Abstract
Neuroscience produces a vast amount of data from an enormous diversity of neurons. A neuronal classification system is essential to organize such data and the knowledge that is derived from them. Classification depends on the unequivocal identification of the features that distinguish one type of neuron from another. The problems inherent in this are particularly acute when studying cortical interneurons. To tackle this, we convened a representative group of researchers to agree on a set of terms to describe the anatomical, physiological and molecular features of GABAergic interneurons of the cerebral cortex. The resulting terminology might provide a stepping stone towards a future classification of these complex and heterogeneous cells. Consistent adoption will be important for the success of such an initiative, and we also encourage the active involvement of the broader scientific community in the dynamic evolution of this project.
The GABA (γ-aminobutyric acid)-ergic interneurons of the cerebral cortex are a diverse population of cells. Their diversity is manifested in every aspect of their phenotype, as evidenced by their different morphological, electrophysiological and neurochemical features. It has long been assumed that neocortical interneurons belong to different classes1, with the variability in their features within a class being much smaller than the differences across classes. We are convinced that the differences between the classes are indeed real and functionally relevant. Thus, identifying classes and subclasses of interneurons is an important step towards understanding how inhibition shapes cortical function.
How to classify neocortical interneurons has been a topic of debate for some time. Nevertheless, classification criteria often seem to be arbitrarily chosen, and the nomenclature that is used to describe their features often varies. Consequently, communication among investigators can suffer. Not only do the different terminologies make comparisons of the findings of different studies difficult, they can even obscure the value of the inquiry.
Cortical interneurons are typically described and classified according to various morphological, molecular and physiological features. Ideally the characterization of a neuron will consider all three sets of criteria, but as neurons do not have an autonomous morphology, molecular biology, or physiology, these multidimensional features are merely probed by specific detection methods. Each investigator might select a particular method to characterize a cell, but there is only one unitary reality behind it all.
Several classification schemes for cortical interneurons have been proposed. Cajal termed these cells “short-axon” neurons2 and distinguished between them on the basis of the morphologies that were revealed by Golgi staining. Lorente de Nó subsequently described dozens of types of short-axon cells in the mouse neocortex3, and similar Golgi-based classification ensued for more than half a century 4–6. Novel labelling methods, such as the use of intracellular horseradish peroxidase (HRP), revealed axonal arbors more completely and led to new efforts to classify interneurons morphologically1,7. The establishment of electrophysiological criteria has resulted in several additional classification attempts1,8–10. Finally, molecular and genetic markers have also been used as bases for classification11–13. These classifications are not always compatible, and interneuron researchers often face problems in describing the cellular subtypes that they investigate.
To aid ongoing efforts towards interneuron classification, to facilitate the exchange of information and to build a foundation for future progress in the field, we propose and publicly endorse a standardized nomenclature of interneuron properties. This proposal arose out of a meeting devoted to this topic in Cajal’s native town, Petilla de Aragón (Navarra, Spain), and is rooted in the collective work that has been performed in many laboratories. We seek agreement on a list of the essential features that differentiate the GABAergic interneurons of the neocortex, and encourage all investigators to use the same terms to describe the same features. We hope that this first step will later lead to a broadly adopted classification. However, before we are able to speak a common language, we need to agree on the words.
Box 1 | The Petilla interneuron nomenclature Group (PING).
PING consists of: Giorgio A. Ascoli, Lidia Alonso-Nanclares, Stewart A. Anderson, German Barrionuevo, Ruth Benavides-Piccione, Andreas Burkhalter, György Buzsáki, Bruno Cauli, Javier DeFelipe, Alfonso Fairén, Dirk Feldmeyer, Gord Fishell, Yves Fregnac, Tamas F. Freund, Daniel Gardner, Esther P. Gardner, Jesse H. Goldberg, Moritz Helmstaedter, Shaul Hestrin, Fuyuki Karube, Zoltán F. Kisvárday, Bertrand Lambolez, David A. Lewis, Oscar Marin, Henry Markram, Alberto Muñoz, Adam Packer, Carl C. H. Petersen, Kathleen S. Rockland, Jean Rossier, Bernardo Rudy, Peter Somogyi, Jochen F. Staiger, Gabor Tamas, Alex M. Thomson, Maria Toledo-Rodriguez, Yun Wang, David C. West and Rafael Yuste.
Our terminology is dynamic. The associated online supplementary material includes the complete terminology as it stands at the time of manuscript submission. Links to the nomenclature that was originally agreed upon in Petilla and the most up-to-date version as it matures are provided in the Further Information. A committee will update this nomenclature as needed. Continuous feedback, constructive criticism and suggestions are invited from the whole scientific community.
Overview of interneuronal features
Even the meaning of terms such as ‘interneuron’ and ‘cortex’ can be sources of debate. This Perspective focuses on the GABAergic cells (both local and projection neurons) of the cerebral cortex, including the neo-, the archi- and the paleo-cortex. A PubMed search using the query ‘cortex interneuron’ returns more than 200 reviews in the past 40 years, ranging from early functional overviews14 to recent specifications of developmental origins15. Most approaches make use of one or more of three essential types of characteristics: morphological, molecular and physiological. We have therefore structured the terminology of the features according to this broad division. The sequence in which these three categories of features are presented does not convey an order of priority. However, each domain is internally ordered in a logically hierarchical fashion. Only the first levels of this organization are illustrated and discussed here. The finer details are included in Supplementary information S2 (box), S3 (box) and S4 (box) and on the Petilla Terminology website. Some terms are self-explanatory or widely agreed and do not require further elaboration. Others, which are either less common or more controversial, are given further explanation.
When used in a scientific document, some terms referring to quantitative features would require the explicit provision of definite numerical criteria. However, such criteria will vary depending on the specific scientific questions and particular lines of investigation. Thus, we refrain from suggesting precise borders between different anatomical attributes, chemical content or electrical activities, leaving this choice as the purview of individual researchers. We recognize the difficulties of comparing morphology or biophysics across laboratories without quantitative guidelines. However, we hope that the future use of this nomenclature for these features will always be accompanied by their quantification, and we envision that the numerical criteria that are needed to distinguish among the different classes of neurons will become clearer with time. It is important to agree on a common terminology when referring to the same features before comparative measurements are attempted.
Although some traditional terms for cortical interneurons (such as basket cells and chandelier cells) are used here for ease of reference and illustration, the nomenclature proposed does not attempt to give names to different classes of interneurons; instead, it defines the features that can be used for their identification. In the following brief description of some of these key features, the actual terms used in the nomenclature are given in quotation marks.
Morphological features
The main structural components of interneurons are the soma, the dendrites, the axon, and their electrical and chemical synaptic connections. Each component is associated with a set of features (BOX 2; Supplementary information S2).
Somata
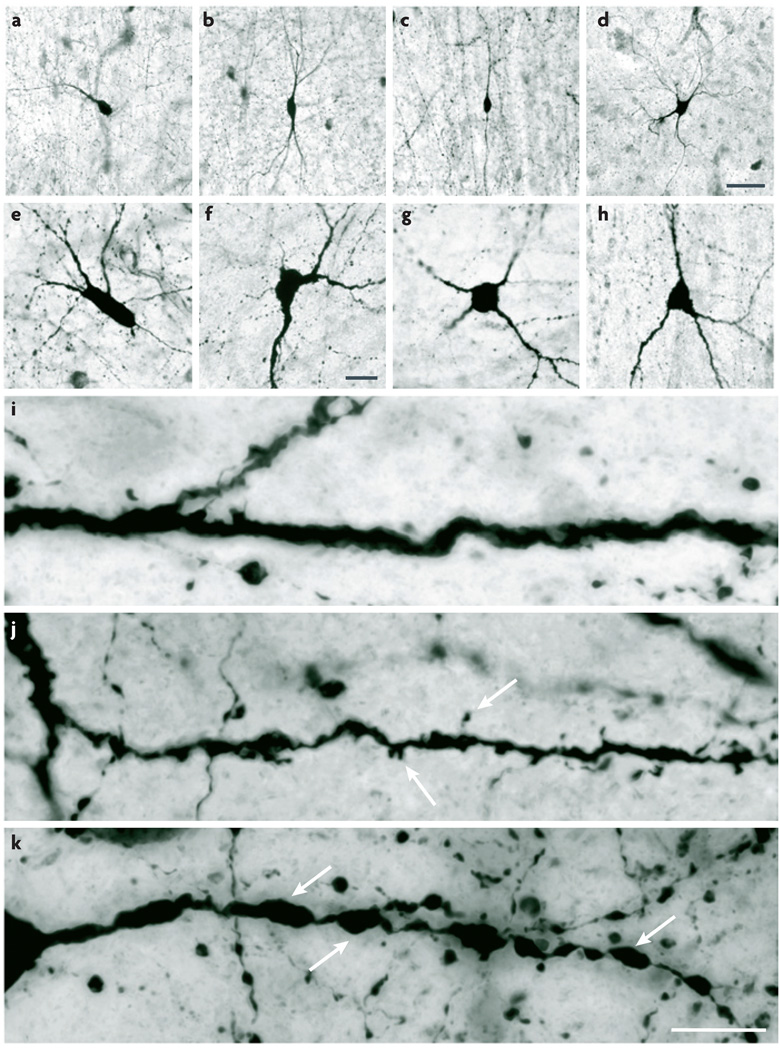
The shape, size and orientation of somata vary greatly. Qualitative shape descriptors (such as ‘round’, ‘fusiform’, ‘triangular’ and ‘polygonal’) are illustrated in FIG. 1 and Supplementary information S5 (figure). Somata typically vary in diameter from 10–30 µm, although more extreme values have been reported. The main axis of somata can be perpendicular (‘radial’) or parallel (‘tangential’) to the pial surface in the coronal plane (or, in the hippocampus, to the cellular layer in the transverse plane).
