The efficacy of a fully human monoclonal antibody to Pseudomonas aeruginosa alginate in preventing infectious and pathologic consequences to the cornea was found to require both PMN and complement for full activity. Neutropenia or an inability to recruit PMN to the eye reduced corneal pathology, but bacterial levels in the tissue were greatly increased with infection spread to the brain and spleen.
Abstract
Purpose.
Ulcerative keratitis due to Pseudomonas aeruginosa is a sight-threatening disease leading to loss of vision due to corneal inflammation. A human IgG1 monoclonal antibody (MAb F429) to the alginate capsule significantly reduces pathology and bacterial burdens in the cornea when applied topically starting 8 hours post-infection. The purpose of this study was to determine whether local polymorphonuclear neutrophils (PMN) recruitment and complement were important lipopolysaccharide co-factors in MAb F429-mediated reductions in P. aeruginosa tissue levels and corneal pathology.
Methods.
MyD88 knock-out mice unable to recruit PMN to tissues, mice depleted of PMNs, or mice depleted of complement component C3 were topically treated with MAb F429 starting 8 hours post-infection and evaluated for bacterial levels and corneal pathology 48 hours after infection with two P. aeruginosa isolates.
Results.
An inability to recruit PMN or systemic PMN depletion plus topical application of MAb F429 resulted in less pathology in the eye, but bacterial burdens were markedly increased in the cornea, brains, and spleens of these mice, indicative of systemic spread. Intraperitoneal injection of cobra venom factor (CVF) reduced C3 levels in the cornea ∼40%, which did not change the beneficial effects of MAb F429. Both systemic injection and topical application of CVF reduced local C3 levels >60%, which eliminated MAb-mediated reductions in corneal pathology and bacterial levels.
Conclusions.
PMN recruitment and complement are both needed for maximal in vivo efficacy of MAb F429 in therapeutically treating P. aeruginosa keratitis, and attempts to reduce pathology by limiting PMN influx could have consequences leading to more extensive local and systemic infection.
Corneal infection due to Pseudomonas aeruginosa can result in devastating tissue destruction within a short period after the initiation of infection, leading to corneal scarification, opacification, and loss of vision.1–4 Numerous patients are at risk for such infections, notably those using extended-wear contact lenses,2,3,5,6 those undergoing various eye surgeries or treatments such as orthokeratology or keratoplasty,7–9 and individuals suffering eye trauma or ocular surface diseases.10 Therapy for such infections must be rapidly instituted after the onset of symptoms to minimize corneal damage, but currently recommended treatments are arduous, consisting of topical antibiotic applications every 5 minutes for 1 hour followed by additional drops every 15 minutes for 24 to 48 hours. More severe infections may require antibiotic injection into the eye itself.
We have recently shown that a fully human monoclonal antibody (MAb F429) specific to the P. aeruginosa alginate surface polysaccharide was highly effective at reducing the infectious burden and corneal pathology in mice treated either prophylactically or therapeutically with the MAb.11 In vitro studies showed that MAb required both phagocytic polymorphonuclear neutrophils (PMN) and complement to mediate bacterial killing, but whether there was a similar requirement for these co-factors in vivo was not determined. Prior work has indicated that human MAbs to P. aeruginosa lipopolysaccharide had different requirements for complement in mediating opsonic killing and protecting against systemic infection, with IgM having an absolute requirement for complement while IgG and IgA did not.12 These studies did not address the role of PMN or other cellular factors. As the PMN-dominated inflammation in the cornea drives the pathology leading to loss of vision from microbial keratitis,13–17 we were interested in determining the contributions of locally-recruited PMN and complement to MAb F429-mediated reductions in bacterial levels and pathology during P. aeruginosa corneal infections. In particular, there was concern that inflammation-associated pathology might be exacerbated by MAb activation of complement and generation of complement split products chemotactic for PMN. Alternately, these may be critical co-factors for the beneficial effects of MAb F429 therapy.
Thus, establishing the proper balance between antibody, PMN, and complement-mediated bacterial clearance while limiting inflammatory pathology is essential for optimizing outcomes in treatment of P. aeruginosa keratitis. In this study, we evaluated the activity of MAb F429 in reducing bacterial burdens and corneal pathology in MyD88 knock-out (KO) mice unable to recruit PMNs to tissue, systemically neutropenic mice, or complement depleted mice, after infection with two different P. aeruginosa strains, one representative of the ExoS+ invasive strains and the other of the cytotoxic ExoU+ strains.
Materials and Methods
Bacterial Strains
For infections we used two clinical isolates of P. aeruginosa obtained from infected corneal ulcers: 6294 (serogroup O6) and 6077 (serogroup O11),18 the former an ExoS+, ExoU− invasive strain and the latter an ExoS−, ExoU+ cytotoxic strain.19 Bacteria were grown overnight at 37°C on trypticase soy agar, resuspended in 1% proteose peptone, and the optical density adjusted to achieve 2 to 9 × 108 cfu/mL for infection of eyes.
Human MAb Production
The fully human IgG1 variant of MAb F429 has been previously described.20 MAb F429 was purified from hybridoma cell supernates over a protein-G column and eluted into buffer to neutralize the acidic eluant to pH 6.5, dialyzed against pH 6.5 PBS, and stored as aliquots at −20°C. The material used here was the same preparation as was used in our prior report on the beneficial effects of MAb F429 administered prophylactically or therapeutically to mice with P. aeruginosa keratitis.11
Mice
C3H/HeN, C57Bl/6, and Balb/C wild-type (WT) mice were obtained from Harlan Laboratories (Somerville, NJ) or Jackson Laboratory (Bar Harbor, ME). MyD88KO mice were initially supplied by Douglas Golenbock from the University of Massachusetts Medical School and subsequently bred in our facility. Mice were maintained in microisolator cages in a barrier facility and fed food and water ad libitum.
Murine Model of P. aeruginosa Keratitis
All animal experiments were performed under an approved protocol from the Harvard Medical Area Institutional Animal Care and Use Committee and adhered to the ARVO statement for the use of animals in ophthalmic and vision research.
