The findings of this study provide the first evidence suggesting that ARMS2 interacts with hormone replacement therapy (HRT) to modulate AMD risk and are consistent with previous reports demonstrating a protective relationship between exogenous estrogen use and neovascular AMD. These results highlight the genetic and environmental complexity of the etiologic architecture of AMD.
Abstract
Purpose.
To investigate whether female reproductive history and hormone replacement therapy (HRT) or birth control pills (BCPs) influence risk for age-related macular degeneration (AMD) and whether genetic factors interact with HRT to modulate AMD risk.
Methods.
Related and unrelated female participants (n = 799) were examined and data were analyzed with generalized estimating equations with adjustment for age and smoking. Individuals with AMD grades 1 to 2 were considered to be unaffected (n = 239) and those with grades 3 to 5 were considered affected (n = 560).
Results.
When comparing all cases with controls, significant inverse associations were observed for HRT (odds ratio [OR] = 0.65, 95% CI 0.48–0.90, P = 0.008) and BCPs (OR = 0.60, 95% CI 0.36–0.10, P = 0.048). When analyses were stratified by AMD severity (early versus geographic atrophy versus neovascular), the inverse association remained significant (HRT OR = 0.45, 95% CI 0.30–0.66, P < 0.0001; BCP OR = 0.55, 95% CI 0.32–0.96, P = 0.036) only when comparing neovascular AMD with the control. All pair-wise HRT-genotype and BCP-genotype interactions were examined, to determine whether HRT or BCP modifies the effect of established genetic risk factors. The strongest interactions were observed for HRT x ARMS2 coding SNP (R73H) rs10490923 (P = 0.007) and HRT x ARMS2 intronic SNP rs17623531 (P = 0.019).
Conclusions.
These findings provide the first evidence suggesting that ARMS2 interacts with HRT to modulate AMD risk and are consistent with previous reports demonstrating a protective relationship between exogenous estrogen use and neovascular AMD. These results highlight the genetic and environmental complexity of the etiologic architecture of AMD; however, further replication is necessary to validate them.
The leading cause of irreversible vision loss among the elderly in the United States today is age-related macular degeneration (AMD).1,2 The rate of vision loss among those with AMD is predicted to double by 2020 due to the fast-growing population of those over 65 years of age in the United States.3 AMD is a complex multifactorial disease consisting of both genetic and environmental risk factors that affect the retina, particularly the macula, and often causes various degrees of central visual loss.4–7 In the absence of treatment, severe and irreversible vision loss can occur, highlighting the importance of identifying risk factors and those at risk for AMD. The strongest known risk factors for AMD include age, cigarette smoking, and variants in the complement factor H (CFH), complement factor B (CFB), and age-related macular degeneration 2 (ARMS2) genes.8–13 These risk factors, however, explain only a portion of AMD risk, and other risk factors have yet to be identified.
Results in studies suggest that exposure to estrogen resulting from both hormone replacement therapies (HRTs) and reproductive factors is associated with a lower prevalence of AMD14,15 and other retinal diseases.16–18 In these studies, an inverse association was observed with current and former use of HRT and/or birth control pill (BCP) use among Caucasian and Latino women with neovascular AMD and pooled analyses including neovascular and non-neovascular AMD. The approximate effect size (OR) was ∼0.50 across studies.14,15,19,20 These studies have not consistently replicated the findings despite comparable sample sizes, possibly because analyses were performed with slightly different definitions of HRT and BCP use (e.g., binary ever/never versus years of HRT or BCP use).21–23 In many of these studies, the sample size was limited for the variables examined. For example in the Eye Disease Case Control study14 fewer than 1% of the women took BCPs. Despite this, an effect size (OR = ∼0.50) comparable to that in studies that have reported statistically significant associations with BCP was observed. The consistency of effects (regardless of statistical significance) suggests that limited power may have been the reason for discrepant results when examining BCP use in AMD. Other studies have observed the associations in univariate but not in multivariate analyses in well age-matched case–control studies, possibly due to loss of power by overfitting the multivariate model.24,25 Another explanation of these discrepancies is that these studies did not look at genetic risk factors that may interact with HRT or BCP. Misspecifying the model-influencing risk of AMD by neglecting an interaction term may explain why these studies had inconsistent results.26,27 Some studies have made adjustments for potential confounders, including genetic risk factors such as Y402H in the CFH gene; however, none of these studies have looked at gene x environment interactions between HRT or BCP and genetic risk factors.
The protective direction of the associations observed with HRT and AMD is relatively consistent across studies and, to some extent, with exogenous estrogen use (e.g., oral contraceptives).21–23 However, research has shown that oral contraceptive has been associated with an increase in risk for some retinal conditions.28 Further studies are warranted to determine what role female reproductive history and HRT have on AMD risk.
Because of the inconsistencies observed in association studies of AMD with HRT and BCPs, we examined female reproductive history and HRT for association with AMD and tested whether these factors might modify the effects of established genetic risk factors: complement factor H (CFH), complement factor H-related proteins (CFH-R1, R3), complement factor B (CFB), age-related macular degeneration 2 (ARMS2), complement component 3 (C3), HtrA serine peptidase 1 (HTRA1), and apolipoprotein E (APOE).