Figure 1. Somatic and dendritic morphology: shape and fine structure.
Human cerebral cortex sections stained by NADPH-diaphorase histochemistry reveal key features of somatic and dendritic morphology. a–h | Examples of soma shape and dendritic arborization polarity. Somata can be fusiform (a–c), polygonal (d), round (g), triangular (h) or shapes that are not described by any of these terms (e,f). Dendritic arborization can be termed monopolar (a), bitufted (b), bipolar (c) or multipolar (d). i–k | Higher-magnification images showing structural details of the dendrites. Dendrites can be fairly regular and aspiny (i), irregular and spiny (j; arrows indicate spines) or beaded and aspiny (k; arrows indicate beads). Scale bars: a–d (shown in d): 50 µm; e–h (shown in f): 20 µm; i–k (shown in k): 10 µm. Images supplied by Javier DeFelipe.
Dendrites
The morphology of dendritic trees is often simpler in cortical interneurons than in principal cells. Moreover, dendrites are typically less elaborate and easier to visualize than axons and thus provide a practical source of morphological features. The polarity of the dendritic arborization16 can be described (FIG. 1a–h; Supplementary information S5). ‘Unipolar’, ‘bipolar’ and ‘multipolar’ cells have one, two or multiple long primary dendrites, respectively, extending from their cell body17,18. ‘Bitufted’ cells have two clusters of branches that originate directly from the soma and extend in opposite directions19. In any interneuron with bidirectional dendrites (be it bipolar or bitufted), the two arbors can extend in radial or tangential directions. In addition to other, shorter dendrites, some interneurons have one thick primary dendrite that resembles that of pyramidal cells. There are also interneurons that have a single dendritic tuft. In addition, some dendrites are confined to specific cortical laminae or columns (‘intralaminar’ and ‘intracolumnar’ dendrites), whereas others are not (‘interlaminar’ and ‘intercolumnar’ dendrites). Somatic shape is to a great extent determined by the number and orientation of the primary dendrites; thus, these two sets of features are not independent.
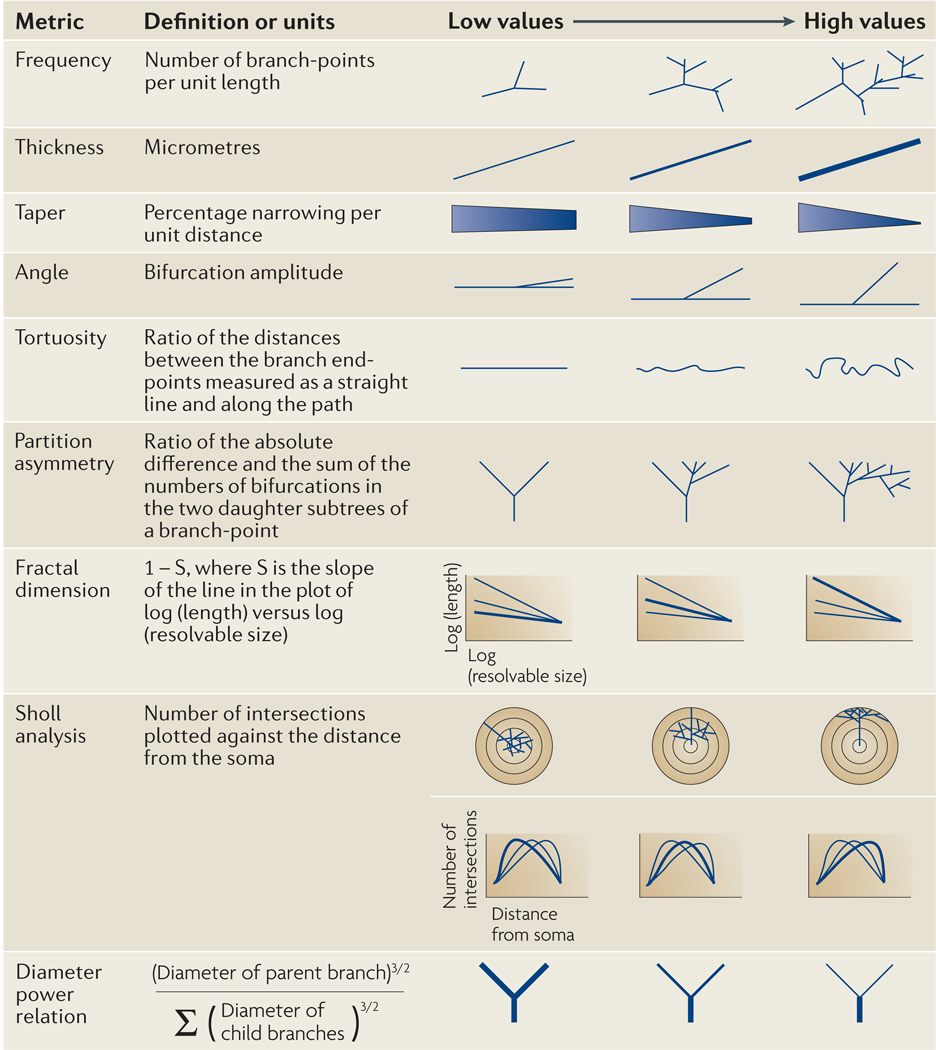
Dendritic branching metrics (FIG. 2) are routinely analysed automatically20. ‘Frequency’ (the ‘number’ or ‘distribution’ of branches, bifurcations and terminals) and ‘size’ (branch diameter, length or surface area) are commonly provided. Other related measurements include the change of diameter along the branch (‘taper’) and at the bifurcations, which can be captured by the ‘power relation’ (REF. 21). ‘Sholl analysis’ measures the number of times that the dendrite intersects a series of consecutive concentric circles22,23. Several variants of this analysis also exist: for example, the percent length of process measured against its distance along the dendritic path. The ‘fractal dimension’ measures a one-dimensional dendrite’s ability to fill a two- or three-dimensional space at finer and finer resolutions, quantifying an important aspect of spatial occupancy24. A related characteristic, ‘tortuosity’, describes dendritic meandering and is captured by the ratio between the straight and the path (along the dendrite) distances between the two ends of the branch25. ‘Partition asymmetry’ analyses the imbalance between the subtrees that stem from a branch in terms of the number of their terminal tips26, and various angles can be used to report the local three-dimensional spatial features.
Figure 2. Basic morphological features that describe neuronal branching.
Dendritic and axonal processes can be quantified with many different metrics. These measurements are illustrated schematically to show the properties of the complex structures that can be captured. Representative exemplars of branches that would give increasing numerical values are shown for each metric. Although some metrics can be understood with little explanation (such as taper), others are not immediately intuitive and must be defined mathematically (such as fractal dimension). Sholl analysis yields a plot rather than a single value. In some cases, cortical interneurons can be distinguished from pyramidal cells and from each other using a combination of these metrics. Images supplied by Adam Packer.
On a finer scale, dendritic spines and beads can be considered (FIG. 1i – k). Dendritic beads are focal swellings that typically contain mitochondria. Many interneurons are spiny early in their development but lose most of their spines as they mature; thus, age should be specified. The total number, density, distribution and shape of spines or beads on the dendrites all vary. These fine structures might have important computational significance. There can also be other structures, such as filopodia. The synaptic inputs that a cell receives on its dendrites also distinguish it from other cell types. ‘Asymmetric’, or Type I, inputs are mostly glutamatergic and excitatory, whereas ‘symmetric’, or Type II, inputs are mostly GABAergic and inhibitory27–29. How densely the inputs are distributed, where they make contact and where they come from are also all important features30.
Axons
Axons are the major determinant of connectivity (Supplementary information S6 (figure)), and their morphological features have traditionally been used as the principal classification criteria2,3,5. Moreover, axonal morphologies are strongly correlated with both the developmental origin of neocortical interneurons31 and their synaptic physiology32. However, rigorously defining quantitative differences between distinct axonal morphologies remains a difficult and open problem.
The axon originates either from the soma or from a primary dendrite and typically has a distinct (short, thin and smooth) initial segment (defined as the portion of the axon that contains a high density of Na+ channels and electron-microscopically recognized plasma-membrane undercoating and fasciculated microtubules). The first bifurcation or collateral can arise at a variable distance from the soma, and this distance should be recorded. Axons can be restricted to their region or layer of origin and can have major branches that travel long distances horizontally. Alternatively, they can course preferentially into deeper cortical layers (‘descending’ towards the white matter) or to more superficial layers (‘ascending’ towards the pial surface). Some axons ascend and descend, and thus form an arch before arborization begins (‘willow axons’). Other axonal arbors (of so-called long-range interneurons) can cover multiple layers and cortical regions33–35. Unfortunately, there is little data about such long-distance GABAergic projections in the cerebral cortex36–38.