The use of anesthetized mice to induce corneal infections after three 1 mM scratches on the corneal epithelium with a small gauge needle has been described.18 Infectious inocula were 3 × 105 to 4.5 × 106 cfu/mouse eye delivered in a 5 μL volume of 1% proteose peptone. As with our prior experience in this model, corneal pathology was maximal after 48 hours of infection and was used as the time point to compare the pathologic changes to the cornea. These were scored by an observer (TSZ) with 16 years of experience using this evaluation system who was unaware of the treatments given to the mice. The scoring system used for quantifying eye pathology was: 0, eye macroscopically identical with the uninfected contralateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; 4, perforation of the cornea, phthisis bulbi (shrinkage of the globe after inflammatory disease), or both.
To determine the infectious level of P. aeruginosa, animals were killed by carbon dioxide overdose, eyes removed, the corneas dissected and used to measure external and internalized bacterial levels as described.11 Briefly, eyes were removed from the mice and corneas carefully dissected. The corneas were briefly rinsed in DMEM to remove non-adherent, external bacteria then placed in 1 mL DMEM and vortexed vigorously for 1 minute to remove the adherent, external bacteria. This sample was diluted and plated for bacterial enumeration. The corneas were then placed in 500 μg gentamicin/mL (old method 300 μg gentamicin/mL) to kill remaining external bacteria, washed three times in DMEM to remove the antibiotic, then homogenized, and the sample with the released, intracellular bacteria diluted and plated for bacterial enumeration. Controls showing this amount of antibiotic was sufficient to kill all bacteria present were included with each assay. We also determined the bacterial levels in the brains and spleens dissected from the mice made neutropenic, MyD88 KO mice, or mice treated with local and systemic cobra venom factor (CVF) to evaluate the spread of P. aeruginosa to these organs. Results shown had smaller-scale (fewer mice) replicate experiments conducted.
Administration of Human MAbs
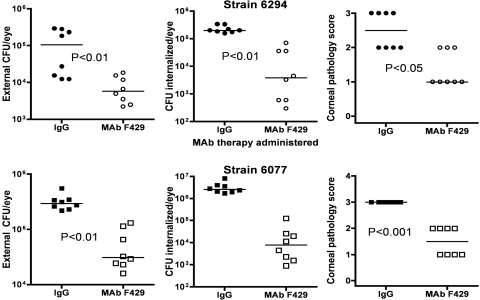
Five μg of MAb F429 in a 5 μL volume, or 5 μg of a control human IgG1 MAb, was applied topically to the eyes of mice lightly anesthetized by isofluorane inhalation 8, 24, and 32 hours post-infection. Although prior work used a 10 μg dose of MAb,11 we found a 5 μg dose to be equally effective at ameliorating the effects of P. aeruginosa keratitis (see Fig. 1).
Figure 1.
Efficacy of MAb F429 specific for the P. aeruginosa alginate surface polysaccharide in reducing infectious levels and corneal pathology due to keratitis induced by P. aeruginosa strains 6294 (upper three panels) or 6077 (lower three panels) in C3H/HeN mice. Points represent individual mice (eight per group), and bars represent the group medians. P values determined by Mann-Whitney U test. X-axis legends indicate if control IgG1 MAb or MAb F429 (5 μg/application) was applied topically starting 8 hours post-infection. These groups were simultaneously tested with the groups shown in Figures 2 and 8 and represent the controls for the activity of MAb F429 for these other studies. Infectious dose: 106 cfu/eye for each P. aeruginosa strain.
Induction of Neutropenia
Twenty-four hours before infection, mice were injected intraperitoneally (IP) with 200 μg of a purified rat anti-mouse Ly6 MAb designated RB6-8C5. Production and purification of this MAb was as described.21 Absolute PMN counts <100/mm3 were obtained and maintained for up to 5 days.21 Counts of mononuclear cells and lymphocytes dropped by approximately 30%.
Depletion of C3 by Cobra Venom Factor
CVF was obtained from CalBiochem (San Diego, CA) and 25 μg injected IP into mice 24 hours before P. aeruginosa infection. In some experiments, 0.5 μg in 5 μL of CVF was also applied topically to the eye at 0, 8, 24, and 32 hours post-infection (along with MAb F429 at 8, 24, and 32 hours post-infection) to ascertain the effect of depletion of C3 in the local environment of the eye. Controls received 5 μL of PBS. CVF applied topically by itself showed no obvious effect on the appearance or clarity of the cornea. Measurement of the level of C3 in the serum and in the corneas after CVF treatment and in comparison to controls was done as follows: For measuring C3 in corneas, tissues were obtained from CVF-treated or untreated control mice 48 hours after bacterial application to the scratch-injured eye. Corneas were excised from enucleated eyes, washed in PBS, and homogenized in PBS. The corneal homogenates were centrifuged and the supernatant used to coat an ELISA plate (Immulon IV; Thermo Fisher Scientific, Waltham, MA), starting with a 1:10 dilution in 0.05 M carbonate buffer (pH 9.6). To measure C3 levels in the sera of control and CVF-treated mice, dilutions of sera in the same carbonate buffer were used to coat an ELISA plate (Immulon IV). The sensitization step proceeded overnight at 4°C, after which the plate was washed in PBS with 0.02% Tween-20, then wells blocked by addition of 200 μL of 1% bovine serum albumin (BSA) in PBS plus 0.002% sodium azide for 2 hours at room temperature. After washing, a goat antibody to mouse C3 (GeneTex, Inc., San Antonio, TX) diluted 1:500 was added to the wells of the plates and incubated for 1 hour at room temperature; a rabbit antibody to goat IgG conjugated to alkaline phosphatase (1:1000 dilution in 1% BSA–0.02% tween-20) was then added and left for 1 hour at room temperature. After washing, the alkaline phosphatase substrate, para-nitrophenylphospate in 0.05 M carbonate buffer (pH 9.6) with 20 mg/L MgCl2, was added, and incubation proceeded for 20 minutes at room temperature. Plates were read at 405 nm and the percentage reduction in C3 determined by comparing serum or corneal samples from CVF-injected mice to the mean values from serum or corneal samples from mice not given CVF.
Histopathology
After euthanatization, eyes were enucleated and fixed in 4% paraformaldehyde. Tissues were embedded in paraffin and anterior sections of the corneal epithelium prepared and stained by hematoxylin and eosin at the Harvard Medical Area rodent pathology core facility. Sections were analyzed by a veterinary pathologist and micrographs prepared.
