Anterior ischemic optic neuropathies result in significantly different disc topography compared with open-angle glaucoma.
Abstract
Purpose.
To compare optic disc topography performed by confocal laser ophthalmoscopy in eyes with nonarteritic anterior ischemic optic neuropathy (NAION), arteritic anterior ischemic optic neuropathy (AAION), and open-angle glaucoma (OAG), adjusting for the amount of retinal ganglion cell (RGC) loss, as measured by nerve fiber layer (NFL) thickness and average visual field loss.
Methods.
At four referral centers, patients who met specific diagnostic criteria for OAG (103 persons, 152 eyes), NAION (53 persons, 57 eyes), or AAION (18 persons, 20 eyes) underwent Heidelberg Retinal Tomography (HRT; Heidelberg Engineering, Heidelberg, Germany), Stratus Optical Coherence Tomography (OCT; Carl Zeiss Meditec, Inc., Dublin, CA), and Humphrey visual field testing (HFA; Carl Zeiss Meditec, Inc.). HRT parameters were compared in univariate and multivariate models, accounting for degree of RGC loss by either OCT NFL thickness or visual field mean deviation (MD). Acute AION occurred at least 6 weeks before testing.
Results.
After adjustment for degree of injury according to either MD or mean NFL thickness, all HRT parameters were significantly different between OAG and both NAION and AAION. With similar damage, OAG eyes had larger, deeper cups; smaller rims; more cup volume; and less rim volume (all P ≤ 0.001). There were differences in disc topography between NAION and AAION, but they were not consistent for both measures of damage. Disc area and MD were also significantly associated with many HRT parameters. NFL thickness was greater at the same MD for both AAION and NAION compared with OAG.
Conclusions.
NAION and AAION cause loss of RGCs, but have significantly different disc topography compared with OAG at a given level of RGC loss.
Optic neuropathies produce characteristic changes in the optic disc that reflect the underlying etiology, as well as the pattern and extent of damage to retinal ganglion cell (RGC) axons. The morphology of the disc in open-angle glaucoma (OAG) is characterized by thinning of the neuroretinal rim, excavation of the optic cup, and a higher cup/disc ratio. However, anterior ischemic optic neuropathies (AION) have also been shown to demonstrate some of these features after the acute optic disc swelling subsides.1–5 In no study to date has the morphology of the optic disc been compared among OAG, arteritic anterior ischemic optic neuropathy (AAION), and nonarteritic anterior ischemic optic neuropathy (NAION) by using laser-based imaging. Unlike OAG, AAION and NAION involve acute injuries to the optic nerve, characterized by sudden loss of vision, optic disc swelling with resolution over weeks, visual field defects, and, ultimately, optic disc pallor and altered optic disc morphology. AAION is caused by an inflammatory vascular occlusion of the posterior ciliary arteries secondary to giant cell arteritis, producing pallid disc swelling. There is no distinctive premorbid disc appearance for AAION, but NAION occurs most commonly in eyes with a small optic disc diameter and a small cup and usually produces hyperemic disc swelling.6–8
In several studies, investigators have documented that, after resolution of optic disc swelling in AION, but particularly in AAION, there is thinning of the neuroretinal rim and enlargement of the cup/disc ratio.1–5,9 Researchers have used the masked assessment of color photographs to evaluate the morphologic differences in the optic disc appearance among these conditions. Discs with cups that seemed larger than average were often said to have “cupping,” although the meaning of this term has rarely been expressed quantitatively. In one study,4 OAG discs differed from those in NAION and AAION, although AAION discs occasionally had a sufficiently large cup size to simulate that in early glaucoma. In another study, also a masked assessment of color photographs, the investigators reported that there was a large cup/disc ratio in 92% of AAION eyes versus 4% of NAION eyes.3 In neither of these studies, however, was adjustment made for the degree of visual field loss in assessing the cup/disc ratio. An important finding noted in both studies was that both forms of AION had pallor of the remaining neuroretinal rim,3,4 whereas in OAG, the remaining rim was most often pink. With Heidelberg Retinal Tomography (HRT; Heidelberg Engineering, Heidelberg, Germany), AAION-affected eyes demonstrated significantly larger cup/disc ratios, thinner neuroretinal rims, and deeper cups than in NAION-affected eyes.5 In another study in which HRT was used in a Japanese population, significant differences between NAION and OAG eyes were found in many HRT parameters, after adjustment for the degree of visual field loss or loss of retinal nerve fiber layer (NFL) thickness. The same investigators also found that the NAION eyes had somewhat smaller disc rims than did the fellow eyes.10,11 This group confirmed that, in NAION, eyes have smaller disc areas than control eyes; however, there were no cases of AAION for comparison in their study.
We extended these analyses by using HRT topography to directly compare, for the first time, the effects of AAION, NAION, and OAG. We also included the important step of adjusting for the degree of RGC loss by determining mean deviation (MD) in the visual field or mean NFL thickness by optical coherence tomography (OCT).