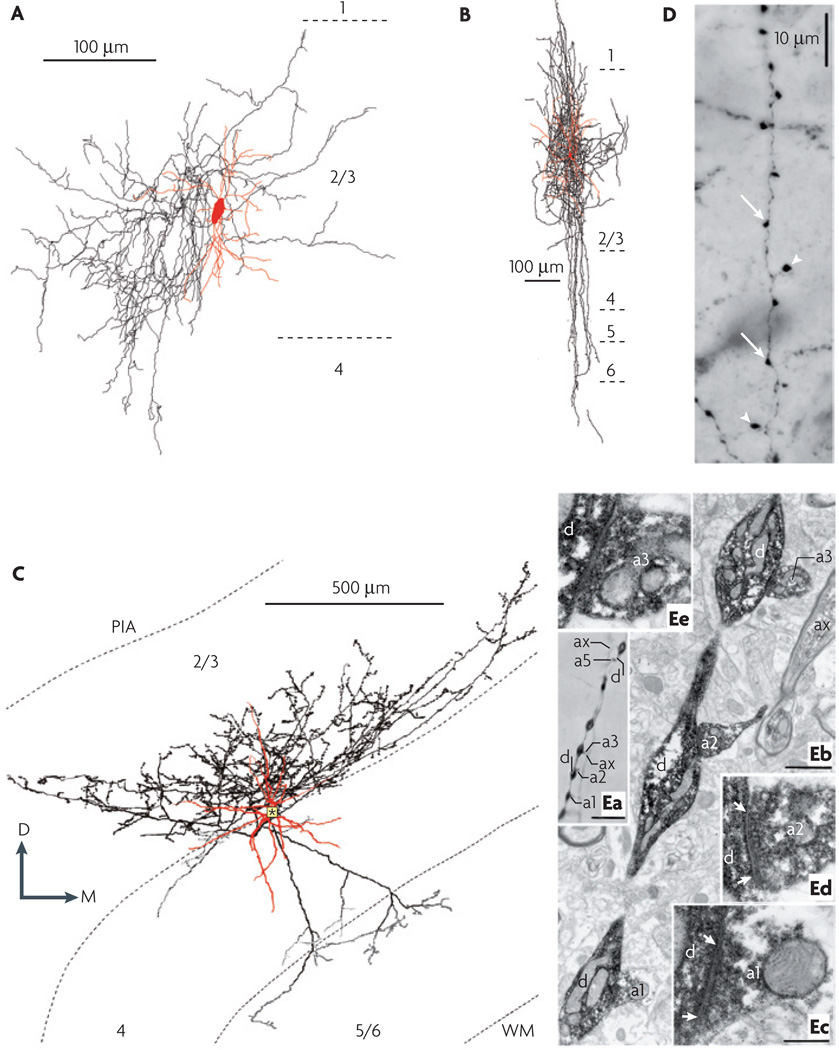
The description of axonal arbors shares some terminology with that of dendrites, but there are also some important differences. In principle, similar branching metrics can be applied. However, unlike most dendrites, some axons are ‘myelinated’. Axon diameter can vary along the length of the axon: broad and myelinated portions alternate with unmyelinated and slender portions. Other axons are unmyelinated and more consistently slender or tapering. A complete description of axonal geometry should therefore include analysis of the relationships between branching patterns, axonal diameters and myelination patterns. The density of axonal arbors as well as their projection pattern (‘radial/oblique’ or ‘tangential’) can be described. Some axons are confined to specific lamina or columns (‘intralaminar’ and ‘intracolumnar’ axons), whereas others are not (‘interlaminar’ and ‘intercolumnar’ axons). Certain patterns of axonal arborization have been assigned specific designations. For example, a plexus of highly branched axons is shown in FIG. 3A. A distinctive pattern of arborization is demonstrated by the type of interneurons that are usually found in neocortical layers 2 and 3 and that have historically been referred to as double-bouquet cells (FIG. 3B; Supplementary information S6). In addition to a dense local plexus in the layer of origin39, these interneurons have bundles of long vertical branches that resemble horsetails40–42 and descend to all deeper layers5,43 to predominantly innervate dendritic spines and shafts2,44. The terminal branches of some axons can be ‘curved’, ‘straight’ or ‘clustered’. The terms curved and straight refer to whether or not the terminal or preterminal branches bend as they approach their target cell(s). Some basket cells (FIG. 3C; Supplementary information S6) provide a clear example of the former case, whereas chandelier or axo-axonic cells exemplify the straight phenotype. Clustered terminals are densely grouped together, as in chandelier cell axons.
Figure 3. Axonal morphology and synaptic structure.
A | An example of a dense plexus of highly branched axons: a neurogliaform cell in the rat somatosensory cortex. The soma and dendrites are shown in black and the axon is shown in red. B | A double-bouquet cell from the cat primary visual cortex. The soma and dendrites are shown in red and the axon is shown in black. Note the characteristic horsetail-like axonal distribution. C | Morphological characteristics of a layer-3 large basket cell from the cat primary visual cortex. In this three-dimensional computer reconstruction, the soma and dendrites are shown in red and the axon is shown in black. The axon arborized profusely in the lower half of layer 3 (black boutons) and had a lateral extent of 1.1 mm from the parent soma (indicated by the asterisk). In addition, two axon collaterals descended into layers 5 and upper 6, each of which provided a small tuft (dark gray boutons). In layer 4, only a few boutons (light gray) were found. The total length of the axon was 44.053 mm, and it had 5,373 boutons. Typically, large basket cell boutons establish multiple axo-somatic contacts on other neurons. D | Fine structure of an axon revealed in a photomicrograph, showing terminal boutons (indicated by arrow heads) and boutons en passant (indicated by arrows). The image was obtained by staining a section of human cerebral cortex using NADPH-diaphorase histochemistry. E | Correlated light and electron microscopy of autapses. Ea | A myelinated axonal branch (labelled ‘ax’) gives rise to six terminal boutons (four of which, a1, a2, a3 and a5, are labelled) that are apposed to the dendrite (labelled ‘d’). Eb | Three of the terminal boutons shown in Ea emerge from the myelinated axonal trunk (ax) and establish synapses (indicated by the arrows) on successive dendritic beads, as illustrated at higher magnification in Ec–e. Scale bars: Ea: 10 µm; Eb: 1 µm; Ec–e (shown in Ec): 0.3 µm. D, dorsal; M, medial; PIA, pia mater; WM, white matter. Part A modified, with permission, from REF. 108 © (2003) American Association for the Advancement of Science. Part B reproduced, with permission, from REF. 39 © (1998) Society for Neuroscience. Part C courtesy of Alex s. Ferecskó. Part D supplied by Javier DeFelipe. Part E reproduced, with permission, from REF. 46 © (1997) Society for Neuroscience.
Not all GABAergic synapses are associated with axonal boutons. Nevertheless, when boutons are present, they can vary in several respects (FIG. 3D,E). Their ‘size’ and ‘density’ can be quantified, as can their clustering patterns (or distribution)45. Boutons can exhibit a particular structure46, such as ‘terminal’ or ‘en passant’, or can be connected by thin stalks or twigs. Electron microscopy can reveal important ultrastructural characteristics, such as the density and type of vesicles that are present in or near the presynaptic terminal, the specific type of synapse, the presence and density of mitochondria in the bouton47 and the number of synapses per bouton.
Connections
The postsynaptic target (be it a pyramidal cell, an interneuron, a glial cell or a component of the vascular system) links the function of an interneuron and the spatial characteristics of its axon. The location of the synapse on the target cell should be identified: are contacts made on the soma, the dendrites (in which case the description should include the order and the distance from the soma) and/or the axon, and are they restricted to specific regions of the dendritic tree (for example, the basal or apical dendrites, the main apical trunk or the apical oblique dendrites)? Furthermore, the proportion of contacts made on to dendritic spines versus dendritic shafts can vary considerably. The final pattern of post-synaptic contacts might present recognizable features. ‘Distributed’ patterns are evenly spaced, whereas in a ‘gradient’ pattern the distribution of contacts changes in a specific direction. ‘Clustered’ terminal branches are often seen in chandelier or axo-axonic cells that innervate pyramidal-cell axon initial segments48. Noticeably, these cells also have such clusters as en passant formations49. The general degree of postsynaptic specificity of GABAergic interneurons, and how it affects cortical microcircuitry, is still debated50.
Cortical interneurons can be coupled electrically through gap junctions51,52. These connections are found in the membranes of somata, dendrites and axons. Their location and distance from the soma can vary53. The distribution of gap junctions can be expressed as the total number and density of contacts and/or the probability that the interneuron of interest will make contact with its neighbouring cells54. If possible, the identity of the connected neurons should also be noted. Finally, neurotransmitters can diffuse upon release and act on other synaptic contacts or on extrasynaptic receptors55,56. Obtaining these data requires difficult experiments, but the available information in this regard should also be noted.
