The authors found that embryonic retinal cells transplanted into the vitreous chamber protect ganglion cells from injury and that the environment provided by the mature eye does not support the differentiation and proliferation of the embryo-derived cells.
Abstract
Purpose.
There is a paucity of neuron replacement studies for retinal ganglion cells. Given the complex phenotype of these neurons, replacement of ganglion cells may be impossible. However, transplanted embryonic cells could provide factors that promote the survival of these neurons. The authors sought to determine whether transplanted embryonic retinal cells from various stages of development influence the survival of mature ganglion cells
Methods.
Acutely dissociated retinal cells, obtained from chick embryos, were transplanted into the vitreous chamber of posthatch chicken eyes after the ganglion cells were selectively damaged. Eight days after transplantation, numbers of ganglion cells were determined
Results.
Embryonic retinal cells from embryonic day (E)7, E10, and E11 promoted the survival of ganglion cells, whereas cells from earlier or later stages of development or from other tissue sources did not. The environment provided by the posthatch eye did not support the proliferation of the embryo-derived cells, unlike the environment provided by culture conditions. Furthermore, cells that migrated into the retina failed to express neuronal or glial markers; those that remained in the vitreous formed aggregates of neuronal and glial cells
Conclusions.
The environment provided within the mature retina does not support the differentiation and proliferation of retinal progenitors. Furthermore, embryo-derived cells likely produce secreted factors that promote the survival of damaged ganglion cells. Therefore, embryonic retinal cells could be applied as a cell-based survival therapy to treat neurodegenerative diseases of the retina.
Replacement of damaged neurons through the transplantation of progenitor cells is a potential treatment of sight-threatening retinal diseases that result from the loss of photoreceptors or ganglion cells. Diseases that result from the loss of photoreceptors include age-related macular degeneration (AMD) and retinitis pigmentosa. Although much research has been conducted to replace photoreceptors, relatively little research has been conducted to replace ganglion cells, which are lost in glaucomatous retinas. The lack of neuron replacement studies in models of glaucoma is likely due to the complex phenotype of ganglion cells and, accordingly, a requirement for an elaborate series of processes to guide the formation of these cells. For example, during the normal course of development, the formation of ganglion cells requires mechanisms to direct cell fate, support differentiation, establish connections with retinal interneurons, guide axons to appropriate targets, and support the survival of cells that have formed proper connections within higher visual centers.
Successful transplantation requires that cells migrate to the appropriate layer of the retina, express appropriate cellular markers, and properly integrate into retinal circuitry. Additionally, transplanted cells must survive for extended periods of time and not elicit immune responses. Unfortunately, most transplantation studies in the retina have demonstrated widespread failure of naive progenitor cells to differentiate and form functional synapses with preexisting circuitry. However, there have been several compelling reports of photoreceptor replacement wherein the donor cells were committed to a photoreceptor fate before transplantation.1,2
Although transplantation studies have demonstrated limited photoreceptor replacement, there is evidence that transplanted cells preserve retinal function by enhancing the survival of host neurons. For example, transplanted retinal sheets improved visual responses as measured by ERG recordings.3,4 These increased responses were attributed to improved survival of host photoreceptors.5 Similarly, transplantation of immature wild-type photoreceptor sheets into the rd mouse (after rod loss but before cone degeneration) increased the number of surviving cone photoreceptors in the host retina.5,6 In addition, nonretinal sources of donor tissue have exhibited protective effects.7 It remains uncertain whether survival of host neurons results from interactions between host and donor cells or whether the perturbations of the host retina promote survival within the host tissue.
Most transplantation studies have been designed to replace lost cells. Therefore, the rescue effects of transplanted cells on host cells have not been well characterized, even though these effects have been noted. The notion of cell-based rescue therapy has been supported by several recent publications. In 2007, Gamm et al.8 explored the possibility of rescuing damaged neurons rather than replacing cells through transplantation of human neural progenitor cells (hNPCs; cortex derived).8 In the RCS rat model of degeneration, subretinal transplantation of hNPCs improved visual function as determined by electroretinography, acuity tested by optomotor responses, and recorded electrophysiologically from the superior colliculi.8 Furthering these findings, the authors showed that transplanted cells maintained the function of host cells for at least 260 days after transplantation, with few aberrant effects on retinal integrity.9 Consistent with the notion of survival supported by diffusible trophic factors, the hNPCs were shown to secrete insulin-like growth factor 1 and fibroblast growth factor 2 (FGF2). Additionally, Bull et al.10 recently showed that transplanted oligodendrocyte precursors promoted the survival of retinal ganglion cells.