Statistical Analysis
A Mann-Whitney U test was used to evaluate differences in corneal pathology scores between two groups. ANOVA was used to determine differences in CFU counts and C3 levels from the eyes of animals, with the Dunn's multiple comparisons test or Bonferroni's multiple comparison test used to determine the significance of differences between different groups in a single experiment. P values determined to be exact are reported using an “=” (equal) sign; P values determined by a Gaussian approximation are indicated by a “<” (less than) sign. P values provided in the results are those determined by the multiple comparison test after an initial analysis indicated that the overall ANOVA for all the data sets had a P value of <0.001.
Results
Efficacy of MAb F429 in Microbial Keratitis
Prior results11 showed that 10 μg of MAb F429 applied topically to the eyes 8, 24, and 32 hours after infection with P. aeruginosa was efficacious in reducing levels of bacterial infection and corneal pathology. We replicated these findings using 5 μg of MAb F429 applied topically (Fig. 1) as controls for experiments involving neutropenic mice (Fig. 2) and complement-depleted mice (see Fig. 6) conducted in the same assay.
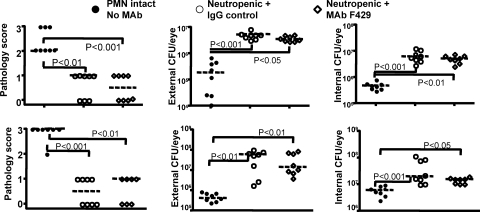
Figure 2.
PMN depletion abrogates the efficacy of human IgG1 MAb F429 in experimental keratitis. Upper panels: strain 6294; lower panels: strain 6077. Legend indicates if the C3H/HeN mice had intact PMN function and were not treated with any MAb (PMN intact, No MAb) or were made neutropenic and further treated by the topical treatment applied to the eye starting 8 hours post-infection of either 5 μg of irrelevant human IgG1 MAb (IgG control) or 5 μg of MAb F429. Points indicate value for an individual mouse (seven to eight mice per group), dashed lines indicate group medians, and P values between the indicated pairs of data determined from multiple comparison tests. The overall ANOVA P value was <0.001 for each data set. Infectious dose: 106 cfu/eye for each P. aeruginosa strain.
Figure 6.
A single systemic 25 μg dose of CVF fails to impact the course of experimental P. aeruginosa keratitis. Upper panels: strain 6294; lower panels: strain 6077. Legend indicates intact C3H/HeN mice not receiving CVF but either topical IgG or MAb F429 (MAb) or receiving CVF IP once and IgG or MAb. Points indicate value for an individual mouse (eight per group), dashed lines indicate group medians, and P values between paired comparisons determined from multiple comparison tests wherein the overall ANOVA P value for the entire group was <0.001. Infectious dose: 106 cfu/eye for each P. aeruginosa strain.
Neutropenia, Corneal Pathology, and Bacterial Levels in MAb F429-Treated Mice
Mice made neutropenic by injection of MAb RB6-8C5, infected with either P. aeruginosa strain 6294 or strain 6077, and then topically treated with either MAb F429 or a control human IgG1 were found to have less severe corneal pathology 48 hours after infection compared with PMN-sufficient control mice also infected with P. aeruginosa (Fig. 2, left-hand panels). There were no significant differences in the corneal pathology scores of the neutropenic mice given control IgG or MAb F429 after challenge with either P. aeruginosa strain. There were significantly higher bacterial counts in the eyes of the neutropenic mice compared with the non-neutropenic controls infected with either strain of P. aeruginosa (Fig. 2, middle and right panels). Thus MAb F429 had no ability to reduce bacterial levels in this setting as it does in mice with intact PMN levels (Fig. 1).
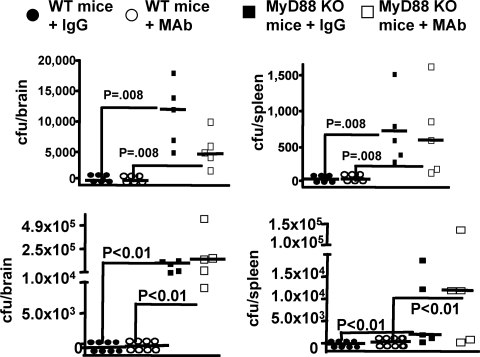
Neutropenia and Local and Systemic Spread of P. aeruginosa from Infected Eyes
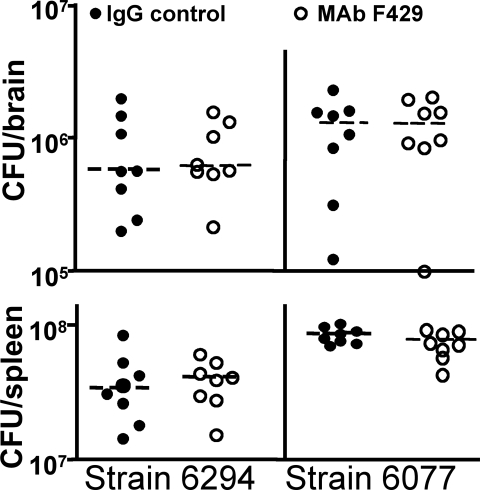
Because of the higher CFU counts in the eyes, we were concerned that neutropenia might allow for local or systemic spread of the P. aeruginosa in the infected mice. We therefore removed the brains and spleens of the neutropenic mice at necropsy and counted the CFU of P. aeruginosa in these tissues. There were very high counts of both P. aeruginosa strains in the brains and spleens (Fig. 3) of the neutropenic mice, and no significant differences (P > 0.05, Dunn's multiple comparisons test) between mice given control IgG or MAb F429, indicating no beneficial effects on systemic bacterial spread from the alginate-specific MAb in the absence of PMN. At the time of the conclusion of the experiment, the mice did not look systemically ill. The brains and spleens of the 16 non-neutropenic control, infected mice had no detectable bacteria except for the brain of one control mouse from which we recovered 34 cfu P. aeruginosa strain 6294. Neutropenia had a positive effect on reducing the inflammatory damage in the cornea from P. aeruginosa infection, but loss of PMNs led to the higher bacterial counts in the corneas, brains, and spleens.
Figure 3.