Materials and Methods
Study Population
Caucasian female participants were recruited into the study by their physicians (EAP, AA, JLK, SGS), through advertisements in waiting rooms and newsletters, presentations to senior groups, and AMD project Web pages. Informed consent was obtained for all patients, and information was collected in compliance with both the internal review boards of all participating institutions and the Health Insurance Portability and Accountability Act of 1996. The study protocol adhered to the tenets of the Declaration of Helsinki.
All AMD patients underwent an eye examination that included color fundus photographs for AMD evaluation. Study ophthalmologists examined the color fundus photographs of all participants and assigned AMD grades, as previously described.29–31 The overall grade assigned each individual was based on the more severely affected eye. Subjects were assigned a grade of 1 through 5, according to a modified classification from AREDS (Age-Related Eye Disease Study), according to both the Wisconsin grading system and the International Classification System.32–34 Individuals with grades 1 and 2 were classified as “unaffected” controls; those with disease grade 3 or higher were considered “affected” cases. A detailed description of the clinical definition for each AMD grade is provided in Table 1.
Table 1.
Description of 560 AMD Cases and 239 Controls
| Clinical Description | AMD Grade | Cases (n) | Controls (n) |
|---|---|---|---|
| No drusen or small (<63 μm), nonextensive drusen without RPE abnormalities | 1 | 0 | 169 |
| Extensive small drusen or nonextensive intermediate drusen (63 μm, 125 μm) and/or RPE hyperpigmentation or hypopigmentation | 2 | 0 | 70 |
| Extensive intermediate drusen or any large soft drusen (125 μm), including drusenoid RPE detachment | 3 | 172 | 0 |
| Geographic atrophy (area of RPE atrophy with sharp margins, usually visible choroidal vessels, at least 175 μm in diameter) | 4 | 75 | 0 |
| Extensive AMD, including nondrusenoid RPE detachment, choroidal neovascularization, subretinal hemorrhage, or fibrosis or photocoagulation scarring consistent with treatment of AMD | 5 | 313 | 0 |
| Total | 560 | 239 | |
| Age at examination, mean y (SD) | 75.5 (8.3) | 66.8 (8.3) | |
| Smoking, % yes, ever | 50 | 41 |
Evaluation of Female Reproductive History
Female participants who filled out the self-administered health and activities questionnaire and answered the questions regarding female reproductive history, HRT, and BCP use were included in the analysis. The questionnaire was formatted in large print to accommodate those individuals with low vision. In situations in which a participant was not able to complete the questionnaire without assistance, a project coordinator helped the patient with the form. Female reproductive history was assessed by a series of five questions: (1) “How old were you when you started having menstrual periods?” (2) “Have you reached menopause?” 2a) “If yes: At what age did your menstrual periods stop?” (2b) “Why did they stop?” (3) “Have you ever used hormone pills, creams, or patches to help stop premenstrual symptoms (PMS) or hot flashes, or to prevent bone loss caused by menopause (the change of life)?” (3a) “If yes, what year did you start?” (3b) “Are you currently taking hormones?” (3c) “If No: What year did you stop?” (4) “You may have started and stopped taking hormones several times. How long overall have you taken hormones?” (5) “Many women take birth control pills, shots, or implants to prevent pregnancy or to regulate their menstrual periods. Have you ever taken birth control pills, shots, or implants for birth control or for any other reason?” (5a) “If yes, what year did you start?” (5b) “Are you currently taking birth control?” (5c) “If no, what year did you stop?” (6) “You may have started and stopped taking birth control several times. How long overall have you taken birth control?”
From these questions, the following variables were constructed for analysis: age at menstrual period start (continuous), has menopause been reached (yes, coded 1/no, coded 0), menstrual period stop age (continuous), why did menstrual period stop (categories included, natural menopause, hysterectomy without oophorectomy, partial hysterectomy, radiation chemotherapy, total hysterectomy), HRT (ever, coded 1/never, coded 0), HRT currently (yes, coded 1/no, coded 0), total years of HRT (continuous), BCP use (ever, coded 1/never, coded 0), and total years of BCP use (continuous). For the variable, why did menstrual period stop, each category was tested in a separate analysis treating each as a risk variable (coded 1) comparing it to the baseline (natural menopause).