Molecular features
In contrast to morphological features, the definition of the molecular features of an interneuron is often unambiguous. Some widely used molecular markers are listed in BOX 2 and in Supplementary information S3. The number of molecular features that could be measured or characterized is obviously enormous and is also rapidly expanding as our knowledge grows. Nevertheless, several families of molecules seem to be particularly important for distinguishing different types of neurons, and cortical interneurons in particular, from each other. We have thus grouped these molecules into categories: transcription factors, neurotransmitters or their synthesizing enzymes, neuropeptides, Ca2+-binding proteins, neurotransmitter receptors, structural proteins, ion channels, connexins, pannexins and membrane transporters. Several prominent members of each group are listed. However, hundreds of molecules might be of interest, and gene expression profiles, which can be generated quickly, are gaining prominence in specifying the molecules of interest in a given interneuron population11,57.
Box 2 | Summary of morphological, molecular and physiological features.
Morphological features
Soma: shape; size; orientation; other
Dendrite: arborization polarity; branch metrics; fine structure; postsynaptic element; other
Axon: initial segment; arbor trajectory; terminal shape; branch metrics; boutons; synaptic targets; other
Connections: chemical and electrical; source; location and distribution; other
Molecular features
Transcription factors
Neurotransmitters or their synthesizing enzymes
Neuropeptides
Calcium-binding proteins
Receptors: ionotropic; metabotropic
Structural proteins
Cell-surface markers
Ion-channels
Connexins
Transporters: plasma membrane; vesicular
Others
Physiological features
Passive or subthreshold parameters: resting membrane potential; membrane time constants; input resistance; oscillation and resonance; rheobase and chronaxie; rectification
Action potential (AP) measurements: amplitude; threshold; half-width; afterhyperpolarization; afterdepolarization; changes in AP waveform during train.
Dendritic backpropagation
Depolarizing plateaus
Firing pattern: oscillatory and resonant behaviour; onset response to depolarizing step; steady-state response to depolarizing step
Response to hyperpolarizing step: rectification; rebound
Spiking recorded extracellularly: phase relationship to oscillations; functional response specificity; cross-correlation and other dynamics
Postsynaptic responses: spontaneous and evoked; ratio of receptor subtypes; spatial and temporal summation; short- and long-term plasticity; gap junctions
Given how quickly this field is moving, one could argue that it might be disadvantageous to put too much emphasis on the molecular analysis of interneuron phenotypes at this early stage. Moreover, even ostensibly similar interneurons (from the biochemical point of view) can be distinguished on the basis of the layer-specific combination of inputs that they receive and on the different regions of postsynaptic cell that they target. However, each day more and more site-specific neurochemical information becomes available58, and considerable effort is focused on investigating the entire genome of single cells59 or populations of identical cells60. In the future, it is therefore likely that these molecular features will be a powerful tool in the classification of interneurons, along with information from studies of the functional and structural features of individual cells and their connections. For example, on the basis of immunocytochemistry, chandelier cells can be chemically defined as GABAergic cells that contain the Ca2+-binding proteins parvalbumin and calbindin but not calretinin, and that might also contain the neuropeptide corticotropin-releasing factor but not other neuropeptides61, namely cholecystokinin, somatostatin, neuropeptide Y, vasoactive intestinal polypeptide and tachykinins. It is also important to recognize that such phenotypes change during development and, quantitatively at least, can be altered plastically.
Physiological and biophysical features
The electrophysiological characteristics of a neuronal population are important in determining what part those cells play in circuit activity and computation. To an extent, neocortical interneurons can be differentiated on the basis of these features alone. However, although some electrophysiological characteristics correlate well with other features, other characteristics do not, and unambiguous identification requires the assessment of other dimensions (see the example in Supplementary information S7 (figure)). Interneurons possess passive properties as well as those that are revealed by stimulation with injections of current. Some spiking information (such as ‘firing frequency’) can also be revealed by extracellular recordings. Postsynaptic responses to stimulation vary in both their time course and the dynamics of transmitter release (BOX 2; Supplementary information S4). When characterizing the electrophysiological properties of cortical interneurons, the advantages and limitations of every technique should be considered. For example, whole-cell patch electrodes are commonly used to record intracellularly, particularly in slice preparations. Although this technique provides stable electrical access to the neuron, it also dializes the neuron’s intracellular milieu, thus potentially altering the response properties of the cell that are modulated or controlled by its intracellular biochemistry. By contrast, sharp-electrode recordings are thought to affect this intracellular biochemistry less, but often do not provide the stability and electrical access of patch electrodes.
Passive and subthreshold properties
The first three measurable passive properties described in the nomenclature are electrical. The ‘membrane potential’, which is usually given in millivolts (mV), reflects the potential difference across the membrane given specific experimental conditions, which should be precisely defined. The membrane potential can resonate or oscillate at a characteristic frequency, particularly when it is close to the firing threshold62–64, which might be related to its function in the circuit49,65. The membrane ‘time constant’, which quantifies the exponential temporal decay of a voltage perturbation, depends on the ‘membrane resistance’ and the ‘membrane capacitance’. The ‘input resistance’ reflects (from an operational point of view) the aggregate electrical resistance of the entire cell (not just the membrane) to current injected through the electrode. A cell’s subthreshold features are its features during the condition in which any stimulus that is being applied does not cause the cell to fire. The ‘rheobase’ is defined as the minimal electrical current (of infinite duration) that is required to bring the cell to its action-potential threshold. The ‘chronaxie’ is the duration of the briefest current of twice the rheobase amplitude that can cause firing.
Action-potential measurements
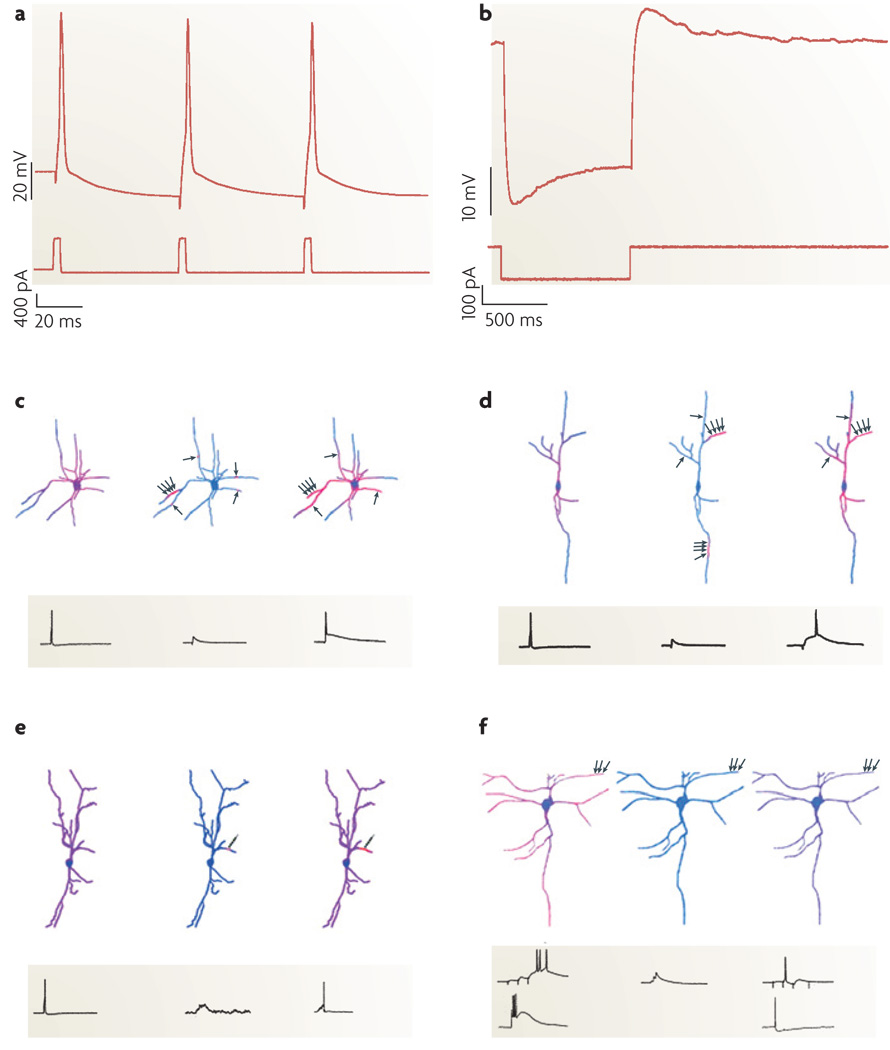
Action potentials have various definable characteristics, including the threshold voltage at which the spike is triggered, as well as the spike amplitude and the half-width. Spike ‘afterhyperpolarizations’ (AHPs) and ‘afterdepolarizations’ (ADPs) are transient changes in membrane potential that follow the action potential (FIG. 4a): they can be simple and close to mono-phasic in shape or can be complex. These waveform characteristics can change during the course of a train of action potentials. If information is available on whether or not backpropagation of the action potential into the dendritic tree from the soma can occur, this should be noted66.