P. aeruginosa dissemination to the brains (upper panels) and spleens (lower panels) of neutropenic C3H/HeN mice 48 hours after induction of keratitis. Legend indicates whether or not mice were topically treated starting 8 hours post-infection with either irrelevant human IgG1 control or MAb F429. In mice with normal PMN levels, there were no bacteria in these tissues except for one mouse with 34 cfu of P. aeruginosa 6294 in the brain. Points indicate value for an individual mouse (eight per group), dashed lines indicate group medians. Infectious dose: 106 cfu/eye for each P. aeruginosa strain.
MyD88KO Mice and P. aeruginosa Keratitis
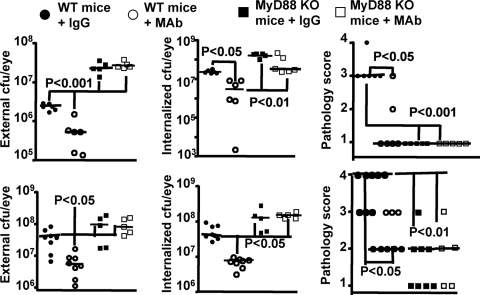
MyD88 is a key signal transduction molecule mediating cellular activation after engagement of TLRs and receptors for IL-1 and TNF,22 and these mice cannot recruit PMN to sites of tissue infection with P. aeruginosa.23 We investigated the effects of MAb F429 in these mice and compared the outcomes with matched WT C57Bl/6 mice, which have a greater susceptibility to P. aeruginosa keratitis.15 MAb F429 reduced external and internalized levels of both P. aeruginosa strains 6294 and 6077 in the WT C57Bl/6 mice (Fig. 4), which did have higher levels of bacteria in the eye compared with the C3H/HeN mice (Fig. 1). The MyD88KO mice behaved just as the neutropenic mice, in that MAb F429 provided no difference in CFU in the eye comparing MyD88KO mice given control IgG with those given MAb F429 (Fig. 4). We could not detect PMN in the corneas of infected MyD88KO mice (data not shown). All the MyD88KO mice had higher levels of P. aeruginosa in the eye compared with WT C57Bl/6 mice (Fig. 4), including WT mice that received only control IgG. Again, as with the neutropenic mice, the MyD88KO mice showed evidence of P. aeruginosa in the brains and spleens (Fig. 5), although these levels were somewhat lower than in the neutropenic mice as there are functional PMN in the blood of MyD88KO mice that likely can control systemic bacterial spread to some degree.
Figure 4.
Inability of MAb F429 to affect the outcome of microbial keratitis in MyD88 knock-out (KO) mice compared to WT C57Bl/6 mice. Upper panels: strain 6294; lower panels: strain 6077. External and internalized levels of indicated P. aeruginosa strain and corneal pathology scores are shown. Points indicate value for an individual mouse (five to eight mice per group), solid lines indicate group medians, and P values between the indicated pairs of data determined from multiple comparison tests. The overall ANOVA P value was <0.002 for all the data sets. Infectious doses: strain 6294: 106 cfu/eye; strain 6077: 1.3 × 106 cfu/eye.
Figure 5.
Dissemination of P. aeruginosa to the brains and spleens of WT C57Bl/6 or MyD88 knock-out (KO) mice with experimental keratitis. Upper panels: strain 6294; lower panels: strain 6077. Legend indicates if the mice were WT or MyD88 KO and further treated by the topical application to the eye starting 8 hours post-infection of either 5 μg of irrelevant human IgG1 (IgG) or 5 μg of MAb F429 (MAb). Points indicate value for an individual mouse (five to eight mice per group), solid lines indicate group medians, and P values between the indicated pairs of data determined from multiple comparison tests. The overall ANOVA P value was <0.002 for all the data sets. Infectious doses: strain 6294: 106 cfu/eye; strain 6077: 1.3 × 106 cfu/eye.
Antibacterial Activity of MAb F429 Plus Complement
We next examined whether intact complement activity was needed for the effects of MAb F429 on reducing bacterial levels and corneal pathology during P. aeruginosa keratitis. Results from control mice not treated with CVF depicted in Figure 1 are replicated in Figure 6 for comparisons with mice treated with CVF. In mice injected once with CVF, there was a significant reduction in bacterial levels and pathology scores for animals topically treated with MAb F429 compared with mice treated with the control IgG1 (Fig. 6). The differences were significant when comparing MAb F429-treated, CVF-injected mice with control IgG1-treated mice with either intact C3 levels or injected with CVF. There were no significant differences in any of the outcomes comparing MAb F429-treated mice with intact C3 or injected once with CVF. Thus, a single injection of CVF did not affect the ability of MAb F429 to reduce bacterial levels and pathology scores from P. aeruginosa keratitis. CVF-treated mice had normal counts of PMNs in their blood.
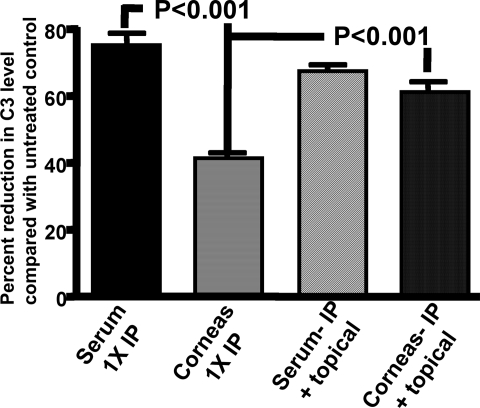
As it was unclear if the lack of an effect of a single CVF injection on the activity of MAb F429 was due to complement-independent in vivo activity or insufficient reductions in local levels of C3, we analyzed the residual C3 levels in serum and corneal tissues from mice injected once with 25 μg of CVF. We found the levels of C3 in mouse blood 48 hours after bacterial inoculation were depleted by ∼75% compared with controls not given CVF, whereas the corneal levels of C3 at the same time point were only depleted 40% (Fig. 7, first two columns on left of graph). As it is known that C3 can be synthesized locally in the eye tissues,24 it appears that the systemic injection of CVF may not be as efficacious in lowering local C3 as it is on lowering blood levels of C3, or, alternately, local synthesis can restore C3 levels more rapidly than can be restored into the blood after a single injection of CVF injection.