Description of the Available Clinical Dataset
As described in Table 1, Caucasian female patients from 492 multiplex and singleton families (560 cases and 239 controls) of non-Hispanic descent who returned the self-administered health and activities questionnaire were included in this analysis. Among these individuals, 392 were unrelated (363 cases and 29 controls), and the remaining individuals made up 100 families, with 66 families containing more than 1 case individual. Of the 239 controls, 169 were classified grade 1 and 70 grade 2. Of the 560 cases, 172 were classified grade 3, 75 grade 4, and 313 grade 5. The mean age at examination for cases (75.5 ± 8.3 years) was higher than the mean age for controls (66.8 ± 8.3 years). Among these individuals, 50% of cases and 41% of controls were smokers (yes, ever). For a more detailed clinical description of the individuals examined in this cohort please refer to Shuler et al.35
Genotyping
Genomic DNA was extracted from whole blood (Puregene; Gentra Systems, Minneapolis, MN). DNA was available on 710 (89%) of the 799 Caucasian women with clinical information. Single nucleotide polymorphisms (SNPs) were examined in CFH (11 SNPs), CFH-R1 (2 SNPs), CFH-R3 (1 SNP), CFB (6 SNPs), ARMS2 (9 SNPs), C3 (2 SNPs), HTRA1 (1 SNP), and APOE (2 SNPs defining the E2, E3, and E4 alleles; Supplementary Table S1, http://www.iovs.org/cgi/content/full/51/4/1873/DC1). The details regarding SNP selection, genotyping, and quality control measures are described elsewhere.11,30,36–40 A genotyping platform (TaqMan; Applied Biosystems, Inc., [ABI], Foster City, CA) was used to genotype the SNPs (model 7900, ABI using Assays on Demand or Assays by Design when necessary, for the majority of SNPs examined. Assays by Design were used when no predesigned assay was available). Probe and primer sequences for the designed assays are available on request. DNA samples from The Foundation Jean Dausset-Centre d'Etude du Polymorphisme Humain (CEPH) families were duplicated between and across plates for use as a quality control, and laboratory personnel were blinded to the affected status of the individuals being genotyped.
Statistical Analysis
Analysis of Female Reproductive History and HRT.
To assess the influence of female reproductive history, HRT, and BCP use on risk of AMD, we implemented population averaged generalized estimating equations (GEEs; SAS, Proc GENMOD, ver. 9.1; SAS Institute, Cary, NC). GEE was used to account for the correlations among related individuals, as the data consisted of a combination of related and unrelated female cases and controls. GEE is a valid test of gene x gene and gene x environment interactions in family data.41 Population-averaged GEEs with an independence correlation matrix to adjust for correlations among related individuals using a robust variance estimator for each term were used for analyses. Each exposure of interest was included in multivariable analyses adjusting for known AMD risk factors, age at examination (continuous), and smoking status (ever smoked at least 100 cigarettes in lifetime, coded 1, vs. never smoked 100 cigarettes in lifetime, coded 0). Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from GEE analyses.
These analyses were performed comparing all AMD cases (moderate AMD,3 geographic atrophy,4 and neovascular AMD5) with controls. However, to investigate whether neovascular AMD significantly differed from early AMD and geographic atrophy AMD, for statistically significant (P < 0.05) associations, we followed up with analyses stratified by AMD grade.
Genetic Analysis.
Tests for deviations from Hardy-Weinberg equilibrium (HWE), calculations of minor allele frequency, and pair-wise linkage disequilibrium (LD) between SNPs were calculated by using Haploview statistical software.42 For these calculations, one case and one control were randomly selected from each family. Haplotype blocks were defined according the Gabriel et al.43 algorithm.
Single-marker tests of association were performed with GEEs, with additive genotypic tests of association used that modeled the minor allele as the risk allele (0, homozygous major allele, vs. 1, heterozygous, vs. 2, homozygous minor allele), with adjustment for age at examination and smoking status. Alleles E2, E3, and E4 at APOE were determined from genotypes at two SNPs and coded into two indicator variables for the effects of E2 and E4. Two analyses were performed for APOE. For the first analysis, genotypes were coded E3/E3 = 0, E2/E3 = 1, and E2/E2 = 2 and for the second analysis genotypes were coded E3/E3 = 0, E4/E3 = 1, and E4/E4 = 2.
Univariate analyses were followed up, and the joint effects of statistically significant variables (from female reproductive history analyses) and established AMD risk genes, including all genotyped SNPs within those genes, were examined for association with AMD susceptibility. When statistically significant interactions were observed, stratified analyses of SNP genotypes and variable subgroups were performed. All SNPs examined are listed in Supplementary Table S1. We performed interaction analyses using additive genotypic models with the minor allele as the risk allele, with the exception of CFH SNP Y402H (rs1061170), ARMS2 SNP rs1040924, and CFB SNP rs641153, which had all been associated with AMD in a dominant model using the major allele homozygote as the referent exposure. All tests were considered significant at a type I error rate of α = 0.05 for the interaction term. These analyses were performed with pooled affected AMD grades and stratified by grade if a significant association was observed in pooled analyses (early AMD,3 geographic atrophy,4 and neovascular AMD5).
Results
Female Reproductive History and Hormone Use Analyses
GEE multivariable analyses of all cases and controls (Table 2) found significant inverse associations of AMD and both HRT (P = 0.008) and BCPs (P = 0.048), adjusting for the effects of age at enrollment and smoking. However, when analyses were stratified by “affected” AMD grades, the inverse associations increased in significance for both HRT and BCP when comparing neovascular AMD (grade 5) with controls (grades 1 and 2; HRT OR = 0.45, 95% CI 0.30–0.66, P < 0.0001; BCP OR = 0.55, 95% CI 0.32–0.96, P = 0.036). BCPs consistently had inverse associations across AMD grades, however, for HRT, the inverse association was only observed for grade 5 and all grades pooled.
Table 2.