Figure 4. Functional characterization of interneurons.
a,b | Electrophysiological protocols used to measure neurons’ after hyperpolarization (AHP) and sag responses. Somatic current injection (bottom trace in both plots) and recorded membrane voltage (top traces) reveal an AHP (a) and a sag response (b)109. c–f | Diverse interneuron subclasses translate distinct patterns of activity into unique Ca2+ signals. The warmth of the colour reflects the magnitude of the Ca2+ accumulations. Axon terminals (indicated by the small black arrows) are positioned adjacent to dendrites to illustrate the spatial arrangement of synaptic activation, which can be in clusters or alone. c | A schematic of dendritic Ca2+ dynamics during a single action potential (left), a subthreshold excitatory postsynaptic potential (EPSP) (centre) and EPSP/action-potential coupling (right) in the dendrites of a multipolar perisoma-targeting fast-spiking cell from neocortical layer 2/3. Note that Ca2+ influx during single action potentials is proximally restricted. During subthreshold synaptic activation, convergent inputs activate dendritic subunits whereas individual inputs cause microdomains of Ca2+ accumulation. During EPSP/action-potential coupling, spikes propagate specifically to synaptically activated synaptic compartments where IA currents are inactivated. d | A schematic of a neocortical layer-2/3 calretinin-positive irregular-spiking interneuron stimulated as in c. The Ca2+ dynamics of bipolar irregular-spiking cells resemble fast-spiking cells, except that they lack Ca2+ microdomains. e | Dendritic Ca2+ signals in a layer-2/3 bitufted cell during action-potential backpropagation (left) and repetitive subthreshold (centre) and suprathreshold (right) activation of a single synapse. The multiple arrowheads on the individual schematic axonal terminals represent repetitive activation of a single synapse. f | Dendritic Ca2+ signals in a layer-5 rebound-spiking dendrite-targeting interneuron. In burst mode (left), synaptic activation (top trace) or somatic current injection (bottom trace) generates rebound spikes and highest Ca2+ signals at distal dendrites, where low-voltage-activated Ca2+ channels are clustered. In tonic mode (right), globally propagating Na+-based action potentials triggered either synaptically (top trace) or by somatic current injection (bottom trace) cause uniform Ca2+ accumulations. Excitation that is subthreshold for rebound-spike or action-potential generation does not cause Ca2+ influx (centre). Parts a and b reproduced from REF. 109. Parts c–f reproduced, with permission, from REF. 110 © (2005) Elsevier Science.
Firing pattern
Interneurons exhibit a wide diversity of spontaneous and evoked firing patterns67. Depolarizing current steps are often used to uncover a cell’s stereotypical response. The response at the onset of a constant somatic depolarizion might or might not resemble the steady-state response (the firing pattern that is observed after an extended current injection)8. The term ‘continuous’ refers to a pattern in which steady-state behaviour is similar to onset behaviour (FIG. 5). An ‘onset burst’ is a train of action potentials that occurs at the beginning of a stimulus and has a shorter ‘interspike interval’ (ISI) than the steady-state trains of spikes. Although the term ‘burst’ is often used simply to indicate higher-frequency firing at the start of a response and/or strong adaptation following the onset, it is also useful to distinguish this from bursts that ride on a depolarizing wave and are nearly identical every time. However, as yet there is no agreement on the terms that should be used to distinguish between these two types of burst. In a ‘delayed onset’ neuron, even when a suprathreshold current is injected, the membrane does not reach the firing threshold until after the moment that would be predicted from the time constant (FIG. 5). In some cells a single spike can be triggered at the onset and firing can cease for an interval before the steady-state behaviour begins. Other onset behaviour is possible as well.
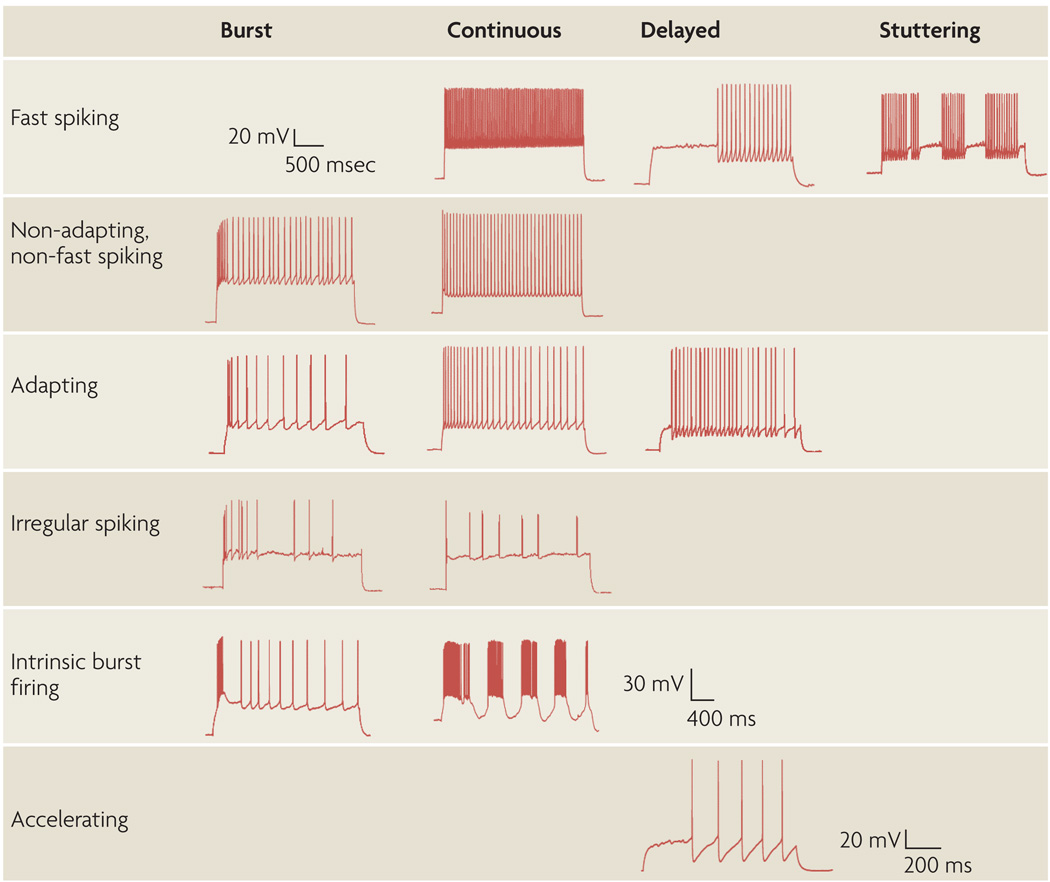
Figure 5. Petilla terminology: types of firing patterns.
Cortical GABAergic interneurons display a vast repertoire of discharge responses. These samples are representative of the most common responses to standardized intrasomatic step-current injections in the rat neocortex. The features of firing patterns in response to step-onset, organized in columns, include bursts, delays and continuous firing, which is neither burst nor delayed. Steady-state patterns, displayed in rows, can be fast spiking, non-adapting non-fast spiking, adapting, irregular spiking, intrinsic burst firing or accelerating. Fast spiking neurons can also display a stuttering or ‘Morse-code-like’ discharge that is characterized by high-frequency spike clusters that are intermingled with unpredictable periods of silence for a wide range of long, sustained, somatic-current injections. Blank areas of the table and boxes containing only scale bars correspond to firing patterns that have not yet been characterized in neocortical interneurons. The scale bar at the top left refers to the traces in the first four rows; the scale bars in the fifth and sixth rows refers to the traces in the fifth and sixth rows, respectively.
Steady-state responses to depolarizing current steps are characterized by different firing patterns. ‘Amplitude accommodation’ is the decrease in amplitude of the action potentials that occurs during a train. ‘Spike frequency adaptation’ (FIG. 5; Supplementary information S8 (figure)) is the decrease in firing frequency that occurs during sustained firing. The maximal sustained firing frequency is called the ‘saturating frequency’. The minimal constantly maintained firing rate is the ‘continuous frequency’. The ‘I-f curve’ is the relationship between the firing frequency (f) and the amount of current injected (I). ‘AHP accommodation’ is the change in the amplitude and/or shape of the spike AHP that occurs during sustained firing. Another type of AHP results from a cumulative and/or voltage-dependent current, which can be particularly slow after a current step.
Even at steady state, the ISI can vary. Action-potential discharges with a broad ISI distribution, denoted ‘variable’ discharges, are commonly termed ‘irregular spiking’ (FIG. 5). Some interneurons display a ‘stuttering’ (sometimes called interrupting) pattern, in which pairs or brief trains of spikes with short and nearly constant ISIs alternate with longer periods of silence (FIG. 5). ‘Fast-spiking’ cells (FIG. 5; Supplementary information S9 (figure)) fire at a continuous (but not necessarily constant) high frequency (greater than 50 Hz, which is often considerably higher than the firing rates of principal cells). These interneurons commonly display a brief action potential with a deep, fast AHP. In healthy adult tissue, many of these cells have subthreshold oscillations. Differences in the degree of spike frequency adaptation (expressed as an adaptation ratio for individual cells68) can be used to distinguish between broad anatomical and physiological classes of interneurons (Supplementary information S8). ‘Silent cells’, as their name implies, exhibit no action potentials, although the existence of these cells is debated. Other firing patterns are also possible.