Methods
Patient Selection
This study adhered to the Declaration of Helsinki and was approved and monitored by the institutional review boards of the Johns Hopkins University School of Medicine, the University of Auckland, the University of Alabama, and the Wills Eye Institute. Patients met formal criteria for the diagnoses of OAG, AAION from biopsy-proven giant cell arteritis, or NAION. We enrolled 103 subjects (152 eyes) with OAG by sequentially soliciting patients at the Wilmer Glaucoma Service. They were included in a previous report comparing OAG and angle-closure glaucoma.12 Their OAG met the criteria of Foster et al.,13 including open angles by gonioscopy and a specifically defined cup/disc ratio and visual field loss in at least one eye. The field loss was defined as a glaucoma hemifield test result outside normal limits and three points abnormal at the 5% level in one hemifield on the pattern deviation plot of a Humphrey Field Analyzer (HFA2; Carl Zeiss Meditec, Inc.), according to the Swedish Interactive Thresholding Algorithm (SITA) Standard 24-2 program. Because there are no specific diagnostic criteria for pigment dispersion and exfoliation syndromes, we included eyes in which either condition was suspected. These patients otherwise met the same diagnostic criteria as other patients with OAG. Patients with secondary causes of glaucoma, other retinal or optic nerve disease, spherical refraction greater than ±5.0 D, or cylinder correction greater than ±3.0 D were excluded.
Eighteen subjects (20 eyes) with AAION and 53 subjects (57 eyes) with NAION were also enrolled. Because these diseases are not common and because testing had to be performed at least 6 weeks after acute optic disc swelling had first been documented, these subjects were identified and recruited both in the clinic and through administrative and clinical records, with some subjects returning for additional examination and testing. The diagnosis of AION was made by a neuro-ophthalmologist based on clinical examination and history. All subjects with AAION or NAION had optic disc swelling observed by one of the authors at the time of onset of visual loss. All cases of AAION were associated with clinical features of giant cell arteritis, and the diagnosis was confirmed by temporal artery biopsy. Subjects were included if they had complete clinical information, an HRT of adequate quality, and either a 24-2 visual field test or an OCT of the peripapillary NFL. Patients who were unable to perform visual field tests because of severe visual field loss had only results of the OCT of the RNFL included.
Examination and Testing
Demographic information was recorded including age, sex, and ethnic derivation (African, Asian, Caucasian, or other), and history of cataract surgery. Subjects underwent a complete ocular examination including Snellen visual acuity, Goldmann applanation tonometry, gonioscopy (glaucoma group only), slit lamp examination, and dilated fundus examination. The refractive error used was the presenting eyeglass correction. Each enrolled eye also underwent visual field testing with the HFA SITA Standard 24-2 algorithm and OCT examination of the peripapillary NFL (Fast RNFL scan, Stratus OCT; Carl Zeiss Meditec, Inc.). Patients were included if either the visual field test result met the reliability criteria or their OCT scans were of good quality (signal strength >4 on three consecutive scans). Scanning laser ophthalmoscopy of the optic disc was performed (HRT2 or -3 with Eye Explorer software version 1.5.1.0; Heidelberg Engineering). Because keratometry was not routinely obtained in all patients, we used the default values for this parameter in the HRT. To determine whether refraction could affect our conclusions, we compared disc area in phakic eyes with that in pseudophakic eyes in patients with OAG. There was no significant difference between the groups (data not shown), which supports the concept that optical differences did not substantially bias the disc diameter measurement.
A single expert (HAQ) identified the optic disc border on all HRT scans. HRT results were assessed according to three criteria: The disc contour had to be drawn accurately in the HRT software, the HRT software had to be able to analyze the images, and the standard deviation of the three scans making up each study had to be <50 mm2. The OCT, visual field, and eye examinations were completed within a 6-month period in each subject and took place at least 6 weeks after the initial observation of disc swelling in the AION groups.
Estimation of RGC Loss
All patients with OAG satisfied visual field criteria for glaucomatous field loss, but not every eye with OAG or AION had a recent analyzable field test of the correct type and quality for inclusion in these data. Eyes with documentation of disease status, but for which there were no acceptable visual field results at the time of the study, were included only if an OCT NFL thickness was obtained as an alternate measure of optic nerve damage. As will be described later in the article, analysis with OCT NFL thickness used as a measure of damage was performed separately from analysis with visual field MD. This approach maximized our ability to detect differences in HRT parameters. When both eyes of a subject qualified for inclusion, data from both eyes were used with appropriate statistical adjustment.
Statistical Analysis
Demographics of the three groups were compared pair-wise by using the t-test for comparing age and Fisher's exact test for eye laterality (right, left, both), sex, and ethnicity. Because some subjects had both eyes enrolled in the study, the generalized estimating equation (GEE)14 was used when analyzing data derived from eyes (as opposed to people). The GEE method accounts for the within-group correlations introduced when some subjects contribute data from both eyes. Differences between groups in numeric variables were assessed with GEE in a Gaussian model. The numeric parameter was the dependent variable in the model, whereas diagnosis was an independent variable. Other dependent variables included intraocular pressure (IOP) at the time of the examination, spherical equivalent refractive error, logarithm of the minimum angle of resolution (logMAR) visual acuity, and HRT disc area. Differences in history of cataract extraction were similarly assessed, using GEE in a binomial model.
The eyes with a valid visual field result were used to compare the HFA MD and pattern standard deviation (PSD) among the three groups by using GEE in a Gaussian model. Similarly, eyes with an OCT NFL scan were used to compare the average NFL thickness among the groups. Finally, we used the same method to compare the values of the HRT parameters in the three groups.