GEE Multivariate Analyses of Female Reproductive History and HRT Use Adjusted for Age at Examination and Smoking Status
| Variable | Cases (n) | Controls (n) | OR | 95% CI | GEE P |
|---|---|---|---|---|---|
| Menstrual period start, y | 478 | 218 | 0.99 | 0.93–1.06 | 0.846 |
| Menopause, yes/no | 527/3 | 232/3 | 2.43 | 0.47–12.53 | 0.290 |
| Menstrual period stop, y | 458 | 210 | 0.99 | 0.96–1.01 | 0.194 |
| Why did menstrual period stop | |||||
| Normal menopause | 301 | 133 | 1.00 | — | — |
| Hysterectomy without oophorectomy | 78 | 28 | 1.66 | 0.98–2.83 | 0.061 |
| Partial hysterectomy | 26 | 9 | 1.29 | 0.55–3.02 | 0.564 |
| Radiation chemotherapy | 2 | 3 | 0.38 | 0.11–1.27 | 0.115 |
| Total hysterectomy | 105 | 45 | 1.02 | 0.71–1.47 | 0.900 |
| HRT (ever/never) | |||||
| All grades | 288/229 | 173/62 | 0.65 | 0.48–0.90 | 0.008 |
| Grade 5 | 135/154 | 173/62 | 0.45 | 0.30–0.66 | <0.0001 |
| Grade 4 | 37/27 | 173/62 | 1.18 | 0.60–2.29 | 0.632 |
| Grade 3 | 116/48 | 173/62 | 1.13 | 0.74–1.73 | 0.571 |
| HRT currently, yes/no | 105/179 | 64/107 | 1.44 | 0.95–2.18 | 0.086 |
| HRT total, y | 199 | 137 | 1.00 | 0.98–1.02 | 0.921 |
| BCP use, ever/never | |||||
| All Grades | 125/381 | 130/102 | 0.60 | 0.36–1.00 | 0.048 |
| Grade 5 | 56/225 | 130/102 | 0.55 | 0.32–0.96 | 0.036 |
| Grade 4 | 12/51 | 130/102 | 0.56 | 0.23–1.35 | 0.195 |
| Grade 3 | 57/104 | 130/102 | 0.61 | 0.36–1.05 | 0.077 |
| BCP use total, y | 78 | 87 | 1.01 | 0.97–1.05 | 0.677 |
Statistically significant (P < 0.05) results are in bold.
Genetic Analysis
We followed-up the significant associations we observed (HRT and BCP) with interaction analyses examining the joint effects of both these variables and established AMD genetic risk factors. A complete list of the SNPs and genes examined is in Supplementary Table S1. Demographic information, single locus tests of association, and characterization of LD structure on all our AMD samples have been published11,30,37; however, in this report, we present the analyses of a subset of those samples (female participants who had been genotyped and completed questions regarding reproductive history, HRT, and BCP use).
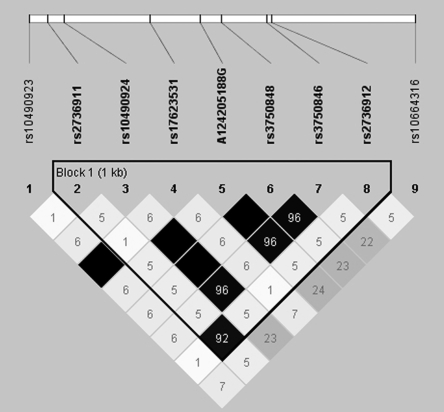
Although we did not observe a statistically significant interaction (P < 0.05) between HRT or BCPs and the specific SNPs previously associated with AMD, we did observe some significant associations at several other markers in those genes. ARMS2 showed the strongest evidence of association, with a statistically significant interaction with HRT at two SNPs (coding SNP R73H rs10490923 x HRT, P = 0.007; intronic SNP rs17623531 x HRT, P = 0.019; Table 3B). These SNPs did not have statistically significant single-marker effects (Table 3A). We did observe, however, five statistically significant associations (rs10490924, A124205188G, rs3750848, rs3750846, and rs10664316; P < 0.01 for all) at other SNPs in ARMS2. None of these SNPs deviated from HWE. In this subset of women, LD analyses showed that rs10490923 and rs17623531 were in perfect LD, with an r2 = 1.0 (Fig. 1), suggesting that these two results probably represent a single interaction with HRT. Although the two SNPs are in complete LD, they do not have identical test results due to a small number of randomly missing genotypes. When analyses were stratified by AMD grade, the interaction with rs17623531 was not significant, but remained for rs10490923 AMD grades 3 (P = 0.046) and 5 (P = 0.044).
Table 3.