Figure 7.
Both systemic and topical CVF treatment is needed for maximal reduction of complement component C3 in corneas of C3H/HeN mice. Murine C3 levels were measured in the serum and the corneas of mice given 25 μg CVF once IP (1 x IP) or both 25 μg once IP and three topical applications of 5 μg at 8, 24, and 32 hours post-infection (IP + topical) and compared with comparably-treated controls given PBS in place of CVF. Bars represent means, and error bars the standard deviations. P values determined by multiple comparisons tests between the indicated pairs of data and the overall ANOVA significance was <0.001. There were no significant differences (P > 0.05) between: the levels of C3 in the sera from mice administered CVF either once IP or IP plus topically applied; between the two serum samples and corneas of mice given CVF both systemically and topically and; between the serum and corneas of mice given CVF both IP and applied topically.
To determine whether we could deplete C3 levels in the corneal tissue to a larger degree, we gave an initial systemic dose of 25 μg of CVF to mice 24 hours before induction of keratitis, and then delivered a dose of 0.5 μg of CVF in 5 μL onto the scratch-injured eye at 0, 8, 24, and 32 hours post-infection. The latter three doses were applied along with 5 μg in 5 μL of MAb F429 or control IgG1. Serum C3 levels at the time of kill were reduced 67% compared with non-CVF–treated controls, which was not significantly different from the C3 levels in serum from mice given one IP dose of CVF (Fig. 7). Notably, the C3 levels in the corneas of mice given both systemic and topical CVF were reduced 61% (Fig. 7), which was a significantly (P < 0.001, Bonferroni multiple comparison test) larger reduction than that achieved in the corneas of mice given a single IP dose of CVF. C3 levels in the sera of mice given either a single IP injection of CVF or both systemic and topical CVF did not differ significantly (P > 0.05, Bonferroni multiple comparison test). Thus, application of CVF onto the cornea was needed to reduce tissue C3 levels to those comparable to that in the serum.
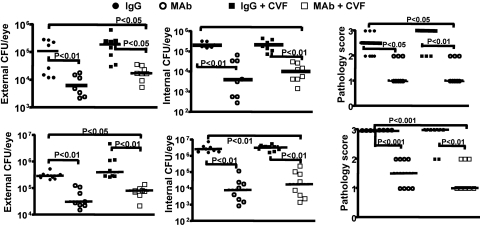
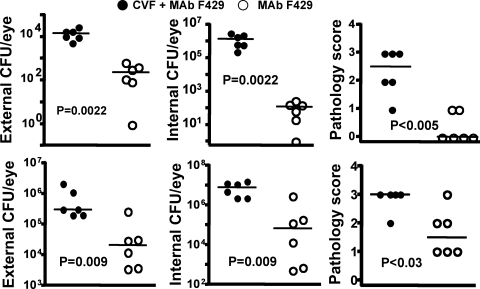
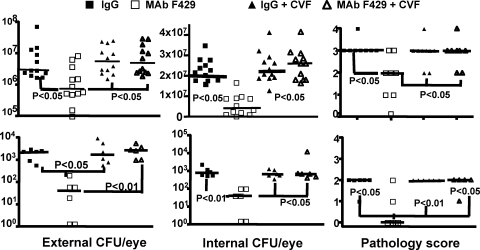
In this setting, complement appeared to be a key co-factor in MAb F429 mediated reductions in bacterial levels and corneal pathology, as the median level of external and internalized CFU of P. aeruginosa were much higher in the MAb F429 plus CVF-treated groups compared with those that got the MAb and had intact complement activity (Fig. 8). Similarly, the corneal pathology scores were also significantly higher with the more vigorous C3 depletion. Comparing the results obtained after a single IP injection of CVF (Fig. 6) with those obtained in the separate, but otherwise comparably executed, experiment incorporating systemic and topical CVF treatments revealed that reducing corneal C3 levels from the topical applications of CVF lead to a higher median CFU of P. aeruginosa in the corneas (Fig. 8). Analysis of brains and spleens found two mice given control IgG plus systemic and topical CVF with low levels of bacteria in the spleen, which was also found in one mouse given the same CVF treatment and MAb F429. No P. aeruginosa were cultured from the brains of any CVF-treated mice nor the spleens of mice with intact complement. Thus, about a 60% depletion of corneal C3 levels by local CVF treatment was needed to document reductions in the efficacy of MAb F429 in bacterial tissue levels and corneal pathology, whereas a 40% reduction in corneal C3 levels did not have any discernable effect under the conditions tested.
Figure 8.
C3H/HeN mice given both systemic injection and topical application of CVF cannot control bacterial survival or pathology due to P. aeruginosa keratitis. Upper panels (closed and open circles): strain 6294; lower panels (closed and open squares): strain 6077. Closed circles indicate animals treated with CVF and topical MAb F429; open circles indicate animals not given CVF but only topical MAb F429. Points indicate value for an individual mouse (six per group), lines indicate group medians, and P values determined by Mann-Whitney U test. Infectious doses: 106 cfu/eye for P. aeruginosa strain 6294, 4.5 × 106 cfu/eye for strain 6077. Results with control mice given irrelevant IgG or MAb F429 in same experiment are shown in Figure. 1.
Antibacterial Efficacy of MAb F429 in Different Mouse Strains
As C57Bl/6 mice have more severe pathology and infectious levels of P. aeruginosa in the setting of keratitis, whereas Balb/C mice have a clearly reduced reaction,15 we analyzed whether these mouse strains also required CVF to mediate the protective effects of MAb F429. C57Bl/6 or Balb/C mice given one systemic injection of 25 μg of CVF followed by the topical applications of 0.5 μg at 0, 8, 24, and 32 hours post-infection along with the MAb F429 or control IgG were compared with mice not treated with CVF. As per prior results, the levels of bacteria and corneal pathology scores of the C57Bl/6 mice given control IgG1 were higher than those of the comparably-treated Balb/C mice (Fig. 9) and even those of C3H/HeN mice given the control IgG1 (see Fig. 1). In both mouse strains, treatment with MAb F429 in the setting of intact complement reduced bacterial levels and corneal pathology scores (Fig. 9). However, in the absence of functional complement, MAb F429 lost protective efficacy, confirming that in these differentially susceptible mouse strains topical application of MAb F429 starting 8 hours after infection can reduce the consequences of P. aeruginosa infection that is maximal when complement activity is fully functional.
