Qualitative evaluation of standard photographs was used to assess the anterior chamber configuration in population-based narrow- and wide-angle cases of glaucoma. Basal iris thickness was found to be relevant in narrow-angle eyes. Other features of the iris and ciliary body were not relevant.
Abstract
Objective.
To classify anatomic features related to anterior chamber angles by a qualitative assessment system based on ultrasound biomicroscopy (UBM) images.
Methods.
Cases of primary angle-closure suspect (PACS), defined by pigmented trabecular meshwork that is not visible in two or more quadrants on static gonioscopy (cases) and systematically selected subjects (1 of every 10) who did not meet this criterion (controls) were enrolled during a population-based survey in Guangzhou, China. All subjects underwent UBM examination. A set of standard UBM images was used to qualitatively classify anatomic features related to the angle configuration, including iris thickness, iris convexity, iris angulation, ciliary body size, and ciliary process position. All analysis was conducted on right eye images.
Results.
Based on the qualitative grades, the difference in overall iris thickness between gonioscopically narrow eyes (n = 117) and control eyes (n = 57) was not statistically significant. The peripheral one third of the iris tended to be thicker in all quadrants of the PACS eyes, although the difference was statistically significant only in the superior quadrant (P = 0.008). No significant differences were found in the qualitative classifications of iris insertion, iris angulation, ciliary body size, and ciliary process position. The findings were similar when compared with the control group of eyes with wide angles in all quadrants.
Conclusions.
Basal iris thickness seems to be more relevant to narrow angle configuration than to overall iris thickness. Otherwise, the anterior rotation and size of the ciliary body, the iris insertion, and the overall iris thickness are comparable in narrow- and wide-angle eyes.
Gonioscopy is currently regarded as the reference standard for clinical assessment of the anterior chamber angle (ACA). However, it is a technically demanding examination that depends on the examiner's subjective judgment. A significant weakness of the technique is that it cannot provide definitive information on the anatomy of structures posterior to the iris that may influence angle width. Although anterior segment optical coherence tomography (ASOCT) is a simple, noncontact alternative for the imaging of the drainage angle, the iris pigment epithelium limits infrared radiation transmission and thus may limit visualization of structures posterior to the iris.1–3
Ultrasound biomicroscopy (UBM) has been shown to be a great asset in assessing ACA structures. Radially oriented scanning through the limbus provides a cross-sectional view of ACA with near-microscopic lateral and axial resolution.4 Examples of the use of UBM in the field of ACA assessment include detection or confirmation of appositional angle closure, verification of the existence of ciliary process position, and identification of other abnormalities related to the drainage angle and ciliary body.5 One of the great merits of UBM is its ability to reveal characteristics of some ACA structures posterior to the iris that are otherwise hidden from clinical observation. Visualization of these structures may help achieve insight into causative factors underlying various angle configurations.
For the purpose of angle-closure research, algorithms and software have been developed to quantify geometric angle width and iris thickness as well as the anatomic relationship between the iris and the ciliary body in UBM images. Although UBM also allows quantitative measurements of the anterior segment to be made, these quantitative parameters alone may not be sufficient to fully describe the features associated with angle-closure. Furthermore, the semiautomated nature of UBM quantitative assessment may compromise its reproducibility and limit its value in clinical practice.6 Therefore, with currently available technology, UBM is used for qualitative observation in most instances.
In the present study, we selected a representative set of UBM images from the database of a population-based study.7 Using this set of images as reference standard photographs, we developed a qualitative assessment system to classify size and rotation of the ciliary body, iris insertion, iris convexity, iris thickness, and iris angulation. We also used this system to describe the characteristics of ACA structures in eyes with different gonioscopic angle width identified in a population-based study conducted in southern China.
Methods
Subjects, Gonioscopy, and UBM Examination
Approval of the ethics of the study protocol was obtained from the Zhongshan University Ethics Review Board, the Ethics Committee of Zhongshan Ophthalmic Center (ZOC), and the Research Governance Committee of Moorfields Eye Hospital. The study was conducted in accordance with the tenets of the World Medical Association's Declaration of Helsinki. Examination of the subjects for the cross-sectional survey was performed from September 2003 to February 2004.