GEE Single Locus Association and Genotype × HRT Interaction Analysis Results for ARMS2
| A. Single-Marker Significance | |||
|---|---|---|---|
| Gene Name | SNP | Allele Frequency (Allele) | GEE P* |
| Age-related macular degeneration 2 (ARMS2) (Chromosome 10) | rs10490923 | 0.12(A)/0.88(G) | 0.116 |
| rs2736911 | 0.11(T)/0.89(C) | 0.641 | |
| rs10490924 | 0.32(T)/0.68(G) | <0.0001 | |
| rs17623531 | 0.12(A)/.088(G) | 0.079 | |
| A124205188G | 0.31(G)/0.69(A) | <0.0001 | |
| rs3750848 | 0.32(G)/0.68(T) | <0.0001 | |
| rs3750846 | 0.30(G)/0.70(A) | <0.0001 | |
| rs2736912 | 0.10(A)/0.90(G) | 0.943 | |
| rs10664316 | 0.34(C)/0.66(A) | 0.001 | |
| B. SNP Interaction | ||||||
|---|---|---|---|---|---|---|
| Gene | SNP Function | AMD Grade | Interaction | OR | 95% CI | GEE p |
| Age-related macular degeneration 2 (ARMS2) (Chromosome 10) | Coding (R73H) | All grades | rs10490923 × HRT | 0.23 | 0.08–0.68 | 0.007 |
| Coding (R73H) | Grade 3 | rs10490923 × HRT | 0.26 | 0.07–0.98 | 0.046 | |
| Coding (R73H) | Grade 4 | rs10490923 × HRT | 0.37 | 0.06–2.52 | 0.313 | |
| Coding (R73H) | Grade 5 | rs10490923 × HRT | 0.25 | 0.06–0.96 | 0.044 | |
| Intron | All grades | rs17623531 × HRT | 0.28 | 0.09–0.81 | 0.019 | |
| Intron | Grade 3 | rs17623531 × HRT | 0.27 | 0.07–1.01 | 0.052 | |
| Intron | Grade 4 | rs17623531 × HRT | 0.44 | 0.07–2.89 | 0.391 | |
| Intron | Grade 5 | rs17623531 × HRT | 0.3 | 0.08–1.18 | 0.084 | |
| C. SNP Significance in Stratified Analysis | ||||
|---|---|---|---|---|
| Subgroup | Variable | OR | 95% CI | GEE P |
| No HRT | rs10490923 | 1.47 | 0.59–3.65 | 0.407 |
| rs17623531 | 1.21 | 0.48–3.09 | 0.686 | |
| HRT | rs10490923 | 0.39 | 0.22–0.67 | 0.001 |
| rs17623531 | 0.38 | 0.22–0.66 | 0.001 | |
| rs10490923 | ||||
| GG | HRT | 0.91 | 0.54–1.53 | 0.710 |
| AG&AA | HRT | 0.50 | 0.30–0.84 | 0.009 |
| rs17623531 | ||||
| GG | HRT | 0.82 | 0.48–1.40 | 0.469 |
| AG&GG | HRT | 0.54 | 0.34–0.87 | 0.011 |
GEE analyses were performed with-adjustment for age-at examiantion and smoking status. Statistically significant results are in bold (P < 0.05).
Figure 1.
ARMS2 r2 LD plot for unaffected control subjects. LD plots were generated in Haploview and are presented for one unaffected subject selected from each family. Within each triangle is the pair-wise correlation coefficient (r2). Standard color coding was used for the Haploview LD plots, for D′ LD plots: white (r2 = 0), shaded gray, (0 < r2 < 1), and black (r2 = 1). Black squares without numbers indicate complete LD (r2 = 1.00).
When stratified analyses were performed (stratifying by both genotypes and HRT) we observed that significance at these SNPs was present only in individuals who indicated use of HRT (rs10490923 OR = 0.39, 95% CI 0.22–0.67, P = 0.0007; rs17623531 OR = 0.38, CI 0.22–0.66, P = 0.0006; Table 3C). We also observed that HRT was most significant with the rs10490923 AG&GG (OR = 0.50, 95% CI 0.30–0.84, P = 0.009) and rs17623531 AG&GG (OR = 0.54, 95% CI 0.34–0.87, P = 0.011) genotypes, for which risk was based on having at least one copy of the minor allele (Table 3C). All gene x environment interaction analyses were performed with adjustment for age at examination and smoking. A significant test statistic for the interaction term would indicate that both were synergistic, inverse associations with AMD.
Discussion
In the present study, we observed significant inverse associations with HRT and BCP use and AMD risk and significant interactions between ARMS2 and HRT. Our strongest interaction was observed with two variants in ARMS2, including one intronic and one coding variant, and HRT adjusted for age at enrollment and smoking. This inverse association was strongest in pooled affected AMD grades 3 to 5. These interactions did not include the specific variants previously associated with AMD risk, although these associations were also in close proximity to the coding variant previously associated with AMD, ARMS2 rs10490924. These results are consistent with some studies that have observed protective effects of HRT and oral contraceptive use in Caucasian and Latino AMD patients14,15,20 and in contrast to others in which these effects have not been observed.21–23 The inconsistent replication of the associations with BCPs and HRT are not attributable to study design differences, as both studies that have replicated and those that have not have consisted primarily of a cohort study design. Our strongest associations with HRT and BCPs were observed in those individuals with grade 5 or neovascular AMD. This effect, specific to those individuals with the neovascular AMD, has also been observed in previous studies (OR = ∼0.50).15
HRT has been associated with reduced risk of cardiovascular disease in postmenopausal women, with evidence coming from both epidemiologic and clinical studies.44,45 However, conflicting results of randomized clinical trials performed by the Women's Health Initiative (WHI) have shown no benefit of oral BCP or HRT on cardiovascular risk46 in some studies and protective effects in others.47 The protective effect that has been observed has been attributed to the lowering of low-density lipoprotein (LDL) cholesterol levels and the elevation of high-density lipoprotein (HDL) cholesterol levels, as well as favorable changes in fibrinogen and PAI-1, enhanced blood flow, and antioxidant properties.48–50 Estrogen has also been associated with lowered risk of mortality among women.15