The pattern of spiking changes in response to different current injections. Specifically, the firing behaviour that results when a small current is injected so that the membrane potential stays near the threshold level can differ from that which is observed with strong current injections. For example, some fast-spiking cells produce near-threshold high-frequency discharges in a stuttering pattern. By contrast, at stronger current injections these cells produce continuous rhythmic spiking. However, the near-threshold pattern varies with cortical area and layer, and the stuttering behaviour is not seen in all cortical locations. Researchers should specify the current that was injected. Furthermore, firing patterns change with maturation, so the age of the animal should be documented.
Characteristic responses to hyperpolarizing steps are described by the rectification and rebound behaviour. The differences between the voltages at the onset of the step and during steady state is defined as the ‘sag’ (FIG. 4b). At the end of a hyperpolarizing step, a depolarizing hump or rebound can appear, which might or might not initiate firing. For example, in burst-firing cells a brief stereotypical high-frequency burst of action potentials is initiated on rebound.
Postsynaptic responses
Interneurons can also be characterized on the basis of their responses to excitatory or inhibitory synaptic input, which can be ‘spontaneous’ or ‘evoked’ by the stimulation of an identified presynaptic cell. The frequency of spontaneous events and the probability of evoking a response upon presynaptic stimulation are highly sensitive to the exact experimental conditions. The properties of the postsynaptic responses of cortical interneurons vary over a wide range69. For example, their ‘rise time’, ‘amplitude’, ‘decay’ (or width at half amplitude), ‘reversal potential’, ‘charge’ and complement of ‘receptors’ that underlie the synaptic current67 can differ. The GABAA receptors that mediate the outputs of interneurons also differ across subtypes70. The ratio of the different receptor subtypes that mediate a combined synaptic current is often of interest. ‘Spatial summation’ and ‘temporal summation’ occur across a cell to differing extents.
Many types of plasticity can occur at the synaptic level. This document does not attempt to incorporate the rich phenomenology of the synaptic plasticity field, which is among the largest and most active in cortical neuroscience71–73. Instead, we provide some guidelines for the types of measurements that can be carried out. In ‘short-term plasticity’, the kinetics of synaptic facilitation/augmentation, depression and post-tetanic potentiation vary considerably74–76. In ‘long-term plasticity’, the same kinetics can be measured, as well as the polarity charge and amplitude77. Furthermore, transmitter release by and on to interneurons is also controlled by neuromodulators, such as acetylcholine, serotonin, noradrenaline and endocannabinoids78–83. Studies have shown that different neuromodulators have differential effects on different types of cells. In the future, this might also help to classify neocortical interneurons.
The electrical properties of gap junctions include how strongly they are coupled — the ‘coupling coefficient’ is the ratio of the voltage response in one cell to the voltage response in another. Another gap-junction property is ‘rectification’, in which a rapid change in conductance in one cell occurs when the difference in voltage between the two coupled cells changes sign, or when the coupling ratio is voltage-dependent78.
Extracellular recordings
Interneurons are embedded in circuits that can modify their firing properties in a state-dependent manner. In addition to intracellular recordings, interneurons can be recorded extracellularly to broadly characterize their spiking patterns during responses to sensory input or in association with oscillatory population activity. An important goal of physiological characterization is to be able to recognize interneuron types by their extracellular features in behaving animals84. The ‘functional response specificity’ of an interneuron refers to its role or function in vivo, such as its receptive-field properties. Several extracellular features, such as spike waveform, firing rate, spike dynamics (including many of the spiking patterns described above), spontaneous and evoked properties and the ‘phase relationship’ between the interneuron spiking and local-field oscillations, can be quantified65,85–87. To date, however, no agreed criteria are available to reliably classify in vivo recorded interneurons. Future research should clarify the relationship between in vitro and in vivo characterization49.
Concluding remarks
A community agreement on the most useful and accurate terms to describe the morphological, molecular and electrophysiological features of cortical GABAergic interneurons is an essential step towards establishing and maintaining effective scientific communication in this field. It will be particularly useful for facilitating the collaboration of investigators from different research subcommunities and scientific backgrounds. A combination of techniques and approaches can aid the characterization of cortical cells (as illustrated in BOX 3 for the Martinotti neuron88).
Box 3 | Petilla terminology in action: the Martinotti cell.
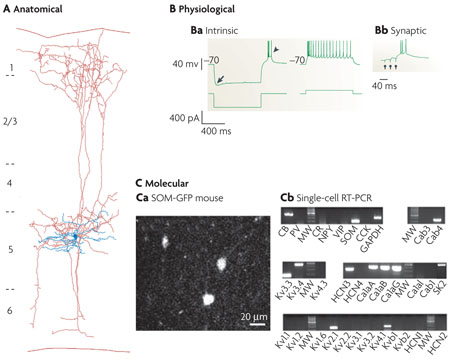
One of the classic interneurons of the cerebral cortex, the Martinotti cell (MC) was first described by Carlo Martinotti104 and was proposed by Ramón y Cajal to play a part in cortical sensorimotor processing2. The MC is characterized anatomically by ascending axonal collaterals that reach layer 1 and then ramify to form a fan-like spread of axonal collaterals5. A biocytin reconstruction of a layer-5 low-threshold spiking MC from mouse somatosensory cortex88 shows its triangular soma and aspiny multipolar dendrites (shown in blue in part A of the figure). Its axon (shown in red) originates at the soma, arborizes locally in layer 5, descends to the white matter, and ascends radially towards layer 1, where it arborizes densely with spine-like boutons, mostly on dendritic shafts of the apical tufts of pyramidal neurons105,106. Although the physiology of MCs can vary106,107, the intrinsic physiology (see figure, part Ba) is characteristic: it has a burst-onset response and, at steady state, shows spike amplitude accommodation and pronounced frequency adaptation. It responds to a hyperpolarizing current step with a sag (indicated by the arrow) and bursts on rebound (indicated by the arrowhead). A common feature of its synaptic physiology, shown in part Bb, is its strong short-term facilitation of slow excitatory postsynaptic potentials and its bursting response to successive synaptic stimulations (indicated by the three circles). Recently, the molecular and genetic details of this phenotype have begun to be clarified (part C). Most consistently, MCs express somatostatin (SOM) and occasionally calbindin (CB), but not parvalbumin (PV) or vasoactive intestinal peptide (VIP)107. Part Ca illustrates somatostatin-positive cells from layers 4 and 5 of the visual cortex of a somatostatin/green fluorescent protein (GFP) transgenic mouse. In part Cb, single-cell reverse transcriptase (RT) PCR analysis of an MC reveals a typical neurochemical profile106, including the expression of SOM and a repertoire of K+ and Ca2+ channels that underlie MC physiological properties. Ca, calcium channel; CCK, cholesystokinin; CR, calretinin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HCN, hyperpolarization-activated cyclic-nucleotide-gated channel; MW, molecular-weight marker; NPY, neuropeptide Y; SK, small conductance Ca2+-activated K+ channel. Parts A and B reproduced, with permission, from REF. 88 © (2004) Cambridge University Press. Part Cb reproduced, with permission, from REF. 106 © (2004) Cambridge University Press.
It is also important to acknowledge again that there might not be full agreement on the terms we have chosen for the nomenclature, and we remain open to suggestions and feedback from all readers and interested researchers. An example of one such area of potential disagreement is the laminar location of the somata of cortical interneurons. Although its importance as a feature is not yet agreed upon, location is included in our nomenclature.
A community-approved terminology for classifying cortical neurons is also a goal of the Neuroscience Information Framework project, which is funded by the US National Institutes of Health Blueprint for Neuroscience. The project intends to decide on a nomenclature tree, with subtypes of cortical neurons defined according to one of the principal schemes (morphological, molecular or physiological), and with the other dimensions or schemes represented as attributes that could potentially modify terms anywhere in the basic tree. A further goal of the project is that the scheme be both adaptive (as more data or more-refined distinctions are introduced) and generalizable to non-cortical cells.
It is tempting at this stage to endeavour to decide the most important properties with which to define a class of interneurons. However, after extensive discussion within our group and with the rest of the community, a strong consensus emerged that this would be counter-productive at this point. Because cortical interneurons are such multifaceted entities, their classification using a few ‘essential’ descriptors (such as morphological descriptors) does not yet match well with classifications using other schemes (such as electrophysiological schemes)89. Ultimately, a simple classification might be based on the role of interneuron subtypes in the circuit. However, such a classification is premature in the absence of a clear functional understanding of the contribution that each element makes to the neocortical network. This work has instead leveraged the expertise from different fields of neuroscience to identify the descriptors that are most likely to lead to a useful multidimensional classification. Efforts are underway to organize future meetings that would serve both as an open platform from which to revise and refine this nomenclature and as a way to move towards the goal of unifying the classification of cortical interneurons.