The purpose of the present study was to investigate whether embryonic retinal cells from different developmental stages protect mature ganglion cells from damage and to assess the influence of the environment provided by the mature eye on the embryo-derived retinal cells.
Methods
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the Ohio State University. Wild-type fertilized eggs were obtained from the Department of Animal Sciences at the Ohio State University. Eggs were incubated between 36.6°C and 37.8°C to the desired developmental stage. Newly hatched Leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University and kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 AM). Chicks were housed in a stainless steel brooder at approximately 28°C and received water and chick starter (Purina, St. Louis, MO) ad libitum. Eyes of Macaca fascicularis were obtained through the Tissue Distribution Program at the Regional Primate Research Center at the University of Washington.
Staging of Embryonic Chicks
Fertilized eggs from GFP- transgenic chickens were obtained from the North Carolina State University Poultry Science Department.11 The developmental stages of chick embryos were determined according to the guidelines established by Hamburger and Hamilton.12,13
Intraocular Injections
Animals were anesthetized using 2.5% isoflurane in oxygen at a flow rate of 1.5 L/min. Injections were made using a Hamilton syringe with a 26-gauge needle through the dorsal eyelid. Treated eyes were injected with 20-μL volumes of colchicine (500 ng) or N-methyl-d-aspartate (NMDA; 283.4 μg) dissolved in sterile saline. Control eyes received injections of saline.
Cocultures
Round coverglasses (12 mm) were individually placed into wells (24-well plates) and coated sequentially with poly-d-lysine (PDL; Sigma, poly 80,000–100,000) and basement membrane matrix (Matrigel; BD Biosciences, Franklin Lakes, NJ). Retinas from embryos were dissociated in cold HBSS+ added with trypsin (0.1 mg/mL) and plated at a density of 1 × 105 cells/well (24-well plates). Embryonic cultures were allowed to settle for 4 hours in media (glutamate- and aspartate-free DMEM-F12 with 0.6% d-glucose, 0.11% sodium bicarbonate, 5 mM HEPES, and 100 U/mL each penicillin and streptomycin supplemented with 5% FBS). Retinas from posthatch chicks, at least 7 days old, were treated with NMDA, isolated 24 hours later, trypsin dissociated, and plated on top of GFP-transgenic embryonic cells at a density of 2 × 105 cells/cm2. Wells containing only embryo-derived cells or mature retinal cells were maintained as controls. After 8 days in vitro, cultures were fixed and immunolabeled.
Transplantation
In some experimental paradigms, the cells to be transplanted were virally infected with a reporter gene. E5 retinas were dissociated and cultured in 75-cm2 flasks in the presence of Ad5-eGFP (7 × 107 TCID50) for 3 to 14 days. Virus was supplemented every third day or as needed with media change. To transfect proliferating progenitor cells, we applied a replication-competent retroviral RCAS vector. One day after dissociation and plating of E5 retinas, cells were transfected (2 μg plasmid/mL media) with RCAS-eGFP by using transfection reagent (FuGENE6; Roche, Basel, Switzerland) and the protocols recommended by the manufacturer. After 2 weeks in vitro, the transfected cells were dissociated and transplanted into the NMDA-injured eyes in 50 μL HBSS+. At different times between 7 and 21 days after transplantation, eyes were enucleated, and retinas were processed for immunolabeling.