Figure 9.
C57Bl/6 (top three graphs) or Balb/C mice (bottom three graphs) are unable to control P. aeruginosa keratitis after reduction of complement component C3B by systemic and local CVF treatment. Open and closed squares indicate WT mice not treated with CVF, whereas open and closed triangles indicate animals treated with CVF. Closed symbols indicate animals treated with control IgG; open symbols indicate animals treated with topical MAb F429. Points indicate value for an individual mouse (6 to 12 per group), lines indicate group medians, and P values determined by Newman-Keuls multiple comparison test. Overall ANOVA for each group, P < 0.01. P values are all compared with WT mice given MAb F429 (second column). Infectious doses: C57 Bl/6 mice: 3 × 105 cfu/eye; Balb/C mice: 106 cfu/eye.
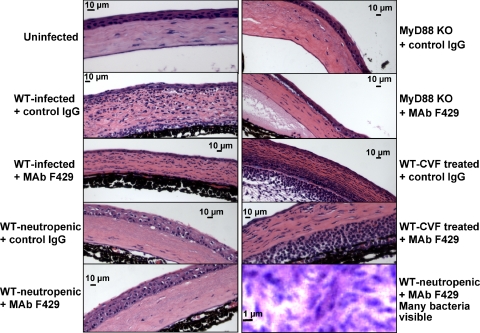
Histopathology of Eyes from Infected Mice
Histopathologic observations were made on corneas from WT C57Bl/6 and MyD88-KO mice; the former animals treated with the various combinations of antibodies and reagents to induce neutropenia or complement depletion. High levels of PMN infiltration were seen in WT infected mice given the control IgG, reduced PMN levels were observed in WT infected mice given MAb F429, and few to no PMNs were seen in WT-neutropenic or MyD88 KO mice regardless of antibody treatment (Fig. 10). In WT mice treated with CVF and control IgG, high levels of PMN were also seen, while slightly reduced levels of PMNs were observed in WT mice treated with CVF. However, these mice did not have reduced bacterial levels or corneal pathology. Higher magnifications of tissues showed many bacteria present in WT-neutropenic mice given MAb F429 (Fig. 10), as well as mice given the IgG1 control (not shown).
Figure 10.
Histopathologic analysis of corneas from indicated mouse strain given the similarly indicated antibody therapy and/or treatment with MAb RB6-8C3 to induce neutropenia or given CVF. High levels of PMN infiltration were seen in WT-infected mice given the control IgG, reduced levels in WT-infected mice given MAb F429, and few to no PMNs seen in WT-neutropenic or MyD88 KO mice regardless of antibody treatment. In WT mice treated with CVF and control IgG, high levels of PMN are seen; slightly reduced levels of PMNs were observed in WT mice treated with CVF, but this was not associated with reduced bacterial levels or corneal pathology.
Discussion
It is critical to understand the interplay of the various immunologic factors involved in both innate and acquired resistance and pathologic responses in the setting of microbial keratitis. Minimizing corneal pathology to preserve ocular clarity, and hence visual acuity, while at the same time limiting bacterial growth, can be competing goals in this setting. An influx of PMNs is needed to phagocytose and kill bacteria but can also cause tissue damage that is very difficult to repair and is often permanent. In this study, we found that limiting PMN influx into the eye during P. aeruginosa infection by making mice neutropenic, or using MyD88KO mice that cannot recruit PMN to tissues, successfully reduced overall corneal pathology. However, these treatments failed to limit bacterial growth in the eye, even when a MAb that is efficacious when administered to WT mice is used to treat the infection. In addition, in the setting of neutropenia and in the MyD88KO mice, there was local and systemic spread of P. aeruginosa to the brains and spleens even when MAb F429 was administered. When complement was depleted by both IP injection and topical application of CVF onto the cornea, MAb F429 had markedly less ability to limit bacterial numbers in the eye and the resultant pathology in C3H/HeN, C57Bl/6, and Balb/C mice. Notably, both systemic and topical C3 depletion in control animals given irrelevant human IgG1 did not limit corneal pathology as did neutropenia, suggesting that complement activation is not a major contributor to pathology in the setting of P. aeruginosa keratitis. Thus, while both PMN depletion or prevention of PMN recruitment reduced corneal pathology during MAb therapy, these conditions also resulted in more severe local and systemic infections, indicating that successful immunologic treatment of eye infections likely requires the coordinated participation of antibody, complement, and phagocytes to be maximally effective.
The treatment with MAb RB6-8C5 to make the mice neutropenic is very effective at the dose used (0.2 mg/mouse) and reduces circulating PMN to extremely low levels that are maintained for 5 days.25 It still might be possible, nonetheless, to use a lower dose of this PMN-depleting MAb to try to have a less severe effect on PMN levels. While theoretically there might be a window wherein PMN levels are sufficient for antibody effector functions but sufficiently limited to minimize corneal pathology, the finding that neutropenic mice had high levels of P. aeruginosa in their brains and spleens would argue that it would be therapeutically very challenging to identify such a window. Older studies examining the role of PMN in innate immune resistance to experimental P. aeruginosa keratitis in guinea pigs made neutropenic by whole body irradiation indicated loss of radiosensitive cells lead to greater bacterial levels in the eye26 but fewer infiltrating PMNs, which are responsible for one of the major pathologic manifestations of keratitis, corneal edema.16 Hazlett et al.27 used cyclophosphamide to induce neutropenia and showed a single strain of P. aeruginosa could cause a lethal sepsis after inoculation of ≥105 cfu onto scratched corneas. However, neither of these results would predict whether MAb F429 required PMN for its beneficial effects on P. aeruginosa keratitis, as it was possible that MAb and complement alone could have direct bactericidal effects that reduced bacterial levels in the cornea and minimized pathology. Notably, both whole body irradiation and cyclophosphamide produce extensive injury to mucosal and epithelial tissues, thus eliciting effects beyond that of merely inducing neutropenia. This makes it difficult to conclude from the older studies that neutropenia alone led to the loss of control of P. aeruginosa replication in the cornea and systemic spread of bacteria and not other factors detrimental to the host associated with radiation and cyclophosphamide administration. As RB6-8C5 depletion of PMN and the inability of MyD88KO mice to recruit PMN to the eye during infection both showed the necessity of PMN for MAb F429's protective activity and control of infection, it is clear from these more specific experimental settings that PMN are critical players in both innate and acquired immunity to P. aeruginosa keratitis.