The Petilla terminology, even before its publication, has already been used as a recognized reference in the recent literature90,91. Ideally, this consensus will foster discussion to identify those features that are most consistently predictive, functionally relevant and empirically useful in characterizing cells in the mammalian nervous system. For example, a pure morphological classification of layer-5 neurons from the mouse primary visual cortex revealed classes of both pyramidal cells and interneurons. Somatic and dendritic metrics similar to those that are described in this document were used to quantitatively distinguish these classes92.
There are likely to be correlations among the morphological, biophysical, molecular and synaptic properties. For instance, the presence of certain molecular markers (such as voltage-gated ion channels or metabotropic receptors) might have specific electrophysiological implications13, whereas other markers (such as cell-adhesion proteins or neurotrophic factors) might correspond to particular morphological phenotypes. Some combinations might be common and others might be rare. The more that these correlations are identified and validated, the faster we will advance towards agreeing on a classification scheme. For example, morphological and electrophysiological measurements of three molecularly identified sets of interneurons uncovered a correlation between synaptic input kinetics and axonal morphologies32.
A number of other aspects should be considered, such as developmental ontogeny31,93 and the function that the interneuron has in the local circuit94. Although these other features are likely to be crucially related to interneuron structure and function, their complexity constitutes a major challenge to categorization, particularly by multiple researchers with different viewpoints. Yet, we consider these criteria to be important and we will not renounce them in future efforts, because research will probably help to clarify which features of these criteria are most important for distinguishing between interneurons. Many cortical interneurons perform multiple functional roles, switching dynamically on the basis of local and afferent activity95. Moreover, intracellular dynamics, such as dendritic Ca2+ signalling (FIG. 4c – f), can also vary substantially in distinct types and even subtypes of cortical interneurons66,88,96–99. Furthermore, properties of cortical GABAergic interneurons might also differ across species, especially between primates and rodents, in which differences in the relative proportions of cell types and their embryonic origins have been reported. For example, a recent study revealed both differences and similarities in the properties of neurogliaform inhibitory neurons in the prefrontal cortex of macaque monkeys and rats100 (BOX 4; Supplementary information S10 (figure)). Thus, the canonical cortical circuit differs in at least some aspects of its constituent elements across species.
Box 4 Species differences: neurogliaform (NGF) cells in primates and rodents.
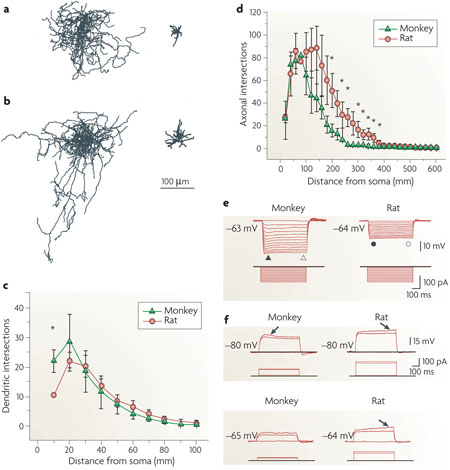
The figure shows axonal and dendritic patterns of NGF cells from the monkey (a) and the rat (b) prefrontal cortex. In both species these interneurons can be readily identified on the basis of their distinctive morphological features. Monkey NGF cells have only a few morphological features that differ from rat NGF cells, including a larger soma, a greater number of dendrites and a more compact axonal field. Parts c and d show Sholl analysis of neuronal arbors from nine monkey and seven rat NGF cells. Average dendritic (c) and axonal (d) intersections are plotted against the distance from the soma (for data marked with an asterisk, p<0.05; error bars represent standard error). Primary dendrites of monkey NGF cells have more intersections than those of rat NGF cells. In contrast to the morphological similarity, whole-cell recordings of the responses to injected current steps reveal a number of differences between monkey and rat NGF cells (see also Supplementary information S10). Monkey NGF cells consistently generate a short-latency first spike that rides on an initial depolarizing hump, whereas in rat NGF cells the first spike appears after a substantial delay riding on a depolarizing ramp that is not seen in monkey NGF cells. Parts e and f show the subthreshold membrane properties of NGF cells in monkey and rat. Voltage responses to the hyperpolarizing current steps are shown in e. Both monkey and rat NGF cells show time-independent inward rectification. Subthreshold voltage responses to depolarizing current pulses are shown in f. The arrows points to the ‘hump’ in the monkey response and to the ‘ramp’ in the rat response. Thus, although rat NGF cells are traditionally classified as late-spiking cells, monkey NGF cells do not meet this physiological criterion. In addition, NGF cells in monkey seem to be more excitable than those in rat, because they display higher input resistance, lower spike threshold and higher firing frequency. Finally, NGF cells in monkey show a more prominent spike frequency adaptation than in rat. Figure reproduced, with permission, from REF. 100 © (2007) American Physiological Society.
Similarly, many of the features that are discussed here vary considerably across age groups, as well as with disease and genetic, pharmacological, surgical and behavioural manipulations. The comprehensive collection of such complex information is likely to go beyond the possibilities of individual laboratories and to require federated programmes101. Ongoing efforts are already striving to gather relevant information from the internet (for example, the Neurogateway), the neuroscience literature (for example, SenseLab), shared digital data102 (for example, NeuroMorpho) or by direct experimental acquisition103. A widespread convergence on a common set of terms is necessary. We look forward to a dynamic evolution of the Petilla nomenclature with the constructive participation of all active researchers.
Supplementary Material
Acknowledgements
The authors are grateful to funding agencies in their respective countries for supporting this work. The Gobierno de Navarra/Nafarroako Gobernua and the town and people of Petilla are acknowledged for graciously hosting the meeting that originated this document. Special thanks are due to A. Rowan, who attended the Petilla meeting and greatly contributed to establishing the initial vision for this report.
Footnotes
FURTHER INFORMATION
Petilla Terminology: http://krasnow.gmu.edu/cng/petilla/
Neuroscience Information Framework: http://nif.nih.gov/
Neurogateway: http://www.neurogateway.org/
Sense Lab: http://senselab.med.yale.edu
NeuroMorpho: http://neuromorpho.org
SUPPLEMENTARY INFORMATION
See online article: S1 (box) | S2 (box) | S3 (box) | S4 (box) | S5 (figure) | S6 (figure) | S7 (figure) | S8 (figure) | S9 (figure) | S10 (figure)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrados (Moya, Madrid, 1899). English translation: Histology of the Nervous System of Man and Vertebrates. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 3.Lorente de Nó R. La corteza cerebral de ratón. (Primera contribución - La corteza acústica). Trabajos del Laboratorio de Investigaciones Biológicas de la Universidad de Madrid 20, 41–78 (1922). English translation: Fairén, A., Regidor, J, & Kruger, L. The cerebral cortex of the mouse (a first contribution - the “acoustic” cortex) Somatosens. Mot. Res. 1992;9:3–36. doi: 10.3109/08990229209144760. [DOI] [PubMed] [Google Scholar]
- 4.Szentagóthai J. The neuron network of the cerebral cortex: a functional interpretation. Proc. R. Soc. Lond. B Biol. Sci. 1978;201:219–248. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- 5.Fairén A, DeFelipe J, Regidor J. In: Cerebral Cortex vol. 1 Cellular Components of the Cerebral Cortex. Peters A, Jones EG, editors. New York: Plenum; 1984. pp. 201–253. [Google Scholar]
- 6.Lund JS. Anatomical organization of macaque monkey striate visual cortex. Ann. Rev. Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- 7.Douglas RJ, Martin KAC. In: The Synaptic Organization of the Brain. Shepherd GM, editor. Oxford: Oxford Univ. Press; 1998. pp. 459–511. [Google Scholar]
- 8.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 9.Lacaille JC, Kunkel DD, Schwartzkroin PA. In: The Hippocampus: New Vistas. Chan-Palay V, Kohler C, editors. Liss; 1989. pp. 287–305. [Google Scholar]
- 10.Maccaferri G, Lacaille JC. Interneuron diversity series: Hippocampal interneuron classifications -making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–571. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Cauli B, et al. Molecular and physiological diversity of cortical nonpyramidal cells. J. Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledo-Rodriguez M, et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb. Cortex. 2004;14:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- 14.Kostyuk PG. Synaptic mechanism of central inhibition. Prog. Brain Res. 1968;22:67–85. doi: 10.1016/S0079-6123(08)63496-2. [DOI] [PubMed] [Google Scholar]
- 15.Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nature Rev. Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- 16.Bayraktar T, Welker E, Freund TF, Zilles K, Staiger JF. Neurons immunoreactive for vasoactive intestinal polypeptide in the rat primary somatosensory cortex: morphology and spatial relationship to barrel-related columns. J. Comp. Neurol. 2000;420:291–304. [PubMed] [Google Scholar]
- 17.Porter JT, et al. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur. J. Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J. Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
- 20.Scorcioni R, Ascoli GA. Algorithmic extraction of morphological statistics from electronic archives of neuroanatomy. Lect. Notes Comput. Sci. 2001;2084:30–37. [Google Scholar]
- 21.Rall W. Electrophysiology of a dendritic neuron model. Biophys. J. 1962;2:145–167. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sholl DA. Dendritic organization in the neurons of the visual cortex and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 23.Sholl DA. The organization of the visual cortex in the cat. J. Anat. 1953;89:33–46. [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon RC, Wheal HV, Turner DA. Dendrites of classes of hippocampal neurons differ in structural complexity and branching patterns. J. Comp. Neurol. 1999;413:619–633. [PubMed] [Google Scholar]
- 25.Li Y, Brewer D, Burke RE, Ascoli GA. Developmental changes in spinal motoneuron dendrites in neonatal mice. J. Comp. Neurol. 2005;483:304–317. doi: 10.1002/cne.20438. [DOI] [PubMed] [Google Scholar]
- 26.Uylings HBM, van Pelt J. Measures for quantifying dendritic arborizations. Netw. Comput. Neural Syst. 2002;13:397–414. [PubMed] [Google Scholar]