Recently, the ARMS2 A69S polymorphism (rs10490924), which has been associated with AMD,12,13 has also been associated with coronary artery disease.51 This association with cardiovascular disease is not surprising, given that AMD is a risk factor for cardiovascular disease and has been positively associated with increased mortality among women with AMD compared with women without AMD.52 ARMS2 is a gene of unknown function on chromosome 10q25. It is located in close proximity to PLEKHA, a phosphoinositol-binding protein, and PRSS11, a protein containing both insulin-like growth factor–binding and serine protease domains. ARMS2 A69S is located between 200 to 500 bp from the ARMS2 variants we observed to interact with HRT, although they are not in high r2 with this variant. Presently, the biology underlying the interaction between ARMS2 and HRT is unknown; however, these results suggest that the impact of menopause on blood lipids and lipoproteins puts women at higher risk of several cardiovascular diseases, and women who have specific ARMS2 variants and who take HRT are at decreased risk of these diseases.53
A biological pathway to AMD that features HRT may include estrogen receptor α (ESR1) and estrogen receptor β (ESR2). Both ESR1 and ESR2 proteins have been observed in the human retina, suggesting that estrogen plays a role in the pathogenesis of AMD.54,55 Estrogen controls expression of chitinase 3-like-1 protein (YKL-40) found in choroidal neovascular membranes, if levels of estrogen are reduced then this can lead to the upregulation of YKL-40 and neovascular AMD.56 Estrogen provides an antioxidant effect by inhibiting lipid peroxidation, which provides protection against oxidative damage in the retina caused by the aging process.57,58 A haplotype (PvuII_XbaI haplotype 1) in ESR1 has recently been identified as being associated with lowered serum estradiol levels.59 Studies by Boekhoorn et al.,60 using the Rotterdam cohort study population, have shown that PvuII_XbaI haplotype 1 in ESR1 is associated with risk for late AMD, with a stronger effect observed for neovascular AMD (hazard ratio = 4.29, 95% CI 1.47–12.49, with adjustment for age, sex, smoking, and CFH).60 They did not, however, test for a direct interaction between this ESR1 haplotype and HRT or oral contraceptive use.
Although these results are interesting, there are some limitations to our study. Although our associations with HRT and AMD and the interaction between ARMS2 and HRT remain significant after an FDR correction (q* = 0.20)61 for the number of variables and genes examined, further studies are necessary to replicate and extend these findings in independent cohorts. Another factor that may affect our findings is the potential bias caused by our case group's being somewhat younger than our control group. To address this potential bias, we included a term in the multivariate model to adjust for confounding by age. This adjustment, however, may not take into account other unobserved factors such as a different medical awareness of the use of HRT and/or cycles of popularity of BCP by age and case/control status. As a result, we also performed analyses in which our data were divided into quartiles (<60, 60–69, 70–79, 80+; results not shown) for the multivariate analyses of BCP and HRT with adjustment for smoking status. In these analyses, we observed that the protective effect was consistent across age ranges for both HRT and BCP, although the significance of the effect was dependent on the number of samples present for that age quartile. Examination of these factors would be beyond the scope of the present study but they are worth examining in follow-up studies of HRT and BCP, to demonstrate that the protective direction of the effects of HRT and BCP was independent of age quartile. In the past, our group has observed an association between ARMS2 A69S and smoking history.37 We directly tested all the ARMS2 SNPs for interaction with smoking and did not observe a statistically significant interaction between the ARMS2 SNPs that interacted with HRT and smoking. Therefore, we did not adjust for a smoking interaction in our models. One factor that may explain why we observed the association in variants in the 5′ region of ARMS2 but not at the previously observed ARMS2 variant rs10490924 may be the SNPs tagging variants upstream in the HTRA1 gene located approximately 6.1 kb downstream of ARMS2. HTRA1 has been associated with AMD in a white and Hong Kong Chinese cohort.62,63 It may be that we are observing associations with ARMS2 due to a functional variant in HTRA1. We did not observe an interaction with the previously associated HTRA1 SNP rs11200638; however, further studies examining the relationship between these ARMS2 variants and variants in HTRA1 should be conducted.
Our data indicate that both HRT and oral contraceptives have significant protective effects in women with AMD, particularly those with the neovascular form. We speculate that this effect may be due to the role that estrogen plays in the eye. These findings also lead us to conclude that HRT in women does not seem to have a significant effect on the occurrence of moderate AMD. If these results are validated, they may indicate that treatment with hormones reduces the risk of soft drusen and could be beneficial for prevention of later-stage AMD. Haan et al.,64 although with a small sample size, supported this conclusion in a study suggesting that HRT in individuals with neovascular AMD reduces their risk of soft drusen. These findings, although inconsistently replicated in the past, support a role for estrogen levels in AMD susceptibility and suggest that interactions with ARMS2 genetic variants may explain a portion of AMD risk. Further studies replicating these effects in an independent cohort are necessary to validate these findings. Expression studies evaluating the effect of variation in the 5′ region of ARMS2 should be conducted to elucidate the mechanism underlying this gene's effect on AMD risk.
Supplementary Material
Acknowledgments
The authors thank the patients, their families, and the control individuals who participated in the study.