Subjects were enrolled during a population-based study conducted in residents aged 50 years and older in the Liwan District of Guangzhou.7 Gonioscopy was performed to identify eyes with primary angle-closure suspect (PACS; defined as those eyes in which the pigmented trabecular meshwork was not visible in two or more quadrants). The detailed protocol for gonioscopy has been described elsewhere.7,8 Any cases with established peripheral anterior synechiae, with or without glaucomatous neuropathy, and those with treatment that alters the anterior segment(i.e., iridectomy, iridotomy, iridoplasty, and cataract surgery) were excluded. All people with PACS in either eye, and 1 of 10 people who did not meet this criterion were invited for UBM examination (P45 Ultrasound Workstation; Paradigm Medical Industries, Salt Lake City, UT) at ZOC. The subjects who did not attend the UBM examination or were not suitable or not able to cooperate during UBM were excluded. UBM examination was conducted at the ZOC's main hospital by a technician (XC) who had 8 years of experience in administering UBM examinations. The technician was masked to the gonioscopic findings. The UBM examination was performed in a dark room with illumination below 5 lux. Images of the four quadrants and one image of the central anterior chamber were acquired in supine subjects. The subjects were asked to fixate on a ceiling target using the contralateral eye. Five target markers (fluorescent papers 5 × 5 cm in size) were set up on the ceiling to guide the patients' direction of gaze for measurement of the superior, inferior, nasal and temporal quadrants, so that the angle between the gaze direction and the measurement axis was standardized to 20° and accommodation was controlled. Saline was used as the coupling agent, and topical anesthesia was used before the examination. The probe was always perpendicular to the ocular surface. To reveal the relationship between the iris and the ciliary body, the technician took care to ensure that the radial perpendicular UBM scans were obtained through a typical ciliary process. The gain was set to between 60 and 80 dB, to have a clear display of the structure and minimize the ultrasound noise simultaneously. The criteria for acceptable images were clear visualization of the scleral spur, angle, ciliary body, and a half chord of the iris. The tangent line of the anterior surface of the lens ideally should be horizontal, to ensure standardization of the layouts of the images.
Definition of Cases and Controls
Only the UBM images of the right eyes of all enrolled subjects were analyzed. Cases were defined as the right eyes of patients with PACS in which the pigmented trabecular meshwork (PTM) was not visible in at least two quadrants on static gonioscopy. Control eyes were defined in two ways: Control-1 was defined as eyes in which the PTM was not visible in less than two quadrants on static gonioscopy. This definition covered all conditions that did not meet the gonioscopic criterion for PACS. Control-2 was defined as eyes in which the PTM was visible in all four quadrants. Thus, control-1 included both control-2 and eyes with a gonioscopically narrow angle in only one quadrant.
Standard Photographs for Qualitative Assessment
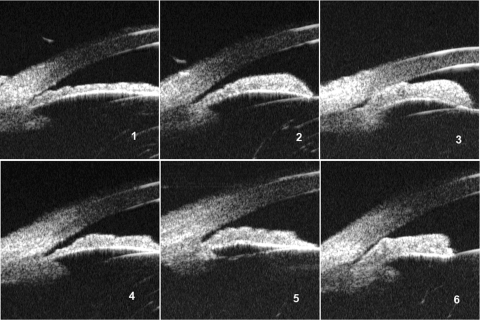
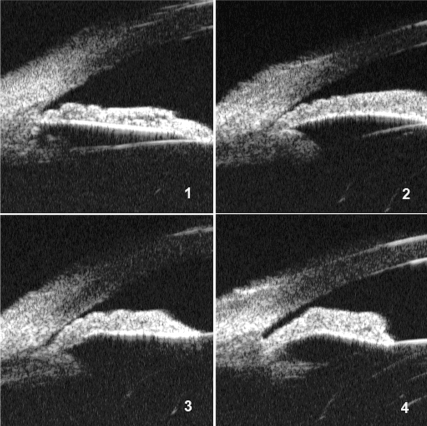
The images were exported from the UBM machine and transferred to a computer for further analysis. Two researchers (MH, PJF) reviewed all images in the database and selected the representative images as standard illustrations of the following six features: (1) iris thickness: the overall thickness and the thickness of the peripheral one third of the iris (termed basal iris thickness), which was graded in reference to the limbal corneal thickness (Fig. 1); (2) iris convexity: judged by the curvature of the posterior surface of the iris (Fig. 2); (3) iris insertion: graded according to the location of the iris insertion into the ciliary body (Fig. 3); (4) iris angulation: identified if the iris made an abrupt change in direction at the point of insertion into the ciliary body (Fig. 3); (5) ciliary body size: identified as the greatest distance in a straight line between the apex of the ciliary body and base, as close as possible to perpendicular to the sclera, in reference to the limbal cornea thickness (small was less than limbal corneal thickness, medium was 1 to 1.99 limbal corneal distance, and large was two or more limbal corneal thicknesses; Fig. 4); and (6) ciliary process position: classified as neutral or anteriorly positioned based on the direction of the axis of the ciliary body (Fig. 4).
Figure 1.
Referring to the limbal corneal thickness: (1) thin overall iris thickness, (2) medium overall iris thickness, (3) thick overall iris thickness, (4) thin basal iris thickness, (5) medium basal iris thickness, and (6) thick basal iris thickness.
Figure 2.
Iris convexity: (1) absent, (2) mild, (3) moderate, and (4) extreme.
Figure 3.
Locations of iris insertion: (1) basal, (2) middle, or (3) apical. Angulation of the iris profile around the location of iris insertion: (4) none, (5) mild, or (6) pronounced.
Figure 4.