For the survival studies, acutely dissociated retinal cells (2 × 106) from E5, E7, E10, E11, and E14 GFP-transgenic embryos were injected into colchicine-treated posthatch eyes. The embryo-derived cells were thoroughly triturated with a Hamilton syringe fitted with a 26-gauge needle before transplantation to ensure that clusters of cells were not present. Plating of these cells in culture confirmed that the cell preparations did not include clusters of more than five cells. The transplants were consistently injected into the dorsal quadrant of the eye, with needle penetration at 4 mm. The contralateral eye received 50 μL HBSS+ as a control. Additionally, embryonic cells (2 × 106) derived from the wing bud (WB) or from the optic tectum (OT) were transplanted to assay for cell-type–specific effects. Eight days after transplantation, retinas were processed for whole-mount immunolabeling with antibodies to Brn3a to detect ganglion cells and antibodies to cleaved-caspase 3 (CC3) to detect apoptotic cells. Dissociated cells (1 × 105/cm2) were cultured, as described, in parallel to transplantation.
Cell Counts
Cocultures were labeled with antibodies to GFP, a neuronal marker (Brn3a, HuC/D, or visinin) and 4′,6-diamidino-2-phenylindole (DAPI). Total numbers of cells and total numbers of neuronal cells per 0.45/mm2 were counted for cocultures and cultures of mature retinal cells alone. Neurons were identified as posthatch derived (GFP negative) or as embryo derived (GFP positive). Numbers of surviving neurons from cocultures and postnatal cultures were compared as percentage increases in survival, and statistical significance was determined using a Student's t-test.
To assess ganglion cell survival, total numbers of Brn3a+ nuclei from four areas (52,900 μm2) per region of the retina (central, temporal, nasal, and ventral) were counted per eye (16 total areas per eye). Retinas from transplanted and control eyes from the same individual were compared pairwise. Data were analyzed separately for each area of the retina using a linear mixed model in which the treatment (transplant or no transplant) was the within-subjects variable, and the developmental stage of the transplant (E5 retina, n = 5; E5 WB, n = 5; E5 OT, n = 6; E7 retina, n = 8; E10 retina, n = 4; E11 retina, n = 9; E11 OT, n = 6; E14 retina, n = 5) was the between-subjects variable. The model includes interactions between the transplants (e.g., E7:E11, E7:E14, E11:E14) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 for all figures).
In Ovo BrdU Injection
The yolks of fertilized eggs were injected with 5 μg BrdU in 20-μL sterile saline and were incubated for 6 hours before kill. The cornea, lens, and vitreous from enucleated eyes were carefully removed in phosphate-buffered saline (PBS; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4). The eyes were placed in fixative for 20 minutes and were processed for immunolabeling as described.
Fixation, Sectioning, and Immunofluorescence
Eyes were enucleated and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, with 3% sucrose for 30 minutes. After three washes in PBS, eyecups were cryoprotected by soaking in 30% sucrose in PBS with 0.01% NaN3 overnight. Transverse sections were cut at 10 μm and thaw mounted onto slides (Superfrost-Plus; Fisher Scientific, Pittsburgh, PA). The slides were air dried and stored at −20°C until use. Sections were warmed to room temperature and ringed with rubber cement. After washing 3× in PBS, slides were incubated overnight under 200 μL primary antibody (antisera diluted in 0.05 M PBS with 0.2% Triton-X with 0.01% NaN3 with 5% blocking serum). For BrdU immunolabeling, slides were washed for 7 minutes in 4 N HCl and then washed in PBS before incubation overnight under primary antibody solution.
For whole-mount preparations of the retina, the pecten was cut away, and the sclera and choroid were dissected from the retina. Retinas, in 30% sucrose in PBS, were frozen and thawed three times before incubation under primary antibody. Retinas were incubated in 300 μL primary or secondary antibodies overnight at room temperature on a rotary platform, similar to previous descriptions.14
Antisera and dilutions used in this study included mouse anti-2M6 at 1:80 (Paul Linser), mouse anti-AP2-α (3B5; Developmental Studies Hybridoma Bank [DSHB]), mouse anti-BrdU at 1:100 (G3G4; DSHB), rat anti-BrdU used at 1:200 (OBT0030S; Accurate Chemicals/Serotec, Raleigh, NC), mouse anti-Brn3a at 1:200 (MAB1585; Chemicon, Temecula, CA), rabbit anti-calretinin at 1:500 (7699/4; Swant Immunochemicals, Bellinzona, Switzerland), rabbit anti-green fluorescent protein (GFP; Luc Berthiaume, University of Alberta), mouse anti-HuC/D used at 1:200 (A21271; Molecular Probes/Invitrogen, Carlsbad, CA), mouse anti-islet1 at 1:50 (40.2D6; DSHB), goat anti-Sox2 used at 1:1000 (Y-17; Santa Cruz Biotechnology, Santa Cruz, CA),; rabbit anti-Sox9 used at 1:2000 (AB5535; Chemicon), mouse anti-vimentin used at 1:100 (H5; DSHB), and mouse anti-visinin used at 1:80 (7G4; DSHB). Nuclei were visualized (ToPro3, 1 μM; Invitrogen) in PBS for 15 minutes, DRAQ5 (5 μM; Biostatus Limited, Leicestershire, UK) with secondary antibody for 1 hour or with DAPI (1 μg/mL; Sigma-Aldrich, St. Louis, MO) for 7 minutes. DNA-strand breaks were detected using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) kit according to the manufacturer's protocol (11767291910; Roche) with the exception that mixed TUNEL reagents were diluted 1:1 with dH2O before incubation at 37°C for 1 hour.
