissonnette EY. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, a component of tobacco smoke, modulates mediator release from human bronchial and alveolar epithelial cells. Clin Exp Immunol. 2005;140(1):46–53. doi: 10.1111/j.1365-2249.2005.02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Therriault MJ, Proulx LI, Castonguay A, Bissonnette EY. Immunomodulatory effects of the tobacco-specific carcinogen, NNK, on alveolar macrophages. Clin Exp Immunol. 2003 May;132(2):232–8. doi: 10.1046/j.1365-2249.2003.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuller HM, Tithof PK, Williams M, Plummer H., III The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a β-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via ß-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–4515. [PubMed] [Google Scholar]
- 36.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, Craig R, Guggino WB. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Naren AP. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol Ther. 2005;108:208–223. doi: 10.1016/j.pharmthera.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 39.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]