The size of the ciliary body was classified according to the relative dimension of the ciliary body in the UBM images compared with limbal corneal thickness: (1) small, (2) medium, or (3) large. The orientation of ciliary body was graded as (4) neutral or (5) anterior.
For the purpose of a reproducibility test, 29 images were randomly selected from our database of UBM images. Each image was independently assessed by two observers (YJ, MH). To reduce the introduction of bias from the previous grading, while masked to the other results, one of the two observers (YJ) performed a second assessment 1 week after the first.
Statistical Analysis
The proportions of anatomic configurations in UBM images that were used for qualitative grading were calculated and compared by χ2 test. The κ statistic was calculated to assess reproducibility. All tests were two-tailed. P = 0.05 or lower was considered statistically significant (all analyses performed with Stata, ver. 10.0; StataCorp., College Station, TX).
Results
Among 186 PACS and 118 normal control subjects identified with gonioscopy, 117 PACS (62.9%) and 57 (48.3%) normal control (n = 57 by control-1 definition, n = 48 by control-2 definition) eyes were successfully examined by UBM. Those who attended the UBM examination tended to be younger: 75.0% in the 50- to 59-year age range and 26.0% in the 80+ group. There was a nonsignificant trend among the women toward a higher attendance rate, compared with the rate of the men (χ2 test, P = 0.660). Reasons for nonparticipation were mainly lack of interest in undergoing further examination (particularly in the normal control groups), inability to tolerate the UBM examination, and limitations in mobility, although free transportation to the ZOC main hospital was offered. However, the ACD (P = 0.550) and Shaffer angle width grades (P = 0.120) of the attendees and absentees did not differ, suggesting that the overall angle status in the attendees and absentees was similar. The demographic characteristics of the control subjects were similar to those of the entire survey population.
The mean ± SD ages of the cases and controls were 68.6 ± 8.0 and 63.2 ± 8.9 years, respectively. There were slightly more women among the cases (65.0% in case versus 56.1% in control), although there was no statistically significant intergroup difference (P = 0.157).
Reproducibility
The κ statistics of the intraobserver and interobserver reproducibility test varied in a range of 0.61 to 0.89 and 0.66 to 0.94 respectively, as shown in Table 1. According to the κ statistic, the intraobserver reproducibility was generally higher than interobserver reproducibility. The interobserver reproducibility of the grading of iris convexity and iris insertion were higher (0.751 and 0.878, respectively) than were those of other features.
Table 1.
κ-Values of the Interobserver and Intraobserver Reproducibility Tests
| Assessed Feature | κ-Value of the Reproducibility Test |
|
|---|---|---|
| Interobserver | Intraobserver | |
| Basal iris thickness | 0.609 | 0.682 |
| Overall iris thickness | 0.878 | 0.874 |
| Iris convexity | 0.751 | 0.847 |
| Iris insertion | 0.878 | 0.940 |
| Iris angulation | 0.657 | 0.843 |
| Ciliary body position | 0.654 | 0.656 |
| Ciliary body size | 0.654 | 0.725 |
Table 2 summarizes proportions of the grades of each feature in the cases and controls.
Table 2.
Qualitative Grading of UBM Images in Cases and Controls (All Quadrants, Right Eyes)
| Number of Eyes (proportion) | Superior |
Nasal |
Inferior |
Temporal |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Control-1 | Control-2 | P* | Cases | Control-1 | Control-2 | P* | Cases | Control-1 | Control-2 | P* | Cases | Control-1 | Control-2 | P* | |
| Iris thickness, basal | ||||||||||||||||
| Thin | 29 (24.79) | 21 (36.21) | 17 (35.42) | 27 (23.08) | 19 (32.76) | 16 (33.33) | 32 (27.35) | 26 (44.83) | 21 (43.75) | 43 (36.75) | 23 (39.66) | 18 (37.50) | ||||