Footnotes
Supported by National Eye Institute Grant EY12118 (MP-V, JLH); National Center for Research Resources Grant M01 RR-00095 to Vanderbilt University; and National Eye Institute Center Grant P30-EY014801; and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York, NY.
Disclosure: D.R. Velez Edwards, None; P. Gallins, None; M. Polk, None; J. Ayala-Haedo, None; S.G. Schwartz, Genentech (F), Pfizer (I), P; J.L. Kovach, None; K. Spencer, None; G. Wang, None; A. Agarwal, P; E.A. Postel, P; J.L. Haines, P; M. Pericak-Vance, P; W.K. Scott, P
References
- 1.Hyman L. Epidemiology of AMD. In: Hampton R, Neslon P. eds. Age-Related Macular Degeneration: Principles and Practice New York: Raven Press; 1992:1–35 [Google Scholar]
- 2.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol 2000;118:819–825 [DOI] [PubMed] [Google Scholar]
- 3.Spencer G. Projections of the population of the United States by age, sex, and race: 1988–1989. Washington, DC: US Bureau of the Census; 1989;P-25:1018 [Google Scholar]
- 4.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:357–364 [DOI] [PubMed] [Google Scholar]
- 5.Klaver CC, Wolfs RC, Assink JJ, et al. Genetic risk of age-related maculopathy: population-based familial aggregation study. Arch Ophthalmol 1998;116:1646–1651 [DOI] [PubMed] [Google Scholar]
- 6.Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol 1998;116:653–658 [DOI] [PubMed] [Google Scholar]
- 7.Tielsch JM, Javitt JC, Coleman A, et al. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med 1995;332:1205–1209 [DOI] [PubMed] [Google Scholar]
- 8.Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48:257–293 [DOI] [PubMed] [Google Scholar]
- 9.Sepp T, Khan JC, Thurlby DA, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci 2006;47:536–540 [DOI] [PubMed] [Google Scholar]
- 10.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697–704 [DOI] [PubMed] [Google Scholar]
- 11.Spencer KL, Hauser MA, Olson LM, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet 2007;16:1986–1992 [DOI] [PubMed] [Google Scholar]
- 12.Jakobsdottir J, Conley YP, Weeks DE, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet 2005;77:389–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 2005;14:3227–3236 [DOI] [PubMed] [Google Scholar]
- 14.Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol 1992;110:1701–1708 [DOI] [PubMed] [Google Scholar]
- 15.Snow KK, Cote J, Yang W, et al. Association between reproductive and hormonal factors and age-related maculopathy in postmenopausal women. Am J Ophthalmol 2002;134:842–848 [DOI] [PubMed] [Google Scholar]
- 16.The Eye Disease Case-Control Study Group. Risk factors for idiopathic macular holes. Am J Ophthalmol 1994;118:754–761 [PubMed] [Google Scholar]
- 17.The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol 1996;114:545–554 [PubMed] [Google Scholar]
- 18.Gray RH, Gregor ZJ, Marsh M. Oestrogens and macular holes: a postal questionnaire. Eye 1994;8:368–369 [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Cho E, Schaumberg DA, et al. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Ophthalmol 2008;126:519–524 [DOI] [PubMed] [Google Scholar]
- 20.Fraser-Bell S, Wu J, Klein R, et al. Smoking, alcohol intake, estrogen use, and age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol 2006;141:79–87 [DOI] [PubMed] [Google Scholar]
- 21.Freeman EE, Munoz B, Bressler SB, West SK. Hormone replacement therapy, reproductive factors, and age-related macular degeneration: the Salisbury Eye Evaluation Project. Ophthalmic Epidemiol 2005;12:37–45 [DOI] [PubMed] [Google Scholar]
- 22.Klein BE, Klein R, Lee KE. Reproductive exposures, incident age-related cataracts, and age-related maculopathy in women: the Beaver Dam Eye Study. Am J Ophthalmol 2000;130:322–326 [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Wang Q, Moss SE. Is age-related maculopathy associated with cataracts? Arch Ophthalmol 1994;112:191–196 [DOI] [PubMed] [Google Scholar]
- 24.Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemons TE, Milton RC, Klein R, et al. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 2005;112:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med 2002;4:45–61 [DOI] [PubMed] [Google Scholar]
- 27.Ioannidis JP. Non-replication and inconsistency in the genome-wide association setting. Hum Hered 2007;64:203–213 [DOI] [PubMed] [Google Scholar]
- 28.Snow KK, Seddon JM. Age-related eye diseases: impact of hormone replacement therapy, and reproductive and other risk factors. Int J Fertil Womens Med 2000;45:301–313 [PubMed] [Google Scholar]
- 29.Postel EA, Agarwal A, Schmidt S, et al. Comparing age-related macular degeneration phenotype in probands from singleton and multiplex families. Am J Ophthalmol 2005;139:820–825 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt S, Saunders AM, De La Paz MA, et al. Association of the apolipoprotein E gene with age-related macular degeneration: possible effect modification by family history, age, and gender. Mol Vis 2000;6:287–293 [PubMed] [Google Scholar]
- 31.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol 1997;123:199–206 [DOI] [PubMed] [Google Scholar]