To compare the differences in HRT parameters among groups, while also controlling for the total amount of damage, we used GEEs to generate multiple regression models in which the HRT parameter was the dependent variable, and diagnosis, HFA MD, HRT disc area, and HRT reference plane height were all independent variables. To determine the effect of age, we generated these models with age as an additional independent variable. Separate models were created with the following dependent variables: cup area, rim area, cup/disc area ratio, cup volume, rim volume, mean cup depth, cup shape measure, and vertical cup/disc ratio. The HFA MD was included to control for the total amount of damage in the eye. We controlled for disc area because it is related to other disc measures.15,16 The reference plane height was included because there may be a greater proportionate loss of macular NFL in AION than in OAG.17 Because reference height is critically dependent on the temporal (macular) zone of NFL, failure to correct for reference height could bias comparisons of HRT data. The same multiple regression analysis of the HRT parameters was repeated using the OCT mean NFL thickness as an alternate measure of total damage (i.e., in place of MD). The MD and NFL thickness measures of damage were thought to be complementary, as one measures functional damage and the other measures structural loss of NFL.
We tested the significance of our regression analyses in which topographical parameters were the dependent variable using both the standard P < 0.05 level of significance and the conservative Bonferroni method. With nine different comparisons of HRT parameters among the disease entities in the univariate regression models, P = 0.05/9 or 0.0055 was significant after the Bonferroni correction. With eight HRT parameters in the multivariate models (not including reference height, which was an independent variable in these models), the Bonferroni-corrected significance level was P = 0.05/8 or 0.0063.
All statistical analyses were performed using R version 2.8.0 (http://www.r-project.org) with the geepack library.18
Results
Comparison of Diagnostic Groups
Subjects with NAION had a significantly younger mean age than in both the OAG and AAION groups (Table 1). More than half the patients with OAG and NAION were men, whereas significantly more patients with AAION were women. Most subjects were European-derived, with the NAION group being significantly more so than the OAG group. The OAG group was significantly more myopic than either AION group, and more were pseudophakic among the patients with OAG than among those with NAION (Table 2). The disc area was significantly smaller in NAION eyes than in either OAG or AAION eyes, and AAION eyes had significantly worse visual acuity than eyes with either NAION or OAG.
Table 1.
Comparison of Subject Characteristics between Groups
| OAG (n = 103) | NAION (n = 53) | AAION (n = 18) | P (OAG vs. NAION) | P (OAG vs. AAION) | P (NAION vs. AAION) | |
|---|---|---|---|---|---|---|
| Age, y (SD) | 69.3 (11.2) | 60.3 (12.0) | 73.0 (7.3) | <0.001 | 0.08 | <0.001 |
| Eye, % with both | 54.4 | 13.2 | 16.7 | <0.001 | 0.002 | 0.93 |
| Sex, % female | 32 | 47 | 67 | 0.08 | 0.008 | 0.18 |
| Ethnicity, % | 0.04 | 0.24 | 0.57 | |||
| African | 13.6 | 5.7 | 0 | |||
| Asian | 3.9 | 0 | 0 | |||
| European | 76.7 | 94.3 | 100 | |||
| Other | 5.8 | 0 | 0 |
Table 2.
Comparison of Eye Characteristics between Groups
| OAG (n = 152) | NAION (n = 57) | AAION (n = 20) | P (OAG vs. NAION) | P (OAG vs. AAION) | P (NAION vs. AAION) | |
|---|---|---|---|---|---|---|
| IOP, mm Hg (SD) | 14.7 (4.5) | 14.8 (3.5) | 14.5 (3.0) | 0.96 | 0.81 | 0.73 |
| n = 56 | ||||||
| Refractive error, D (SD) | −2.0 (3.0) | 0.21 (1.9) | −0.29 (2.0) | <0.001 | 0.002 | 0.50 |
| n = 93 | n = 55 | n = 18 | ||||
| Acuity, logMAR (SD) | 0.27 (0.44) | 0.42 (0.66) | 1.1 (1.0) | 0.13 | <0.001 | 0.006 |
| Pseudophakia, % | 45 | 18 | 35 | 0.001 | 0.40 | 0.06 |
| Disc area, mm2 (SD) | 1.8 (0.41) | 1.7 (0.37) | 1.84 (0.34) | 0.03 | 0.69 | 0.04 |
Univariate Analysis of Imaging Parameters
AAION eyes had significantly more damage, as judged by visual field MD, than did either OAG or NAION eyes, whereas MD in OAG and NAION eyes was similar. By contrast, the mean OCT NFL thickness was similar in OAG and AAION eyes, and eyes with either of these disorders had significantly thinner NFL than did NAION eyes (Table 3).
Table 3.
Visual Field and OCT NFL Thickness Parameters for the Three Groups
| OAG | NAION | AAION | P (OAG vs. NAION) | P (OAG vs. AAION) | P (NAION vs. AAION) | |
|---|---|---|---|---|---|---|
| MD, dB (SD) | −10.0 (7.2) | −10.8 (8.4) | −18.6 (9.6) | 0.23 | <0.001 | 0.003 |
| n = 135 | n = 54 | n = 16 | ||||
| PSD, dB (SD) | 8.7 (3.8) | 8.8 (4.0) | 8.7 (3.9) | 0.90 | 0.87 | 0.92 |
| Average NFL thickness, μm (SD) | 60.3 (14.1) | 71.3 (21.5) | 56.8 (21.1) | 0.008 | 0.38 | 0.022 |
| n = 147 | n = 49 | n = 16 |
Significant P-values are shown in bold.