| Medium | 69 (58.97) | 20 (34.48) | 16 (33.33) | P1 = 0.008 | 54 (46.15) | 22 (37.93) | 18 (37.50) | P1 = 0.365 | 49 (41.88) | 18 (31.03) | 14 (29.17) | P1 = 0.068 | 51 (43.59) | 24 (41.38) | 21 (43.75) | P1 = 0.932 |
| Thick | 19 (16.24) | 17 (29.31) | 15 (31.25) | P2 = 0.009 | 36 (30.77) | 17 (29.31) | 14 (29.17) | P2 = 0.370 | 36 (30.77) | 14 (24.14) | 13 (27.08) | P2 = 0.108 | 23 (19.66) | 11 (18.97) | 9 (18.75) | P2 = 0.990 |
| Iris thickness, overall | ||||||||||||||||
| Thin | 5 (4.27) | 3 (5.17) | 3 (6.25) | 7 (5.98) | 3 (5.17) | 3 (6.25) | 14 (11.97) | 10 (17.24) | 9 (18.75) | 20 (17.09) | 13 (22.41) | 11 (22.92) | ||||
| Medium | 76 (64.96) | 34 (58.62) | 28 (58.33) | P1 = 0.716 | 63 (53.85) | 36 (62.07) | 30 (62.50) | P1 = 0.677 | 68 (58.12) | 34 (58.62) | 27 (56.25) | P1 = 0.535 | 74 (63.25) | 33 (56.90) | 26 (54.17) | P1 = 0.651 |
| Thick | 36 (30.77) | 21 (36.21) | 17 (35.42) | P2 = 0.691 | 47 (40.17) | 19 (32.76) | 15 (31.25) | P2 = 0.645 | 35 (29.91) | 14 (24.14) | 12 (25.00) | P2 = 0.488 | 23 (19.66) | 12 (20.69) | 11 (22.92) | P2 = 0.535 |
| Iris convexity | ||||||||||||||||
| Absent | 3 (2.56) | 5 (8.62) | 5 (10.42) | 2 (1.71) | 6 (10.34) | 6 (12.50) | 2 (1.71) | 4 (6.90) | 4 (8.33) | 4 (3.42) | 9 (15.52) | 9 (18.75) | ||||
| Mild | 96 (82.05) | 43 (74.14) | 34 (70.83) | 95 (81.20) | 37 (63.79) | 31 (64.58) | 92 (78.63) | 44 (75.86) | 37 (77.08) | 94 (80.34) | 46 (79.31) | 38 (79.17) | ||||
| Moderate | 18 (15.38) | 10 (17.24) | 9 (18.75) | P1 = 0.174 | 18 (15.38) | 15 (25.86) | 11 (22.92) | P1 = 0.011 | 21 (17.95) | 10 (17.24) | 7 (14.58) | P1 = 0.253 | 18 (15.38) | 3 (5.17) | 1 (2.08) | P1 = 0.009 |
| Extreme | 0 (0.00) | 0 (0.00) | 0 (0.00) | P2 = 0.078 | 2 (1.71) | 0 (0.00) | 0 (0.00) | P2 = 0.010 | 2 (1.71) | 0 (0.00) | 0 (0.00) | P2 = 0.160 | 1 (0.85) | 0 (0.00) | 0 (0.00) | P2 = 0.001 |
| Iris insertion | ||||||||||||||||
| Basal | 75 (64.10) | 38 (65.52) | 32 (66.67) | 61 (52.14) | 27 (46.55) | 24 (50.00) | 72 (61.54) | 36 (62.07) | 32 (66.67) | 48 (41.03) | 25 (43.10) | 23 (47.92) | ||||
| Middle | 39 (33.33) | 19 (32.76) | 16 (33.33) | P1 = 0.934 | 51 (43.59) | 29 (50.00) | 23 (47.92) | P1 = 0.721 | 45 (38.46) | 21 (36.21) | 16 (33.33) | P1 = 0.355 | 66 (56.41) | 32 (55.17) | 25 (52.08) | P1 = 0.918 |
| Apical | 3 (2.56) | 1 (1.72) | 0 (0.00) | P2 = 0.531 | 5 (4.27) | 2 (3.45) | 1 (2.08) | P2 = 0.733 | 0 (0.00) | 1 (1.72) | 0 (0.00) | P2 = 0.535 | 3 (2.56) | 1 (1.72) | 0 (0.00) | P2 = 0.423 |
| Iris angulation | ||||||||||||||||
| None | 84 (71.79) | 39 (67.24) | 33 (68.75) | 84 (71.79) | 43 (74.14) | 33 (68.75) | 76 (64.96) | 38 (65.52) | 32 (66.67) | 65 (55.56) | 42 (72.41) | 35 (72.92) | ||||
| Mild | 24 (20.51) | 13 (22.41) | 11 (22.92) | P1 = 0.779 | 23 (19.66) | 12 (20.69) | 12 (25.00) | P1 = 0.725 | 29 (24.79) | 16 (27.59) | 13 (27.08) | P1 = 0.741 | 32 (27.35) | 10 (17.24) | 8 (16.67) | P1 = 0.098 |
| Pronounced | 9 (7.69) | 6 (10.34) | 4 (8.33) | P2 = 0.925 | 10 (8.55) | 3 (5.17) | 3 (6.25) | P2 = 0.694 | 12 (10.26) | 4 (6.90) | 3 (6.25) | P2 = 0.709 | 20 (17.09) | 6 (10.34) | 5 (10.42) | P2 = 0.117 |
| Ciliary body size | ||||||||||||||||
| Small | 41 (35.04) | 17 (29.31) | 10 (20.83) | 37 (31.62) | 21 (36.21) | 12 (25.00) | 31 (26.50) | 18 (31.03) | 13 (27.08) | 17 (14.53) | 5 (8.62) | 0 (0.00) | ||||
| Medium | 49 (41.88) | 27 (46.55) | 25 (52.08) | P1 = 0.742 | 61 (52.14) | 28 (48.28) | 28 (58.33) | P1 = 0.830 | 52 (44.44) | 24 (41.38) | 21 (43.75) | P1 = 0.819 | 56 (47.86) | 26 (44.83) | 23 (47.92) | P1 = 0.383 |
| Large | 27 (23.08) | 14 (24.14) | 13 (27.08) | P2 = 0.198 | 19 (16.24) | 9 (15.52) | 8 (16.67) | P2 = 0.688 | 34 (29.06) | 16 (27.59) | 14 (29.17) | P2 = 0.996 | 44 (37.61) | 27 (46.55) | 25 (52.08) | P2 = 0.013 |
| Ciliary body rotation | ||||||||||||||||
| Neutral | 46 (39.32) | 21 (36.21) | 19 (39.58) | P1 = 0.690 | 31 (26.50) | 20 (34.48) | 19 (39.58) | P1 = 0.274 | 69 (58.97) | 27 (46.55) | 25 (52.08) | P1 = 0.120 | 51 (43.59) | 30 (51.72) | 28 (58.33) | P1 = 0.310 |