- 27.Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscopic study. J. Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 28.Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- 29.Peters A, Palay SL, Webster HdeF. The Fine Structure of the Nervous System. Neurons and their Supporting Cells. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 30.Gulyás AI, Megías M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J. Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Dumitriu D, Cossart R, Huang J, Yuste R. Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cereb. Cortex. 2007;17:81–91. doi: 10.1093/cercor/bhj126. [DOI] [PubMed] [Google Scholar]
- 33.Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- 34.Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J. Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sik A, Penttonen M, Buzsáki G. Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur. J. Neurosci. 1997;9:573–588. doi: 10.1111/j.1460-9568.1997.tb01634.x. [DOI] [PubMed] [Google Scholar]
- 36.Jinno S, et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyashita T, Rockland KS. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur. J. Neurosci. 2007;26:1193–1204. doi: 10.1111/j.1460-9568.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- 38.Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J. Comp. Neurol. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- 39.Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J. Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somogyi P, Cowey A. Combined Golgi and electron microscopic study on the synapses formed by double bouquet cells in the visual cortex of the cat and monkey. J. Comp. Neurol. 1981;195:547–566. doi: 10.1002/cne.901950402. [DOI] [PubMed] [Google Scholar]
- 41.Somogyi P, Cowey A. In: Cerebral Ccortex vol. 1 Cellular Components of the Cerebral Cortex. Peters A, Jones EG, editors. New York: Plenum; 1984. pp. 337–360. [Google Scholar]
- 42.White EL. Cortical Circuits. Synaptic Organization of the Cerebral Cortex. Boston: Birkhauser; 1989. [Google Scholar]
- 43.Valverde F. The organization of area 18 in the monkey: Golgi study. Anat. Embryol. 1978;154:305–334. doi: 10.1007/BF00345659. [DOI] [PubMed] [Google Scholar]
- 44.Ballesteros-Yánez I, et al. The double bouquet cell in the human cerebral cortex and a comparison with other mammals. J. Comp. Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- 45.Binzegger T, Douglas RJ, Martin KA. Stereotypical bouton clustering of individual neurons in cat primary visual cortex. J. Neurosci. 2007;27:12242–12254. doi: 10.1523/JNEUROSCI.3753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J. Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters A, Harriman KM. Different kinds of axon terminals forming symmetric synapses with the cell bodies and initial axon segments of layer II/III pyramidal cells. I. Morphometric analysis. J. Neurocytol. 1990;19:154–174. doi: 10.1007/BF01217295. [DOI] [PubMed] [Google Scholar]
- 48.DeFelipe J, Hendry SH, Jones EG, Schmechel D. Variability in the terminations of GABAergic chandelier cell axons on initial segments of pyramidal cell axons in the monkey sensory-motor cortex. J. Comp. Neurol. 1985;231:364–384. doi: 10.1002/cne.902310307. [DOI] [PubMed] [Google Scholar]
- 49.Somogyi P, Klausberger T. Defined types of cortical interneuron structure space and spike timing in the hippocampus. J. Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeFelipe J, editor. J. Neurocytol. Vol. 31. 2002. pp. 181–416. [DOI] [PubMed] [Google Scholar]
- 51.McBain CJ, Fisahn A. Interneurons unbound. Nature Rev. Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 52.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 53.Tamas G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nature Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda T, Kosaka T. Ultrastructural study of gap junctions between dendrites of parvalbumin-containing GABAergic neurons in various neocortical areas of the adult rat. Neuroscience. 2003;120:5–20. doi: 10.1016/s0306-4522(03)00328-2. [DOI] [PubMed] [Google Scholar]
- 55.Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- 56.Vizi ES. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol. Rev. 2000;52:63–89. [PubMed] [Google Scholar]
- 57.Monyer H, Markram H. Interneuron diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur. J. Neurosci. 2005;21:3277–3290. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 59.Kamme F, et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J. Neurosci. 2003;23:3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugino K, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nature Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 61.DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 62.Llinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc. Natl Acad. Sci. USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–222. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- 64.Pike FG, et al. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J. Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klausberger T, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J. Physiol. 2003;551:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali AB, Bannister AP, Thomson AM. Robust correlations between action potential duration and the properties of synaptic connections in layer 4 interneurones in juvenile and adult neocortical slices. J. Physiol. 2007;580:149–169. doi: 10.1113/jphysiol.2006.124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kröner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb. Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- 69.Thomson AM, West DC, Deuchars J. Properties of single axon EPSPs elicited in spiny interneurones by action potentials in pyramidal neurones in slices of rat neocortex. Neuroscience. 1995;69:727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 70.Ali AB, Thomson AM. Synaptic a5 subunit containing GABAA receptors mediate IPSPs elicited by dendrite-targeting cells in rat neocortex. Cereb. Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- 71.Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 72.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nature Rev. Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 73.Pelletier JG, Lacaille JC. Long-term synaptic plasticity in hippocampal feedback inhibitory networks. Prog. Brain Res. 2008;169:241–250. doi: 10.1016/S0079-6123(07)00014-3. [DOI] [PubMed] [Google Scholar]
- 74.Thomson AM, Deuchars J, West DC. Single axon EPSPs in neocortical interneurones exhibit pronounced paired pulse facilitation. Neuroscience. 1993;54:347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- 75.Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurones in layers 2 to 5 of adult rat and cat neocortex: triple intracellular recordings and biocytin-labelling in vitro. Cereb. Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- 76.West DC, Mercer A, Kirchhecker S, Morris OT, Thomson AM. Layer 6 cortico- thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb. Cortex. 2006;16:200–211. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- 77.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 78.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J. Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 80.Férézou I, et al. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 82.Bodor AL, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J. Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J. Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 84.Buzsáki G. Large-scale recording of neuronal ensembles. Nature Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 85.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barthó P, et al. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- 87.Klausberger T, et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nature Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 88.Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J. Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tyner CF. The naming of neurons: applications of taxonomic theory to the study of cellular populations. Brain Behav. Evol. 1975;12:75–96. doi: 10.1159/000124141. [DOI] [PubMed] [Google Scholar]
- 90.Bota M, Swanson LW. The neuron classification problem. Brain Res. Rev. 2007;56:79–88. doi: 10.1016/j.brainresrev.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsiola A, Hamzei-Sichani F, Peterlin Z, Yuste R. Quantitative morphologic classification of layer 5 neurons from mouse primary visual cortex. J. Comp. Neurol. 2003;461:415–428. doi: 10.1002/cne.10628. [DOI] [PubMed] [Google Scholar]
- 93.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nature Rev. Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 94.Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front. Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb. Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- 96.Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 97.Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+ dynamics. J. Physiol. 2003;551:67–78. doi: 10.1113/jphysiol.2003.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaiser KM, Zilberter Y, Sakmann B. Back-propagating action potentials mediate calcium signalling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex. J. Physiol. 2001;535:17–31. doi: 10.1111/j.1469-7793.2001.t01-1-00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaiser KM, Zilberter Y, Sakmann B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J. Neurosci. 2004;24:1319–1329. doi: 10.1523/JNEUROSCI.2852-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Povysheva NV, et al. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J. Neurophysiol. 2007;97:1030–1039. doi: 10.1152/jn.00794.2006. [DOI] [PubMed] [Google Scholar]
- 101.Ascoli GA. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nature Rev. Neurosci. 2007;7:318–324. doi: 10.1038/nrn1885. [DOI] [PubMed] [Google Scholar]
- 102.Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. J. Neurosci. 2007;27:9247–9251. doi: 10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Markram H. The Blue Brain Project. Nature Rev. Neurosci. 2006;7:153–160. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- 104.Martinotti C. Contributo allo studio della corteccia cerebrale, ed all’origine centrale dei nervi. Ann. Freniatr. Sci. Affini. 1889;1:14–381. [Google Scholar]
- 105.Marin-Padilla M. In: Cerebral Cortex: Cellular Components of the Cerebral Cortex. Peters A, Jones EG, editors. New York: Plenum; 1984. pp. 447–478. [Google Scholar]
- 106.Wang Y, et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 108.Tamás G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 109.Toledo-Rodriguez M. Thesis. Weizmann Inst. Sci.; Genetical, Anatomical and Electrical Determinants of Neuronal Diversity. [Google Scholar]
- 110.Goldberg JH, Yuste R. Space matters: local and global dendritic Ca2+ compartmentalization in cortical interneurons. Trends Neurosci. 2005;28:158–167. doi: 10.1016/j.tins.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.