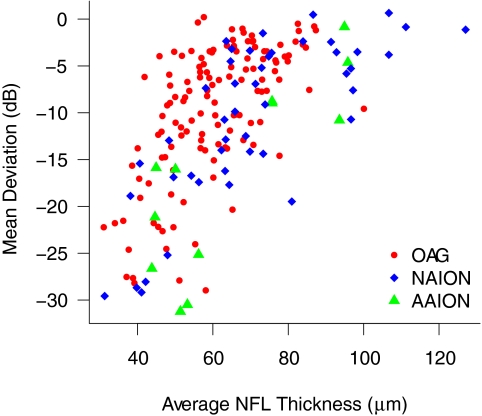
In the comparison between OAG and either NAION or AAION, diagnosis was a significant predictor of OCT NFL thickness when controlling for MD (Table 4). For a given MD, NFL thickness was greater in NAION or AAION than in OAG, but there was no significant difference between the AAION and NAION groups. The relationship between MD and mean OCT NFL thickness in the three groups is shown in Figure 1.
Table 4.
Multiple Regression Coefficients for Diagnosis and Visual Field MD with OCT RNFL as the Dependent Variable
| OAG/NAION | OAG/AAION | NAION/AAION | |
|---|---|---|---|
| Diagnosis | 11.5 (p < 0.001) | 11.4 (P = 0.01) | 5.3 (P = 0.22) |
| MD | 1.4 (P < 0.001) | 1.2 (P < 0.001) | 2.0 (P < 0.001) |
Figure 1.
Visual field MD versus average NFL thickness for the three groups in the study.
In the univariate analysis of HRT parameters (Table 5), there were significant differences between OAG and NAION for all parameters, using the corrected significance level of 0.0055. NAION and AAION differed at the corrected significance level for cup area, cup/disc area ratio, cup volume, rim volume, and vertical cup/disc ratio, and at P < 0.05 for all other structural measures except reference height. OAG and AAION differed at the corrected significance level in rim area, cup volume, mean cup depth, and reference height.
Table 5.
Univariate Comparison of HRT Parameters between Eyes
| OAG (n = 152) | NAION (n = 57) | AAION (n = 20) | P (OAG vs. NAION) | P (OAG vs. AAION) | P (NAION vs. AAION) | |
|---|---|---|---|---|---|---|
| Cup area, mm2 | 0.94 (0.48) | 0.29 (0.29) | 0.66 (0.39) | <0.001 | 0.019 | <0.001 |
| Rim area, mm2 | 0.89 (0.32) | 1.4 (0.33) | 1.2 (0.38) | <0.001 | 0.003 | 0.01 |
| Cup/disc area ratio | 0.49 (0.20) | 0.16 (0.15) | 0.35 (0.22) | <0.001 | 0.023 | <0.001 |
| Cup volume, mm3 | 0.26 (0.24) | 0.05 (0.07) | 0.12 (0.09) | <0.001 | <0.001 | <0.001 |
| Rim volume, mm3 | 0.19 (0.11) | 0.37 (0.15) | 0.25 (0.15) | <0.001 | 0.137 | 0.004 |
| Mean cup depth, mm | 0.28 (0.13) | 0.13 (0.08) | 0.17 (0.07) | <0.001 | <0.001 | 0.009 |
| Cup shape measure, mm | −0.08 (0.08) | −0.17 (0.06) | −0.13 (0.08) | <0.001 | 0.017 | 0.03 |
| Vertical cup/disc ratio | 0.70 (0.23) | 0.21 (0.25) | 0.50 (0.31) | <0.001 | 0.033 | <0.001 |
| Reference height, mm2 | 0.38 (0.15) | 0.24 (0.13) | 0.29 (0.10) | <0.001 | <0.001 | 0.066 |
Data are expressed as the mean (SD). Bold indicates that the difference exceeded the corrected significance level of 0.0055.
Multivariate Analysis of HRT Parameters
In multivariate models of HRT parameters, we accounted for either MD or average OCT NFL thickness (but not both in the same model) as independent variables to adjust for degree of RGC loss. Disc area and reference height were also included in each model, as they are known to be related to other HRT parameters. In the models with MD as the damage criterion, OAG eyes differed significantly from both NAION and AAION eyes in all HRT parameters, having a larger cup area, smaller rim area, larger cup/disc ratio, larger cup volume, smaller rim volume, and greater cup depth (at the corrected significance level). When comparing NAION to AAION and controlling for RGC loss using MD, only cup volume achieved significance at P < 0.0063. At uncorrected P < 0.05, eyes with NAION had a significantly smaller cup area, a larger rim area, and a larger cup/disc ratio (Table 6). In the models with OAG against the two forms of AION, MD was independently associated with cup and rim area, cup/disc area ratio, and rim volume but not with cup volume, mean cup depth, or cup shape. In those same models, disc area was significantly associated with all HRT parameters except rim volume. When age was added to the multivariate model, the difference between AAION and NAION decreased, but remained significant (at the uncorrected significance level) when controlling for OCT RNFL thickness (data not shown).
Table 6.