| Anterior | 71 (60.68) | 37 (63.79) | 29 (60.42) | P2 = 0.975 | 86 (73.50) | 38 (65.52) | 29 (60.42) | P2 = 0.097 | 48 (41.03) | 31 (53.45) | 23 (47.92) | P2 = 0.417 | 66 (56.41) | 28 (48.28) | 20 (41.67) | P2 = 0.085 |
Data are presented as counts (proportions) by column. The grading is based on standard photographs of all quadrants in the right eyes.
The P-values of Pearson χ2 test for the comparison of proportions of UBM features in cases and controls. P1 is for comparison between cases and the controls defined by pigment trabecular meshwork not visible in less than two quadrants (control-1); P2 was for comparison between cases and the controls defined by PTM visible in all quadrants (control-2).
Iris Thickness
The overall thickness of the iris was classified as either medium or thick in 80% to 90% of all eyes. However, when the peripheral one third of the iris was considered alone, the proportion with medium or thick grades tended to be much less (50%–80%). The difference was not statistically significant between the overall iris thickness of cases and controls (χ2 test, cases versus control-1: P = 0.716 for superior, 0.677 for nasal, 0.535 for inferior, and 0.651 for temporal; cases versus control-2: P = 0.691 for superior, 0.645 for nasal, 0.488 for inferior, 0.535 for temporal). However, the thickness of the peripheral one third of the iris (basal iris thickness, as defined in the present study) tended to be higher in the cases in all quadrants than in the controls, although the differences in proportions of basal iris thickness grades between the cases and controls were statistically significant only in the superior quadrant (cases versus control-1: P = 0.008 for superior quadrant, 0.365 for nasal, 0.068 for inferior, and 0.932 for temporal; cases versus control-2: P = 0.009 for superior, 0.370 for nasal, 0.108 for inferior, and 0.990 for temporal).
Iris Convexity
Iris convexity was classified as mild in most of the eyes in both the cases and controls. The proportions of absent iris convexity were generally higher in all quadrants in the controls than in cases, although the difference was significant only in the nasal (cases versus control-1: P = 0.011, cases versus control-2: P = 0.010) and temporal (cases versus control-1: P = 0.009, cases versus control-2: P = 0.001) quadrants. Extreme iris convexity was seen only in the case group.
Iris Insertion
Basal iris insertion, defined in the present study as the iris insertion located near the base of the ciliary body, was commonly observed in all four quadrants in both the cases and controls. The proportions of basal iris insertion in the cases and controls did not differ significantly in all quadrants (P = 0.059–0.830). In contrast, apical iris insertion, with the iris root inserted far more toward the apex of the ciliary body, was very rarely seen in both groups.
Iris Angulation
Unexpectedly, we found that mild or pronounced iris angulation was not a unique feature of eyes in the case group. In all four quadrants, both mild and pronounced iris angulation had almost equal proportions in the cases and controls. However, for both the cases and controls, iris angulation was not observed in most eyes.
Ciliary Body Size
No significant differences were found in the classification of ciliary body size and position of the ciliary process between the cases and controls. In both cases and controls, the highest proportions (∼50%) of eyes were classified as having ciliary bodies of medium size. The difference in ciliary body size between cases and open-angle eyes (control-2) was found to be statistically significant only in the temporal quadrant (P = 0.013). Otherwise, no significant intergroup difference was found in the proportions of various grades of ciliary body size. Although there were no significant differences between the cases and controls, the differences between quadrants was significant in all groups (P < 0.05). Another interesting finding is that in both the cases and controls, a large ciliary body was comparatively more frequently observed in the inferior and temporal quadrants.