Microscopy
High-resolution (5.4 MP) fluorescent micrographs were taken with a digital camera (DC500; Leica, Wetzlar, Germany) and a microscope (DM5000B; Leica). Color and brightness were optimized for all images with a graphics editing software (Photoshop 6; Adobe, Mountain View, CA).
BrdU Labeling
Nuclei were stained (To-Pro3, 1 μM final concentration; Invitrogen) in PBS. Computing software (ImagePro 6.2; Media Cybernetics, Silver Spring, MD) was used to tally the pixels occupied by BrdU fluorescence and the pixels occupied by the nuclear counterstain. Enumerated areas were defined by pixel values between 80 and 255 (0, black; 255, saturation), excluding any areas with fewer than 50 clustered pixels to exclude debris. The total area for the BrdU label was divided by the total area for the nuclear label and converted to a percentage. To verify the computing software (ImagePro 6.2; Media Cybernetics) data, cells that incorporated BrdU were counted manually and expressed as a percentage of total cells per area (n > 600 cells per area). Computed and manual counts were not significantly different (not shown).
Results
Survival of Mature Retinal Neurons in Culture with Embryonic Retinal Cells
The first insight that embryo-derived cells support the survival of mature neurons came from culture experiments in which embryonic chick retina served as a feeder layer for mature primate retina. Coculture of mature (∼8 years of age) macaque retina with E7 chick retina resulted in an eightfold increase of surviving photoreceptors, immunolabeled for recoverin, compared with macaque retina cultured without a feeder layer (Supplementary Fig. S1; all Supplementary Figures available at http://www.iovs.org/cgi/content/full/51/4/2208/DC1). To further study the survival-promoting effects of embryo-derived cells on mature retinal neurons, we cocultured chick cells from mature retina with GFP-transgenic retinal cells obtained from E7 or E10 embryos. After 7 days in vitro, cells were fixed and labeled for markers of ganglion cells (Brn3a), ganglion and amacrine cells (HuC/D), or photoreceptors (visinin) and GFP (embryo-derived cells). Few neurons from mature retina survived when cultured alone (Supplementary Figs. S2a–S2d). However, when cocultured with E7 or E10 retinal cells, there were significantly more ganglion cells, amacrine cells, and photoreceptors from the mature retina that survived when compared with cultures of mature retinal cells alone (Supplementary Figs. S2e–S2r). Brn3a+ cells did not survive in the cocultures of E10 and mature retina, suggesting diminished survival of ganglion cells with increased developmental age of the embryonic cells.