We are not certain as to the route taken by the bacteria into the brain, but the most parsimonious explanation is local spread either via the optic nerve or other routes through the head that connect brain tissue with eye tissues. As the MyD88KO mice do have functional PMN in the blood, the fact that bacteria spread to the brains of these mice also indicates a non-vascular route of infection of the brain. A local spread from eye to brain was found to be the path taken by Herpes Simplex Virus-1 in neutropenic mice initially infected in the anterior chamber of the eye.28 We did find one, untreated, non-neutropenic mouse with a low level of P. aeruginosa in their brain at kill, suggesting that P. aeruginosa keratitis might also lead to spread of the pathogen locally even in a host with intact PMN levels and complement function. There are only a few case reports associating P. aeruginosa eye infections with systemic infections, meningitis, or brain abscesses,29–31 and these are almost all in pre-term infants weighing <1000 g. In some cases, it appears the initial clinical presentation was an eye infection, although in others, a systemic infection might have preceded the seeding of the eye and development of P. aeruginosa conjunctivitis, keratitis, and/or endophthalmitis. Nonetheless, there is some concern raised by our findings that P. aeruginosa could spread from the eye to the brain and blood, particularly during neutropenia, and interfering with host inflammatory reactions, while potentially preserving corneal clarity, could make for significantly worse clinical issues if brain infections or systemic bacterial spread develops.
A single injection of 25 μg of CVF reduced circulating C3 levels by ∼75%, but local levels of C3 in the cornea were only reduced by approximately 40%. Cleveland et al.24 injected CVF into non-immune DBA/2 mice, which are normally resistant to P. aeruginosa keratitis and restore corneal clarity after infection with a high bacterial dose (1.25 × 108 cfu/eye). They found CVF treatment inhibited the recovery of corneal clarity under these experimental conditions, indicating that reduction in C3 levels by systemic CVF treatment could alter the course of keratitis in non-immune mice challenged with high levels of highly virulent P. aeruginosa. However, this article did not report on bacterial levels in the infected cornea, so it cannot be concluded that C3 depletion actually affected microbial growth and persistence. It is possible that the inability of the CVF-treated mice to recover corneal clarity was unrelated to loss of the effects of complement on reducing bacterial levels or inflammation, but possibly related to the role of complement in tissue responses that affect tissue repair, which could be compromised in CVF-treated animals.32 Verhagen et al.33 found systemic CVF treatment of rats reduced the inflammatory response and manifestations of keratitis after a single injection of heterologous rabbit serum into the corneas of animals with no prior sensitization to rabbit serum. This type of keratitis is associated with development of antibodies to the heterologous serum proteins and takes 5 days for clinical signs to appear that are maximal by day 11 in intact rats. On secondary challenge, there was no difference in the development of inflammatory keratitis between CVF-treated or untreated rats, wherein both groups had a rapid onset of inflammation in either the same eye or the contralateral eye of a rat previously injected with rabbit serum. This finding suggests that the systemic CVF treatment did not reduce C3 levels in the eyes sufficiently to reduce the consequences of immune-complex–mediated inflammation in the presence of preexisting antibody, a finding consistent with our inability to show a significant effect of a single systemic CVF injection on the activity of MAb F429 in treatment of P. aeruginosa keratitis.
We did find, however, that a combination of systemic injection and topical application of CVF to the cornea during the infectious process reduced local C3 levels >60%. With this level of local C3 depletion, the expected differences in bacterial levels and pathology scores normally obtained when comparing mice given MAb F429 with those given irrelevant IgG1 were not achieved in either C3H/HeN, C57Bl/6, or Balb/C mice that have differing levels of susceptibility to P. aeruginosa eye infections.15 Thus it appears that to show that C3 is important in acquired immunity to P. aeruginosa keratitis mediated by this human MAb to alginate, one has to administer the CVF locally as well as systemically to deplete C3 to levels that can compromise the efficacy of MAb. Hazlett and Berk34 found a single injection of CVF altered PMN infiltration into the cornea of non-immune mice. The reason it took both injected and topically applied CVF to document a loss of the effects of MAb F429 was that the 40% reduction in local levels of C3 from the single systemic injection of CVF left sufficient complement present for the MAb to mediate opsonic killing of bacteria. When the C3 levels were depleted >60%, there was insufficient C3 activity for the MAb to mediate its effects, which was found in three different mouse strains. While the overall differences in C3 levels achieved with the two different treatments might not seem large, it is clear from the results that this difference had a profound effect on the efficacy of therapeutic treatment of P. aeruginosa keratitis with MAb F429 in three distinct mouse genetic backgrounds. This might be explained, in part, by the likelihood that C3 depletion can also lead to decreased PMN infiltration into the cornea, and this also contributed to loss of effective bacterial clearance when CVF was topically applied.
In conclusion, optimal therapy of P. aeruginosa keratitis in a murine model with a human MAb to alginate appears to require some level of PMN function and around 60% of normal levels of C3 in the cornea, wherein complement can be a contributing factor to the salutary effects of MAb F429. It was notable that MAb treatment does lead to some residual inflammation in the treated eyes, which could be reduced by eliminating PMNs. However, this resulted in vastly increased bacterial levels in the eye, and local and systemic spread of P. aeruginosa to the brain and spleen, indicating that MAb and complement alone are insufficient to control P. aeruginosa levels in tissues. Also, it appears there could be quite severe consequences from trying to limit corneal pathology by manipulating PMN levels. Complement depletion with CVF to a level (>60%) showing reduced efficacy of MAb F429 required both systemic injection and local application of CVF onto the cornea, indicating a role for this co-factor in MAb efficacy. However, C3 levels of ∼60% of normal were nonetheless adequate for MAb F429 to reduce bacterial burdens and tissue pathology. From these results, it appears that the optimal therapeutic results from MAb F429 therapy of P. aeruginosa keratitis will require reasonably intact PMN levels and high levels of C3 in the cornea to reduce bacterial levels and minimize corneal pathology.