Ciliary Process Position
Approximately one half to three fourths of the ciliary bodies were classified as anteriorly positioned. In contrary to large ciliary body size, the proportions of anterior ciliary process position were lower in the inferior and temporal quadrants than in the other two quadrants in all groups, although the interquadrant difference was significant only in the case group (P < 0.001). Except for the inferior quadrant, the proportion of anteriorly positioned ciliary processes was higher than that of neutral ciliary body position in the cases.
Discussion
This study, to the best of our knowledge, is the first to describe a comprehensive method of qualitative assessment of ACA structures, including both iris and ciliary body, by evaluating images acquired by UBM. By using this qualitative assessment system, we described the characteristics of ACA structures in a group of Chinese people who were classified as diagnosed PACS or as normal control. In accordance with previous expectations, we found that a thick peripheral iris (basal iris thickness, as defined in our study) was disproportionately seen in narrow-angle eyes. However, some anatomic features that were previously believed to be associated with angle closure, such as a convex iris, iris angulation, and a large and anteriorly positioned ciliary body were seen comparably in both narrow- and wide-angle eyes. That the subjects were enrolled from a population-based study may favor the generalizability of our results.7,8
The intraobserver and interobserver agreement of the qualitative assessment system described in the present study was relatively greater than prior reports of quantitative measurements.9,10 This finding is in agreement with those of Spaeth et al.,4 although their definition of iris insertion was different, and the classification of iris configuration was less detailed than was the system described in the present study. In their study, two experienced clinicians qualitatively graded iris insertion and peripheral iris configuration. Their unweighted κ statistics showed high reproducibility in both intraobserver (κ = 0.83–0.89 for iris insertion, κ = 0.92 for iris configuration) and interobserver (κ = 0.79 for iris insertion, κ = 0.84 for iris configuration) analyses.4 Semiautomated quantitative measurement of UBM images requires a subjective identification of anatomic landmarks (e.g., the scleral spur) as reference points. Inaccurate determination of these landmarks may result in measurement errors. Before elimination of observer participation in the measurement, caution is still warranted when applying the quantitative assessment of UBM in clinical practice. Qualitative assessment, however, although it appears to be more subjective or observer dependent, is actually less susceptible to variability. Simple qualitative grading of a set of standard photographs is technically less demanding. Reasonable individual variation is usually not likely to result in major errors in qualitative assessment. Furthermore, appropriate training including more detailed and straightforward description of the grading method may further diminish errors introduced by observers' variable interpretation of the UBM images. Compared with currently available semiautomated quantification, comprehensive and detailed qualitative assessment can be equally informative. The high reproducibility of qualitative assessment may increase its value in clinical practice. However, it should be mentioned that the reproducibility assessment is largely for the same image. The variation on UBM image acquisition itself should also be considered.
So far, there has been no evidence from population-based studies of an association between greater peripheral iris thickness with narrow angles. A bulky peripheral iris may occupy a large proportion of the drainage angle recess, therefore narrowing the angle. This finding was confirmed in the present study. According to our data, although cases and controls shared roughly similar distribution of qualitative overall iris thickness grading, the peripheral iris was thicker in eyes with gonioscopically narrower angles. This observation was shown to be a general trend in all quadrants, although the difference was statistically significant only in the superior quadrant. Of note, the proportion of eyes with the iris graded as thick in the control groups (29.31% in control-1, 31.25% in control-2) was even greater than in the case group (16.24%), but in the superior quadrant only. There is no definitive explanation for this observation but it suggests that a thick iris may not be the only factor that contributes to angle closure.
It is noteworthy that all UBM images in our study were obtained in the dark. The influence of illumination on iris thickness has drawn the attention of some investigators.11–14 Pavlin et al.12 reported that in the dark, when angles are narrower, eyes in their study showed thickened and shortened irises. Iris convexity also increased. This finding was confirmed by Woo et al.,14 who showed a decrease in angle width when the illumination changed from light to dark. They attributed this phenomenon to increased iris thickness and anterior iris bowing in the dark. Hence, in the present study, it was difficult to tell whether the higher proportion of thick peripheral irises in narrow-angle eyes resulted from a static intrinsic anatomic feature or a dynamic change of iris thickness in response to illumination.
Compared with the method Spaeth et al.4 for qualitatively describing iris configuration, we used a more detailed classification method in which we defined iris curvature and iris angulation as two separate entities and then further subcategorized each feature into qualitative grades according to the degree of iris convexity or angulation. A pressure gradient between the posterior and anterior chambers (presumably resulting from pupil block) may induce an anterior convexity of the iris, which then may result in narrowing of the angle or appositional angle closure. Barkana et al.15 even included characteristic iris convexity in UBM images in the criteria for identifying the pupil block mechanism of angle closure. In the present study, we found that proportions of absent iris convexity (in other words, flat iris) were consistently more common in all quadrants of the controls compared with the cases. However, of interest, mild or even moderate iris convexity was also identified in a considerable proportion of eyes in the control group. This result suggests that despite being a major causative factor in angle closure, pupil block may not be uniquely associated with closed or occludable angles. It is possible that relative pupil block may also occur in eyes with wide and open angles in static gonioscopy. Presumably, the determining factor in development of pathologic angle closure is the existence of relative pupil block in an already crowded anterior segment. Because of the limited sample size of the control group in the present study, care should be exercised when interpreting data about iris convexity in control eyes.