Multivariate Models of HRT Parameters with Diagnosis as the Independent Variable and Controlling for MD, Disc Area, and Reference Height
| Cup Area | Rim Area | Cup-Disc Area Ratio | Cup Volume | Rim Volume | Mean Cup Depth | Cup Shape | Vertical Cup-Disc Ratio | |
|---|---|---|---|---|---|---|---|---|
| OAG (n = 135) vs. NAION (n = 54) | ||||||||
| Diagnosis | −0.63* | 0.62* | −0.35* | −0.22* | 0.22* | −0.13* | −0.092* | −0.47* |
| MD | −0.010* | 0.01* | −0.005* | −0.003 | 0.004* | 0.001 | −0.002† | −0.004 |
| Disc area | 0.74* | 0.26* | 0.19* | 0.30* | 0.03 | 0.14* | 0.055* | 0.23* |
| Reference height | −0.30 | 0.30 | −0.15 | −0.18† | 0.28* | 0.13† | 0.012 | −0.04 |
| OAG (n = 135) vs. AAION (n = 16) | ||||||||
| Diagnosis | −0.56* | 0.56* | −0.30* | −0.25* | 0.16* | −0.14* | −0.097* | −0.34* |
| MD | −0.013* | 0.013* | −0.007* | −0.004 | 0.004* | 0 | −0.002† | −0.006† |
| Disc area | 0.81* | 0.19* | 0.19* | 0.35* | 0.018 | 0.16* | 0.062* | 0.22* |
| Reference height | −0.39† | 0.39† | 0.24† | −0.18 | 0.33* | 0.12 | 0.007 | −0.21 |
| NAION (n = 54) vs. AAION (n = 16) | ||||||||
| Diagnosis | 0.20† | −0.21† | 0.098 | 0.068* | −0.065 | −0.023 | −0.004 | 0.17† |
| MD | −0.004 | 0.004 | −0.002 | −0.001 | 0.005* | 0.001 | −0.002* | 0.00 |
| Disc area | 0.50* | 0.50* | 0.18* | 0.097* | 0.042 | 0.30* | 0.042† | 0.31* |
| Reference height | 0.026 | −0.028 | 0.008 | −0.36* | 0.21 | 0.11 | −0.005 | −0.22 |
Data are the regression coefficients for each of the model variables.
P ≤ 0.0063.
P ≤ 0.05.
In multivariate models with mean OCT RNFL thickness used as the measure of damage, the findings were similar to those with MD. Again, OAG eyes differed significantly from both NAION and AAION eyes in all HRT parameters (Table 7), with the former showing larger cup area and volume, smaller rim area and volume, greater cup depth, and a more positive cup shape measure. The coefficients in the model comparing NAION and AAION were generally larger in magnitude than those in the HFA MD analysis. The significant differences between NAION and AAION eyes were a smaller cup volume and smaller vertical cup/disc ratio in NAION. OCT RNFL thickness was associated only with cup shape measure.
Table 7.
Multivariate Models of HRT Parameters with Diagnosis Used as the Independent Variable and Controlling for OCT RNFL, Disc Area, and Reference Height
| Cup Area | Rim Area | Cup-Disc Area Ratio | Cup Volume | Rim Volume | Mean Cup Depth | Cup Shape | Vertical Cup-Disc Ratio | |
|---|---|---|---|---|---|---|---|---|
| OAG (n = 147) vs. NAION (n = 49) | ||||||||
| Diagnosis | −0.54* | 0.54* | −0.30* | −0.18* | 0.18* | −0.13* | −0.072* | −0.45* |
| RNFL average | −0.006* | 0.006* | −0.003* | −0.002* | 0.002* | 0.00 | −0.002* | −0.003* |
| Disc area | 0.77* | 0.23* | 0.20* | 0.30* | 0.015 | 0.14* | 0.067* | 0.24* |
| Reference height | −0.21 | 0.21 | −0.11 | −0.13 | 0.26* | 0.14* | 0.019 | −0.035 |
| OAG (n = 147) vs. AAION (n = 16) | ||||||||
| Diagnosis | −0.36* | 0.36* | −0.19* | −0.17* | 0.11* | −0.11* | −0.071* | −0.23* |
| RNFL average | −0.01* | 0.01* | −0.005* | −0.003* | 0.003* | −0.001 | −0.002* | −0.005* |
| Disc area | 0.83* | 0.17* | 0.19* | 0.34* | −0.005 | 0.15* | 0.07* | 0.22* |
| Reference height | −0.30 | 0.30 | −0.22† | −0.13 | 0.30* | 0.12 | 0.008 | −0.24† |
| NAION (n = 49) vs. AAION (n = 16) | ||||||||
| Diagnosis | 0.34† | −0.34 | 0.17† | 0.11* | −0.10 | 0.038 | 0.01 | 0.30* |
| RNFL average | −0.001 | 0.001 | −0.001 | 0.001 | 0.004 | 0.00 | −0.001* | −0.001 |
| Disc area | 0.36 | 0.66 | 0.12† | 0.10* | 0.299 | 0.07† | 0.035 | 0.10 |
| Reference height | −0.15 | 0.16 | −0.03 | −0.53* | 0.462 | 0.14† | 0.013 | 0.010 |
Data are the regression coefficients for each of the model variables.
P ≤ 0.0063.
P ≤ 0.05.
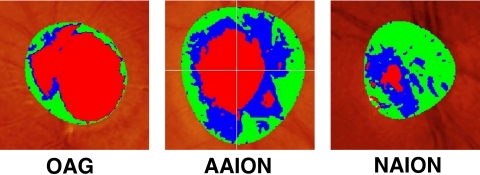
To illustrate the average difference between HRT findings in OAG, NAION, and AAION eyes, we selected from each disorder typical HRT disc image examples that had an average 25-dB loss in MD (Fig. 2). The size of the cup, shown in red, was proportionately much greater in the OAG than the AAION or NAION eyes.
Figure 2.