- 32.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications: AREDS report no. 1. Control Clin Trials 1999;20:573–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991;98:1128–1134 [DOI] [PubMed] [Google Scholar]
- 34.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367–374 [DOI] [PubMed] [Google Scholar]
- 35.Shuler RK, Jr, Schmidt S, Gallins P, et al. Phenotype analysis of patients with the risk variant LOC387715 (A69S) in age-related macular degeneration. Am J Ophthalmol 2008;145:303–307 [DOI] [PubMed] [Google Scholar]
- 36.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308:419–421 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt S, Hauser MA, Scott WK, et al. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet 2006;78:852–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer KL, Hauser MA, Olson LM, et al. Haplotypes spanning the complement factor H gene are protective against age-related macular degeneration. Invest Ophthalmol Vis Sci 2007;48:4277–4283 [DOI] [PubMed] [Google Scholar]
- 39.Spencer KL, Olson LM, Anderson BM, et al. C3 R102G polymorphism increases risk of age-related macular degeneration. Hum Mol Genet 2008;17:1821–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer KL, Hauser MA, Olson LM, et al. Deletion of CFHR3 and CFHR1 genes in age-related macular degeneration. Hum Mol Genet 2008;17:971–977 [DOI] [PubMed] [Google Scholar]
- 41.Hancock DB, Martin ER, Li YJ, Scott WK. Methods for interaction analyses using family-based case-control data: conditional logistic regression versus generalized estimating equations. Genet Epidemiol 2007;31:883–893 [DOI] [PubMed] [Google Scholar]
- 42.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263–265 [DOI] [PubMed] [Google Scholar]
- 43.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science 2002;296:2225–2229 [DOI] [PubMed] [Google Scholar]
- 44.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 1995;273:199–208 [PubMed] [Google Scholar]
- 45.Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 1996;335:453–461 [DOI] [PubMed] [Google Scholar]
- 46.Stefanick ML, Prentice RL, Anderson G, et al. Reanalysis of the Women's Health Initiative oral contraceptive data reveals no evidence of delayed cardiovascular benefit. Fertil Steril 2005;83:853–854 [DOI] [PubMed] [Google Scholar]
- 47.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291:2959–2968 [DOI] [PubMed] [Google Scholar]
- 48.Mosca L, Manson JE, Sutherland SE, et al. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Writing Group. Circulation 1997;96:2468–2482 [DOI] [PubMed] [Google Scholar]
- 49.Subbiah MT. Mechanisms of cardioprotection by estrogens. Proc Soc Exp Biol Med 1998;217:23–29 [DOI] [PubMed] [Google Scholar]
- 50.Women's Health Initiative Study Group. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized clinical trial. JAMA 2002;288:321–333 [DOI] [PubMed] [Google Scholar]
- 51.Pulido JS, McConnell JP, Lennon RJ, et al. Relationship between age-related macular degeneration-associated variants of complement factor H and LOC387715 with coronary artery disease. Mayo Clin Proc 2007;82:301–307 [DOI] [PubMed] [Google Scholar]
- 52.Buch H, Vinding T, la CM, et al. Age-related maculopathy: a risk indicator for poorer survival in women: the Copenhagen City Eye Study. Ophthalmology 2005;112:305–312 [DOI] [PubMed] [Google Scholar]
- 53.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med 1997;336:1769–1775 [DOI] [PubMed] [Google Scholar]
- 54.Munaut C, Lambert V, Noel A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol 2001;85:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogueta SB, Schwartz SD, Yamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci 1999;40:1906–1911 [PubMed] [Google Scholar]
- 56.Rakic JM, Lambert V, Deprez M, et al. Estrogens reduce the expression of YKL-40 in the retina: implications for eye and joint diseases. Invest Ophthalmol Vis Sci 2003;44:1740–1746 [DOI] [PubMed] [Google Scholar]
- 57.Akcay T, Dincer Y, Kayali R, et al. Effects of hormone replacement therapy on lipid peroxides and oxidation system in postmenopausal women. J Toxicol Environ Health A 2000;59:1–5 [DOI] [PubMed] [Google Scholar]
- 58.Subbiah MT, Kessel B, Agrawal M, et al. Antioxidant potential of specific estrogens on lipid peroxidation. J Clin Endocrinol Metab 1993;77:1095–1097 [DOI] [PubMed] [Google Scholar]
- 59.Schuit SC, de Jong FH, Stolk L, et al. Estrogen receptor alpha gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol 2005;153:327–334 [DOI] [PubMed] [Google Scholar]
- 60.Boekhoorn SS, Vingerling JR, Uitterlinden AG, et al. Estrogen receptor alpha gene polymorphisms associated with incident aging macula disorder. Invest Ophthalmol Vis Sci 2007;48:1012–1017 [DOI] [PubMed] [Google Scholar]
- 61.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284 [DOI] [PubMed] [Google Scholar]
- 62.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006;314:989–992 [DOI] [PubMed] [Google Scholar]
- 63.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006;314:992–993 [DOI] [PubMed] [Google Scholar]
- 64.Haan MN, Klein R, Klein BE, et al. Hormone therapy and age-related macular degeneration: the Women's Health Initiative Sight Exam Study. Arch Ophthalmol 2006;124:988–992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.