Large, anteriorly positioned ciliary processes are associated with plateau iris or the non–pupil-block mechanisms of angle closure.16,17 Barkana et al.15 used the characteristic configuration of large, anteriorly positioned ciliary processes obliterating the ciliary sulcus as the single UBM criterion for the diagnosis of plateau iris configuration. To the contrary, however, and in agreement with data from one of our previous studies,5 we found that both larger size and anterior rotation of the ciliary body process can be seen in eyes with relatively wider ACA in gonioscopy as well. Although the definition of open-angle eyes in that study differed from that of control eyes in the present study, we also found that anterior ciliary body position was common in both open- and closed-angle eyes. Our data suggest that neither of the two ciliary body features alone is adequate to cause narrow angle configuration. Even the coexistence of both large and anteriorly positioned ciliary processes is not a unique characteristic of narrow-angle eyes with plateau iris configuration. It is possible that only in combination with other ACA structures such as anterior iris insertion, thick peripheral iris, and moderate or pronounced iris angulation, can the large anteriorly positioned ciliary body contribute to a narrow angle. This possibility may explain the high proportion of plateau iris configuration in the case series of Barkana et al.15 Therefore, when intending to identify the existence of plateau iris configuration or non–pupil-block mechanisms of angle closure, it may be reasonable to employ more comprehensive and stringent diagnostic criteria that cover the status of iridotrabecular contact, iris profile, and features of the ciliary body.
As we have pointed out elsewhere,5 because the UBM imaging was performed in supine patients, the interquadrant variation in UBM ACA structures was unlikely to result from effects of gravity and thus was unlikely to be an artifact. There may be some intrinsic differences in ACA structure between the quadrants. Also, although care was taken to ensure that the radial perpendicular UBM scans were obtained through a ciliary process, it is possible that some misaligned images were taken through the side instead of the apex of the ciliary process; these images would not represent the real size of the ciliary body and processes. Another possibility is that the numerous ciliary processes in a single human eye can vary greatly in size and can produce an intrinsic difference in the size and rotation status of ciliary body among quadrants. Finally, the translimbal UBM scanning through a single ciliary process may not reflect the general anatomic feature of the ciliary body within the range of a certain quadrant. These reasons are all possible explanations of the significant differences in ciliary body size and rotation among quadrants.
It is noteworthy that when we changed the definition of controls from non-PACS eyes (control-1) to eyes having wide open angles in all four quadrants (control-2), the results of the comparison between the cases and controls remained largely unchanged. The only change occurred in the difference in ciliary body size between the cases and controls. However, the difference was found to be statistically significant only in the temporal quadrant. Considering that the aforementioned factors may affect the appearance of the ciliary processes in UBM images, caution is advised in interpreting this finding.
Sampling from a population-based study increased the strength of the data in the present study, which may be representative of the situation in the population. However, considering the following limitations, caution should be used when interpreting the results of our study. The classification of a narrow angle and a control angle was based an arbitrary definition on the visibility of PTM on gonioscopy, because the gonioscopic definition is the current acknowledged standard for diagnosis and treatment.18–20 It is possible that the comparison of extremely narrow and wide angles would generate different results. However, our data suggest that the results are consistent in the comparison of narrow angles in at least two quadrants versus the narrow angle in one or fewer quadrants or versus those with wide angle in all quadrants. This result underscores that the identified qualitative features may occur independent of the degree of narrow angle. On the other hand, the classification method of the qualitative assessment system described in the present study was arbitrarily selected. Difficulty in identifying some features such as the position of the ciliary processes and iris angulation may affect the classification result. In addition, the inadequate sample size, particularly in the control group, may limit the power to detect the significance of differences between the case and control groups. Thus, it may be that the study results should be reconfirmed in studies with larger sample sizes. Finally, our study lacked UBM images acquired in the light. Although ACA features in the light are less important in the formation of appositional angle closure, it may help us to better understand the mechanism of angle closure by looking at the possible association between dynamic changes in peripheral iris thickness and angle configuration.
In summary, the present study described a method of qualitative assessment of a set of standard photographs obtained by UBM. Reproducibility testing of this classification system showed acceptable intra- and interobserver reproducibility. Compared with the controls, the cases, identified by two quadrants of invisible PTM in static gonioscopy, seemed to have thicker peripheral irises around the insertion spot, although the difference is statistically significant only in superior quadrant. The considerable proportion of mild or moderate iris convexity suggests the pupil block may not uniquely exist in narrow-angle eyes. According to data from the present study, factors believed to be associated with non–pupil-block mechanisms of angle closure—such as apical iris insertion, large ciliary body, and anteriorly positioned ciliary body—were found to have similar proportions in gonioscopically narrow- and wide-angle eyes. Our data strongly suggest that angle closure results from the coexistence of more than one abnormal anatomic characteristic.