Representative HRT images from each of the three groups. The images were selected from the patient in each group with MD closest to −25 dB. The cup was clearly much larger in the glaucomatous eye. In this particular example, the disc diameter was largest in the AAION eye, although the mean disc diameters of OAG and AAION eyes were similar and both larger than the mean NAION disc diameter.
Discussion
In this study, eyes with OAG, NAION, and AAION had different topography of the optic disc, as measured by scanning laser ophthalmoscopy. When we used RNFL thickness or MD as a surrogate measure of loss of RGC, discs affected by OAG had significantly less disc rim tissue and a dramatically deeper cup than either NAION or AAION. When comparing the disc topography of OAG and AION, we also controlled for disc area. It is well known that disc area affects cup size and rim area, presumably because smaller discs have, in general, fewer nerve fibers,19 and NAION eyes have smaller disc diameter than the general population. In eyes with NAION, disc area was significantly smaller than that in either AAION or OAG. We found some differences in disc topography between AAION and NAION; however, neither NAION nor AAION eyes exhibited the significant deepening of the cup that was seen in OAG. Our univariate analysis revealed that OAG discs had almost seven times greater cup volume and twice the cup depth as NAION discs and twice the cup volume and one-third greater cup depth than AAION discs. Of note, the cup area and the cup/disc area ratio are not significantly different between OAG and AAION, suggesting that, although there is enlargement of the cup size and thinning of the rim area in both disorders, the posterior excavation is far more dramatic in OAG eyes than in AAION eyes. For a given level of field loss, our multivariate models showed that both forms of AION had cups that were not as deep and had less volume than those in OAG. Our multivariate analysis also showed some differences between AAION and NAION when examined by HRT. In both the models using MD and those using mean NFL thickness, AAION eyes had larger cup volumes than did eyes with NAION.
A possible hypothesis for our findings is that OAG, NAION, and AAION result in damage to the prelaminar and laminar zones of the optic nerve head that differs in severity and in the specific structures most damaged. The prelaminar zone consists largely of RGC axons (with some astrocytes and capillaries), whereas the laminar zone consists of load-bearing laminar connective tissues through which pass the axon bundles.20,21 Our findings suggest that OAG affects the laminar connective tissues much more than either NAION or AAION. All three disorders lead to RGC axon loss and thus are likely to produce some prelaminar tissue loss, but the increased cup depth and volume in OAG—with the same degree of RGC axon loss as in NAION or AAION—suggests that the supporting connective tissues of the lamina are retrodisplaced and/or thinned much more in OAG. At similar injury levels (defined by NFL loss or MD), NAION and AAION eyes have relative preservation of the neuroretinal rim compared with OAG eyes, which may reflect the optic disc pallor and RGC loss seen in these eyes.
The results do not mean that vascular insults do not affect laminar connective tissues to some degree, but they do indicate that the displacement of the lamina and widening of the posterior scleral canal that characterizes glaucoma does not happen to any detectable degree in NAION and only marginally in AAION. Laminar deformation has been shown in experimental monkey glaucoma22 and human23 by histologic study, but no well-documented specimens of human AION have been studied in a manner that shows laminar architecture. It is feasible that some reorganization of laminar connective tissues occurs in the two forms of AION; however, the type and severity of laminar deformation must be substantially smaller than in OAG, based on our HRT data.
In both the models using MD and the models using mean NFL thickness as measures of damage, AAION eyes had significantly larger cup volumes than did eyes with NAION. These differences between AAION and NAION eyes may result from differences in pathologic events or from relative differences in the baseline structure of the optic disc. Because NAION eyes had a somewhat smaller disc diameter than AAION eyes, it is possible that NAION eyes also have a different composition of laminar connective tissue—perhaps showing greater resistance to deformation—than eyes with AAION. Alternatively, AAION is associated with occlusion of the major arterial input to the eye (retina, choroid, and ciliary), whereas the vascular changes that occur in NAION appeared to be limited to the nerve head area. This effect may lead to more profound changes in optic nerve tissues in AAION than in NAION. Of interest, animal models of vascular occlusion of the optic disc have never shown changes in connective tissue within the affected region similar to those in eyes with OAG. The recent development of a nonhuman primate model simulating aspects of NAION24 could be used to investigate this question.
We found that for the same amount of visual field loss as judged by MD, OAG eyes had a greater reduction in mean NFL thickness than AION eyes. Although there have been several studies of the structure–function relationship in OAG,25 few reports have compared this relationship between AION and OAG. Hood et al.26 compared OCT-measured RNFL thickness in upper and lower poles with corresponding visual field locations in 24 AION eyes and 15 OAG eyes. The type of AION was not indicated. These investigators stated that the relationship in these sectors of the RNFL and field between their patients with AION and those with OAG was similar; however, they presented no formal statistical comparisons to support their conclusions. They also identified that OCT RNFL thickness, after extensive RGC damage, retained a value of nearly 50 μm. This apparent floor effect may reflect the contribution of nonaxonal tissue to the measure or an artifact in the way the image is segmented by the analysis software. In our study, eyes with AAION had significantly more visual field damage than did eyes with either OAG or NAION; however, due to the floor effect in RNFL thickness—approximately 40 μm—their structural damage may be underestimated in the multivariate analysis. Nevertheless, our comparisons show statistically significant differences in the MD-RNFL relationship between OAG and both NAION and AAION, using overall field and NFL data instead of the sectoral data used by Hood et al. We therefore conclude that when a similar estimated loss of RGCs has occurred, OAG has a significantly greater effect on RNFL thickness, in addition to greater loss of disc rim tissue and a greater deepening of the cup, than does either AAION or NAION.