Differentiation of Transplanted Embryonic Progenitors
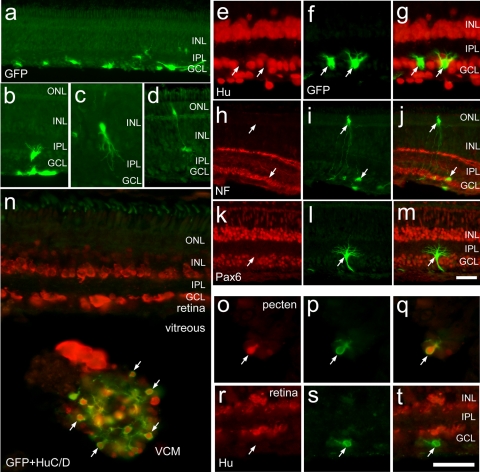
Unlike the microenvironment provided by the embryonic retina or culture conditions, the damaged mature retina may not provide a microenvironment that supports the neuronal differentiation of naive progenitors. For example, in acutely damaged retinas, Müller glia dedifferentiate and undergo one round of cell division, and most of the glia-derived cells remain as undifferentiated progenitor-like cells for at least several weeks after division.15–17 It has been proposed that the failure of Müller glia-derived cells to differentiate occurs because the cues to promote differentiation are absent in the mature damaged retina (for a review, see Fischer18). To test whether embryo-derived progenitors are capable of differentiating within the mature damaged retina, E5 retinal progenitors (cells that normally generate neurons) were infected with adenovirus (Ad5-eGFP) for short-term culture paradigms or with RCAS-GFP for long-term culture paradigms and were transplanted into postnatal eyes with injured retinas. Numerous embryo-derived retinal cells integrated into NMDA-damaged retina (Fig. 1a), whereas no transplanted cells migrated into undamaged retinas (data not shown). Many cells that migrated into the retina acquired neuronal morphology (Figs. 1b–d), but none (n = 394) expressed neuronal markers such as HuC/D, neurofilament, or Pax6 (Figs. 1e–m). By comparison, when transplanted cells reaggregated to form vitreal cellular masses (VCMs), the cells differentiated to express neuronal markers such as HuC/D (Fig. 1n). In addition, we found embryo-derived cells that migrated into the pecten, a pigmented vascular structure in the ventral chick eye; these cells differentiated to express neuronal markers such as HuC/D (Figs. 1o–q). In the same eye, however, transplanted cells that migrated into the neural retina, either within the nerve fiber layer or the ganglion cell layer, failed to express HuC/D (Figs. 1r–t). These findings are consistent with the hypothesis that the mature damaged retina does not provide a microenvironment that supports the differentiation of neural progenitors.
Figure 1.
Transplanted cells that migrate into NMDA-damaged retinas fail to express neuronal markers. (a–m) retinal progenitors (obtained from E5 chick embryos) infected with RCAS-GFP and transplanted into the postnatal chicken eye that has been damaged by NMDA. (a–d) Representative images of transplanted cells that migrated to the retina. (e–m) Embryo-derived cells that migrated into the retina and acquired neuronal morphology but did not express markers of mature neurons, such as HuC/D (e–g), neurofilament (h–j), and Pax6 (k–m). (n–t) Representative images of E5 retinal cells infected with adenovirus serotype 5 CMV-eGFP (Ad5-eGFP) that migrated into the postnatal retinas that were injured with NMDA. Arrows: GFP+, embryo-derived cells that migrated to the host retina. (n) Small VCM labeled with antibodies to HuC/D (red) and GFP (green). Arrows: transplanted cells within the VCM that express the neural marker HuC/D. (o–q) Representative micrographs of transplanted cells that migrated into the pecten. These cells were labeled with antibodies to HuC/D and GFP. (r–t) Transplanted cell that migrated into the retina that failed to express HuC/D. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Colchicine-Mediated Death of Ganglion Cells
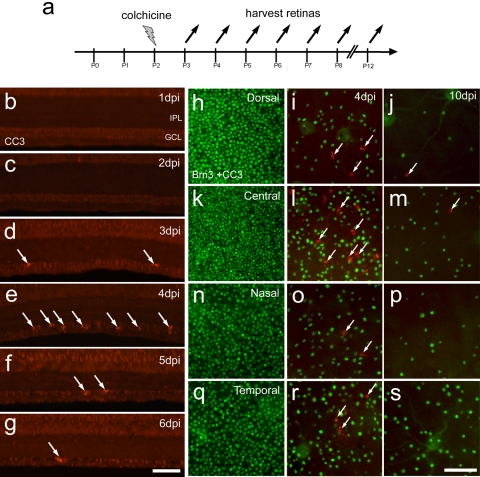