Footnotes
Supported by a UCL Graduate School Research Scholarship and UCL Overseas Research Scholarship 2001061054, Grant 2005B30901008 from Scientific and Technology Foundation of Guangdong Province, and Grant NCET-06-0720 from the Program for New Century Excellent Talents in University, Ministry of Education (MH); and by Medical Research Council Grant G0401527, The Wellcome Trust Grant 075110, and The Richard Desmond Charitable Foundation (via Fight for Sight) (PJF).
Disclosure: Y. Jiang, None; M. He, None; W. Huang, None; Q. Huang, None; J. Zhang, None; P.J. Foster, None
References
- 1.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 2005;123:1053–1059 [DOI] [PubMed] [Google Scholar]
- 2.Mansouri K, Burgener ND, Bagnoud M, Shaarawy T. A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology following laser iridotomy in European eyes. Eye Published online January 9, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg 2007;33:837–840 [DOI] [PubMed] [Google Scholar]
- 4.Spaeth GL, Azuara-Blanco A, Araujo SV, Augsburger JJ. Intraobserver and interobserver agreement in evaluating the anterior chamber angle configuration by ultrasound biomicroscopy. J Glaucoma 1997;6:13–17 [PubMed] [Google Scholar]
- 5.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in eyes with narrow drainage angles: ultrasound biomicroscopy outcomes: The Liwan Eye Study. Ophthalmology 2007;114:1513–1519 [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol 2000;11:133–139 [DOI] [PubMed] [Google Scholar]
- 7.He M, Foster PJ, Ge J, et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci 2006;47:2782–2788 [DOI] [PubMed] [Google Scholar]
- 8.He M, Foster PJ, Ge J, et al. Gonioscopy in adult Chinese: The Liwan Eye Study. Invest Ophthalmol Vis Sci 2006;47:4772–4779 [DOI] [PubMed] [Google Scholar]
- 9.Tello C, Liebmann J, Potash SD, Cohen H, Ritch R. Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest Ophthalmol Vis Sci 1994;35:3549–3552 [PubMed] [Google Scholar]
- 10.Urbak SF, Pedersen JK, Thorsen TT. Ultrasound biomicroscopy, II: intraobserver and interobserver reproducibility of measurements. Acta Ophthalmol Scand 1998;76:546–549 [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Esaki K, Liebmann JM, Uji Y, Ritch R. Ultrasound biomicroscopy dark room provocative testing: a quantitative method for estimating anterior chamber angle width. Jpn J Ophthalmol 1999;43:526–534 [DOI] [PubMed] [Google Scholar]
- 12.Pavlin CJ, Harasiewicz K, Foster FS. An ultrasound biomicroscopic dark-room provocative test. Ophthalmic Surg 1995;26:253–255 [PubMed] [Google Scholar]
- 13.Sakuma T, Sawada A, Yamamoto T, Kitazawa Y. Appositional angle closure in eyes with narrow angles: an ultrasound biomicroscopic study. J Glaucoma 1997;6:165–169 [PubMed] [Google Scholar]
- 14.Woo EK, Pavlin CJ, Slomovic A, Taback N, Buys YM. Ultrasound biomicroscopic quantitative analysis of light-dark changes associated with pupillary block. Am J Ophthalmol 1999;127:43–47 [DOI] [PubMed] [Google Scholar]
- 15.Barkana Y, Dorairaj SK, Gerber Y, Liebmann JM, Ritch R. Agreement between gonioscopy and ultrasound biomicroscopy in detecting iridotrabecular apposition. Arch Ophthalmol 2007;125:1331–1335 [DOI] [PubMed] [Google Scholar]
- 16.Congdon NG, Youlin Q, Quigley H, et al. Biometry and primary angle-closure glaucoma among Chinese, white, and black populations. Ophthalmology 1997;104:1489–1495 [DOI] [PubMed] [Google Scholar]
- 17.Kumar RS, Baskaran M, Chew PT, et al. Prevalence of plateau iris in primary angle closure suspects an ultrasound biomicroscopy study. Ophthalmology 2008;115:430–434 [DOI] [PubMed] [Google Scholar]
- 18.Aung T, Chan YH, Chew PT. Degree of angle closure and the intraocular pressure-lowering effect of latanoprost in subjects with chronic angle-closure glaucoma. Ophthalmology 2005;112:267–271 [DOI] [PubMed] [Google Scholar]
- 19.Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol 2003;87:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijaya L, George R, Arvind H, et al. Prevalence of primary angle-closure disease in an urban south Indian population and comparison with a rural population: The Chennai Glaucoma Study. Ophthalmology 2008;115:655–660 e651 [DOI] [PubMed] [Google Scholar]