Although not all AAION eyes could perform visual field tests, our data may point to a fundamental difference in how RGCs are affected by NAION and AAION, compared with OAG. First, this difference may derive from a differential susceptibility of RGC types to injury in the two forms of AION, or it may be a result of methodological differences in laser imaging. Clinically, visual acuity, hence, macular RGC function, is more often affected in AION than in OAG for the same degree of overall field loss. The macular RGCs have thinner axons and contribute less to overall NFL thickness as measured by OCT, perhaps explaining our finding. A second methodologic explanation of our findings of greater mean NFL thickness in eyes with NAION or AAION compared with OAG is the possibility that the NFL in eyes with NAION and AAION remained swollen from the acute event longer than the 6-week minimum that we allowed. Most of our imaging studies were performed longer than 6 weeks after presentation, with 52 (91%) of 57 NAION and 16 (80%) of 20 AAION eyes imaged with HRT and/or OCT 3 months or more after the event. Although one recent report suggests that swelling after NAION should have resolved by this time,27 another publication indicated that RNFL thinning may not fully plateau until 6 months.28 Hence, there may have been mild further thinning of RNFL thickness in NAION and AAION eyes that has not been considered in our analysis. It is possible that further studies of NFL and field correlations in NAION, such as that performed by Alasil et al.28 will provide a more complete explanation of this relationship. Finally, the different pathophysiology of these three optic neuropathies may have differential effects on retinal glial cells and blood vessels and structures that make variable contributions to the RNFL profile.
To further evaluate the role of the interval from the ischemic event in either type of AION to the date of testing, we reran our analyses including only those subjects with an interval of at least 3 months (data not shown). All statistically significant differences between the three groups of subjects remained but the smaller disc area in the NAION eyes was nonsignificant in the reanalysis. In the univariate analysis of HRT parameters, OAG remained different from NAION in all cases. There was also no change in the significant differences between OAG and AAION and between NAION and AAION. Furthermore, the differences between OAG and NAION in the multivariate analysis were all unchanged, and the differences found between OAG and AAION changed only slightly: The difference in rim volume became nonsignificant at the corrected probability when we used MD and controlled for damage and the same was true for vertical cup/disc ratio when we used NFL thickness controlled for damage. As expected, the probabilities for differences between NAION and AAION decreased in this analysis, with none reaching the corrected significance level. Based on this analysis, we remain confident in our primary conclusion regarding differences in disc topography between OAG and either form of AION at a given level of ganglion cell loss.
This study has several limitations. First, we assumed that both RNFL thickness and visual field tests in AION and OAG are adequate methods for determining the number of RGCs, even though the disorders may affect the structure of the optic disc, visual function, or both in quite different ways. The single episode of NAION or AAION typically results in sudden loss of central visual acuity and color vision, whereas OAG spares visual acuity until very late in the process. It is possible that a different population of RGCs is damaged in OAG than in eyes with AION. Various populations of RGCs have different axon diameters and different topographic distributions near the optic disc. These considerations could influence the RNFL thickness measurement. If indeed a larger number of smaller diameter macular fibers are affected in AION than in OAG, then RNFL thickness could underestimate RGC loss in NAION and AAION compared with OAG. This underestimation could have contributed to the difference in the MD-RNFL relationship between AION and OAG observed in our study. Furthermore, Humphrey visual field testing emphasizes the superior and inferior arcuate zones and minimizes testing of the papillomacular bundle, which is more damaged in AION than in OAG. These factors may result in an underestimation of the amount of RGC damage (as reflected in the MD) in AION. In addition, RNFL thickness represents the sum of two components: (1) the thickness derived from an age-dependent population of RNFL axons and (2) the thickness of nonneural, glial tissue, which partly compensates for the age-related decrease in axons in the NFL. In OAG, an increase in nonneural, glial tissue has been suggested in histologic studies of postmortem human eyes. Hence, it is likely that in advanced disease, OAG and AION differ in the relative amount of remaining glial tissue.29 Another limitation is that many of our patients with OAG had undergone visual field testing before entering the study, whereas subjects with AION were unlikely to have undergone any field testing before their acute loss of vision. In addition, all patients with OAG were from one center, whereas subjects with AAION and NAION were recruited from four centers. Finally, we excluded patients for whom we were not able to obtain reliable imaging or visual field testing. This predominantly affected the patients with AAION, who often had profound visual field loss to levels of hand movements or worse. Hence, our AAION population may not be representative of the degree of damage that normally occurs in these patients. On the other hand, the measures of damage (OCT RNFL thickness, field MD) showed significantly worse effects in the AAION group, suggesting that we did capture some of the differences in disease severity.
In summary, we found that the topography of the optic disc differed significantly between eyes with OAG and eyes with either NAION or AAION, demonstrating greater excavation, greater thinning of the neuroretinal rim, and more RNFL loss after adjusting for the estimated degree of RGC loss. The consistent difference between AAION and NAION when controlling for total damage was in cup volume.
Footnotes
Supported in part by Grants EY01765 (Wilmer Eye Institute) and EY015025 (MVB) from the National Institutes of Health, Saranne and Livingston Kosberg (HAQ), Eyesight Foundation of Alabama (CAG), and the Donegan Fund for Ischemic Optic Neuropathy Research (NRM).