We next sought to determine whether the neuroprotective properties of embryonic retinal cells were maintained in an in vivo model. We used a damage paradigm in which ganglion cells degenerate. The eyes of posthatch day (P)2 chicks were injected with colchicine and harvested between 4 and 10 days after treatment to assay for cell death (Fig. 2a). Colchicine-treatment at P2 resulted in the apoptosis of cells in the ganglion cell layer (GCL; Figs. 2b–g). Apoptotic cells, immunolabeled for cleaved caspase 3 (CC3), were first detected in the GCL at 3 days postinjury (dpi), and the abundance of CC3+ cells was greatest at 4 dpi (Figs. 2d, 2i, 2l, 2o, 2r). The abundance of apoptotic cells progressively decreased through 5 and 6 dpi (Figs. 2e, 2f). By 10 dpi, very few apoptotic cells were found (Figs. 2j, 2m, 2p, 2s). Ganglion cells in different regions of the retina were differentially sensitive to colchicine-induced injury. The most colchicine-resistant ganglion cells were found in the temporal retina, with a loss of 36% ± 10% of cells at 4 dpi and a loss of 82% ± 11% by 10 dpi (Figs. 2q–s). Similarly, other regions of the retina had significant losses in Brn3a+ ganglion cells. The dorsal, nasal, and central regions lost 56% ± 12%, 46% ± 22%, and 54% ± 9% of the ganglion cells at 4 dpi and 97% ± 4%, 92 ± 8%, and 86 ± 12% at 10 dpi, respectively. These findings are consistent with previous reports of the loss of ganglion cells in retinas treated with colchicine.19,20
Figure 2.
Colchicine-mediated death of ganglion cells. (a) Injection and harvesting paradigm for colchicine-induced retinal damage. (b–g) One to 6 days after colchicine-treatment at P2, transverse sections of central retina were labeled with antibodies to CC3 (red). (h–s) Flat-mount retinas labeled with antibodies to Brn3a (green) and CC3 (red). Representative micrographs were taken from dorsal (h–j), central (k–m), nasal (n–p), and temporal (q–s) regions of the retina from uninjured P2 retina (h, k, n, q), retina 4 days after P2 colchicine injection (i, l, o, r), or from retinas 10 days after colchicine injection (j, m, p, s). Arrows: CC3+ cells. IPL, inner plexiform layer; GCL, ganglion cell layer.
Transplantation of Embryonic Cells into the Vitreous Chamber
We began these studies by using viral transfection to “tag” the donor cells for transplantation. This paradigm requires periods of culture before transplantation to facilitate viral transfection. Our data suggest that culture of the donor cells somehow enhances the integration of donor cells into host retina. We chose to use acutely dissociated cells from transgenic embryos for the survival studies because without culture these cells do not migrate into the host retina.
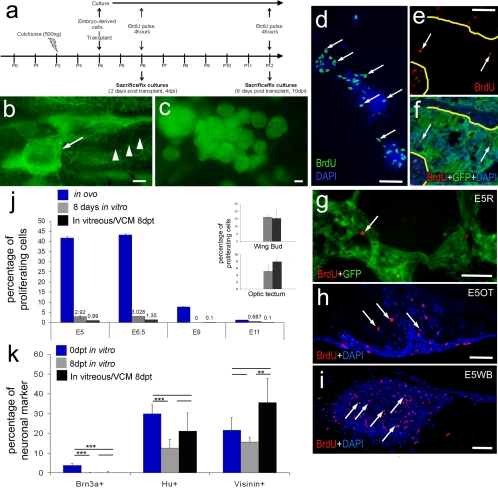
eGFP-transgenic embryonic retinal cells were acutely dissociated and injected into the vitreous chamber of colchicine-treated eyes (Fig. 3a). Retinas were harvested (4 hours after BrdU treatment to label proliferating cells) 8 days after transplantation (Fig. 3a). Although migration of the transplanted cells into the retina was prevalent in NMDA-injured retinas, few transplanted cells migrated into colchicine-injured retinas. The few transplanted cells that migrated into the retina failed to acquire neuronal morphology and failed to express neuronal or glial markers (Supplementary Fig. S3). The transplanted cells were adherent to the pecten (Fig. 3b) or formed free-floating aggregates of cells within the vitreous (Fig. 3c). These cell masses were reminiscent of the transplants described in studies by Ehlrich et al.21 The masses of cells in the vitreous resulted from reaggregation because clusters of cells (>5 cells) were never observed for acutely dissociated cells that were used for transplantation (not shown).