Acknowledgments
The authors thank Lisa Cavacini and Marshall Posner from the Beth Israel/Deaconess Medical Center, Harvard Medical School, for deriving the original hybridomas used to produce MAb F429 and Akinobu Kamei and Andrew Koh for provision of MAb RB6-8C5.
Footnotes
Supported by National Institutes of Health Grant EY016144 from the National Eye Institute.
Disclosure: T.S. Zaidi, None; T. Zaidi, None; G.B. Pier, P
References
- 1.Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem Immunol Allergy 2007;92:185–194 [DOI] [PubMed] [Google Scholar]
- 2.Robertson DM, Petroll WM, Jester JV, Cavanagh HD. Current concepts: contact lens related Pseudomonas keratitis. Cont Lens Anterior Eye 2007;30:94–107 [DOI] [PubMed] [Google Scholar]
- 3.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis 2007;20:129–141 [DOI] [PubMed] [Google Scholar]
- 4.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci 2007;84:273–278 [DOI] [PubMed] [Google Scholar]
- 5.Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens-related infiltrates. Optom Vis Sci 2007;84:257–272 [DOI] [PubMed] [Google Scholar]
- 6.Fleiszig SM. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom Vis Sci 2006;83:866–873 [DOI] [PubMed] [Google Scholar]
- 7.Vajpayee RB, Sharma N, Sinha R, Agarwal T, Singhvi A. Infectious keratitis following keratoplasty. Surv Ophthalmol 2007;52:1–12 [DOI] [PubMed] [Google Scholar]
- 8.Wroblewski KJ, Pasternak JF, Bower KS, et al. Infectious keratitis after photorefractive keratectomy in the United States Army and Navy. Ophthalmology 2006;113:520–525 [DOI] [PubMed] [Google Scholar]
- 9.Swarbrick HA. Orthokeratology review and update. Clin Exp Optom 2006;89:124–143 [DOI] [PubMed] [Google Scholar]
- 10.Panda A, Satpathy G, Nayak N, Kumar S, Kumar A. Demographic pattern, predisposing factors and management of ulcerative keratitis: evaluation of one thousand unilateral cases at a tertiary care centre. Clin Experiment Ophthalmol 2007;35:44–50 [DOI] [PubMed] [Google Scholar]
- 11.Zaidi T, Pier GB. Prophylactic and therapeutic efficacy of a fully human immunoglobulin G1 monoclonal antibody to Pseudomonas aeruginosa alginate in murine keratitis infection. Infect Immun 2008;76:4720–4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pier GB, Thomas D, Small G, Siadak A, Zweerink H. In vitro and in vivo activity of polyclonal and monoclonal human immunoglobulin G, M and A against Pseudomonas aeruginosa lipopolysaccharide. Infect Immun 1989;57:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun 2008;76:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szliter EA, Barrett RP, Gabriel MM, Zhang Y, Hazlett LD. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens 2006;32:12–18 [DOI] [PubMed] [Google Scholar]
- 15.Hazlett LD. Role of innate and adaptive immunity in the pathogenesis of keratitis. Ocul Immunol Inflamm 2005;13:133–138 [DOI] [PubMed] [Google Scholar]
- 16.Chusid MJ, Nelson DB, Meyer LA. The role of the polymorphonuclear leukocyte in the induction of corneal edema. Invest Ophthalmol Vis Sci 1986;27:1466–1469 [PubMed] [Google Scholar]
- 17.Thakur A, Xue M, Stapleton F, Lloyd AR, Wakefield D, Willcox MD. Balance of pro- and anti-inflammatory cytokines correlates with outcome of acute experimental Pseudomonas aeruginosa keratitis. Infect Immun 2002;70:2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston MJ, Fleiszig SMJ, Zaidi TS, et al. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun 1995;63:3497–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiszig SMJ, Wiener-Kronish JP, Miyazaki H, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun 1997;65:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pier GB, Boyer D, Preston M, et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J Immunol 2004;173:5671–5678 [DOI] [PubMed] [Google Scholar]
- 21.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 2008;4:e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell 2006;125:834–836 [DOI] [PubMed] [Google Scholar]
- 23.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem 2004;279:49315–49322 [DOI] [PubMed] [Google Scholar]
- 24.Cleveland RP, Hazlett LD, Leon MA, Berk RS. Role of complement in murine corneal infection caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci 1983;24:237–242 [PubMed] [Google Scholar]
- 25.Koh AY, Priebe GP, Pier GB. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect Immun 2005;73:2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chusid MJ, Davis SD. Experimental bacterial keratitis in neutropenic guinea pigs: polymorphonuclear leukocytes in corneal host defense. Infect Immun 1979;24:948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazlett LD, Rosen DD, Berk RS. Pseudomonas eye infections in cyclophosphamide-treated mice. Invest Ophthalmol Vis Sci 1977;16:649–652 [PubMed] [Google Scholar]
- 28.Zheng M, Fields MA, Liu Y, Cathcart HM, Richter E, Atherton SS. Neutrophils protect the retina of the injected eye from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Invest Ophthalmol Vis Sci 2008;49:4018–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SS, Gallagher PG. Complications of conjunctivitis caused by Pseudomonas aeruginosa in a newborn intensive care unit. Pediatr Infect Dis J 1998;17:97–102 [DOI] [PubMed] [Google Scholar]
- 30.Shah SS, Gloor P, Gallagher PG. Bacteremia, meningitis, and brain abscesses in a hospitalized infant: complications of Pseudomonas aeruginosa conjunctivitis. J Perinatol 1999;19:462–465 [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe M, Nolan L, Lanigan B, Murphy J. Pseudomonas aeruginosa endophthalmitis in a preterm infant. J AAPOS 2005;9:288–289 [DOI] [PubMed] [Google Scholar]
- 32.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 2007;171:715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhagen C, Breebaart AC, Kijlstra A. The effects of complement depletion on corneal inflammation in rats. Invest Ophthalmol Vis Sci 1992;33:273–279 [PubMed] [Google Scholar]
- 34.Hazlett LD, Berk RS. Effect of C3 depletion on experimental Pseudomonas aeruginosa ocular infection: histopathological analysis. Infect Immun 1984;3:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]