Disclosure: H.V. Danesh-Meyer, Alcon New Zealand (F), Allergan New Zealand (F); M.V. Boland, Microsoft (F); P.J. Savino, None; N.R. Miller, None; P.S. Subramanian, Pfizer (F); C.A. Girkin, Heidelberg Engineering (F), Carl Zeiss Meditec, Inc. (F), Alcon (C), Allergan (C), Pfizer (C); H.A. Quigley, Alcon (C, F), Allergan (C, F), Pfizer (C, F), Carl Zeiss Meditec, Inc. (F)
References
- 1.Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology 2001;108:1586–1594 [DOI] [PubMed] [Google Scholar]
- 2.Sebag J, Thomas JV, Epstein DL, Grant WM. Optic disc cupping in arteritic anterior ischemic optic neuropathy resembles glaucomatous cupping. Ophthalmology 1986;93:357–361 [DOI] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV, Savino PJ, Sergott RC. The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2001;108:593–598 [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Anderson DR. Cupping of the optic disc in ischemic optic neuropathy. Trans Am Acad Ophthalmol Otolaryngol 1977;83:755–762 [PubMed] [Google Scholar]
- 5.Danesh-Meyer H, Savino PJ, Spaeth GL, Gamble GD. Comparison of arteritic and nonarteritic anterior ischemic optic neuropathies with the Heidelberg Retina Tomograph. Ophthalmology 2005;112:1104–1112 [DOI] [PubMed] [Google Scholar]
- 6.Beck RW, Savino PJ, Repka MX, Schatz NJ, Sergott RC. Optic disc structure in anterior ischemic optic neuropathy. Ophthalmology 1984;91:1334–1337 [DOI] [PubMed] [Google Scholar]
- 7.Mansour AM, Shoch D, Logani S. Optic disk size in ischemic optic neuropathy. Am J Ophthalmol 1988;106:587–589 [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Xu L. Optic disc morphology in eyes after nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 1993;34:2260–2265 [PubMed] [Google Scholar]
- 9.Miller S. The enigma of glaucoma simplex. Trans Ophthalmol Soc U K 1972;92:561–584 [PubMed] [Google Scholar]
- 10.Saito H, Tomidokoro A, Sugimoto E, et al. Optic disc topography and peripapillary retinal nerve fiber layer thickness in nonarteritic ischemic optic neuropathy and open-angle glaucoma. Ophthalmology 2006;113:1340–1344 [DOI] [PubMed] [Google Scholar]
- 11.Saito H, Tomidokoro A, Tomita G, Araie M, Wakakura M. Optic disc and peripapillary morphology in unilateral nonarteritic anterior ischemic optic neuropathy and age- and refraction-matched normals. Ophthalmology 2008;115:1585–1590 [DOI] [PubMed] [Google Scholar]
- 12.Boland MV, Zhang L, Broman AT, Jampel HD, Quigley HA. Comparison of optic nerve head topography and visual field in eyes with open angle and angle closure glaucoma. Ophthalmology 2008;115:239–245 [DOI] [PubMed] [Google Scholar]
- 13.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002;86:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060 [PubMed] [Google Scholar]
- 15.Quigley HA, Coleman AL, Dorman-Pease ME. Larger optic nerve heads have more nerve fibers in normal monkey eyes. Arch Ophthalmol 1991;109:1441–1443 [DOI] [PubMed] [Google Scholar]
- 16.Budenz DL, Anderson DR, Patella VM, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007;114:1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quigley HA, Miller NR, Green WR. The pattern of optic nerve fiber loss in anterior ischemic optic neuropathy. Am J Ophthalmol 1985;100:769–776 [DOI] [PubMed] [Google Scholar]
- 18.Halekoh U, Højsgaard S, Yan J. The R Package geepack for generalized estimating equations. J Stat Software 2006;15:1–11 [Google Scholar]
- 19.Caprioli J, Miller JM. Optic disc rim area is related to disc size in normal subjects. Arch Ophthalmol 1987;105:1683–1685 [DOI] [PubMed] [Google Scholar]
- 20.Anderson DR. Ultrastructure of human and monkey lamina cribrosa and optic disc. Arch Ophthalmol 1969;82:800–814 [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci 2007;48:4597–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005;24:39–73 [DOI] [PubMed] [Google Scholar]
- 23.Quigley HA, Hohman RM, Addicks EM, Massof RS, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 1983;95:673–691 [DOI] [PubMed] [Google Scholar]
- 24.Chen CS, Johnson MA, Flower RA, Slater BJ, Miller NR, Bernstein SL. A primate model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2008;49:2985–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strouthidis NG, Vinciotti V, Tucker AJ, Gardiner SK, Crabb DP, Garway-Heath DF. Structure and function in glaucoma: the relationship between a functional visual field map and an anatomic retinal map. Invest Ophthalmol Vis Sci 2006;47:5356–5362 [DOI] [PubMed] [Google Scholar]
- 26.Hood DC, Anderson S, Rouleau J, et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy: a test of a linear model. Ophthalmology 2008;115:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol 2008;246:641–647 [DOI] [PubMed] [Google Scholar]
- 28.Alasil T, Tan O, Lu AT, Huang D, Sadun AA. Correlation of Fourier domain optical coherence tomography retinal nerve fiber layer maps with visual fields in nonarteritic ischemic optic neuropathy. Ophthalmic Surg Lasers Imaging 2008;39:S71–S79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harwerth RS. Charles F. Prentice Award Lecture 2006: a neuron doctrine for glaucoma. Optom Vis Sci 2008;85:E436–E444 [DOI] [PubMed] [Google Scholar]