Figure 3.
Cells derived from embryonic retina, OT, and WB remain as masses of cells within the vitreous. (a) Injection and harvesting paradigm for experiments involving colchicine-induced retinal damage and transplantation of cells from GFP-transgenic embryos. (b) Representative micrograph of transplanted cells (arrow) adherent to the pecten (arrowheads). (c) Representative micrograph of aggregates of transplanted cells in the vitreous. Numbers of proliferating retinal cells significantly decreased after transplantation into the vitreous. (d–i) Representative micrographs of proliferating cells exposed to BrdU for 4 hours immediately before fixation. Cells were labeled with DAPI (blue) and with antibodies to GFP (green) and BrdU (red). (d) Cultured E7 retinal cells. (e, f) Sections through an aggregation of transplanted retinal cells adherent to the pecten. Arrows: BrdU+ cells. Yellow lines: border between the transplanted cells and the pecten. (g) Section through a VCM from transplanted E7 retinal cells. (h) Section through a VCM of transplanted E5 optic tectal cells. (i) Section through a VCM of transplanted E5 WB cells. (j) Histogram of the percentages of cells that incorporate BrdU in the vitreous after transplantation (black), in vitro (gray), and in ovo (blue). The percentage of proliferating cells is represented on the y-axis, and the developmental stage of the cells is represented on the x-axis. (j, insets) Percentage of proliferating cells from WB and OT. (k) Histogram of the percentages of neuronal markers in culture compared with transplanted cells within the VCM. Scale bars, 50 μm. **P ≤ 0.01; ***P ≤ 0.001.
To characterize the influence of the environment provided by the posthatch eye on the transplanted cells, we assayed for proliferation of the embryonic cells in ovo, in vitro, and after transplantation. Both transplanted and cultured cells were exposed to BrdU, to label cells in S-phase, for 4 hours before fixation (Figs. 3d–i). Eight days after dissociation, 3% of the E6.5 retinal cells that were maintained in vitro incorporated BrdU (Figs. 3d, 3j), whereas only 1.4% ± 0.7% of the transplanted cells incorporated BrdU in the vitreous after 8 days (Figs. 3d–g, 3j). By comparison, more than 40% of the cells in the intact E6 retina were proliferating (Fig. 3j), indicating a decrease in the relative abundance of cells that proliferate with culture or transplantation into a mature eye. Unlike the retina-derived cells, cells derived from embryonic optic tectum (OT) or wingbud (WB) continued to proliferate in significant numbers after transplantation into the posthatch eye (Figs. 3h–j). The percentage of WB- and OT-derived cells that were proliferating after 8 days in vitro or after transplantation was not significantly different (Fig. 3j).
To assess the influence of the different conditions on embryonic retinal cells, we compared the relative abundance of Brn3a+, HuC/D+, and visinin+ cells on the day of transplantation to the relative abundance of cells in the vitreous and those cells maintained in culture. No Brn3a+ cells were found within the VCM 8 days after transplantation, whereas Brn3a+ cells were identified in vitro on the day of transplantation (data not shown), suggesting that embryo-derived ganglion cells do not survive the transplantation procedures. Compared with the numbers of HuC/D+ cells on the day of transplantation/culture, there was a significant reduction in the numbers of HuC/D+ cells after 8 days in vitro. However, the numbers of HuC/D+ cells on the day of transplantation were not significantly different from the relative abundance of HuC/D+ cells within the VCM 8 days after transplantation (Fig. 3k). HuC/D is expressed by most, if not all, amacrine and ganglion cells in the chick retina.15 Given the absence of Brn3a+ ganglion cells, the HuC/D+ cells were likely to be amacrine cells. No difference was seen in the relative abundance of visinin+ cells on the day of transplantation compared with 8 days in vitro (Fig. 3k). However, there were significantly more visinin+ cells in the VCM compared with the relative abundance of these cells after 8 days of culture conditions (Fig. 3k), suggesting that the survival of visinin+ cells was supported within the vitreous of the postnatal eye. There was not a significant difference between the relative abundance of visinin+ cells among cells initially transplanted and those that remained within the VCM (Fig. 3k).
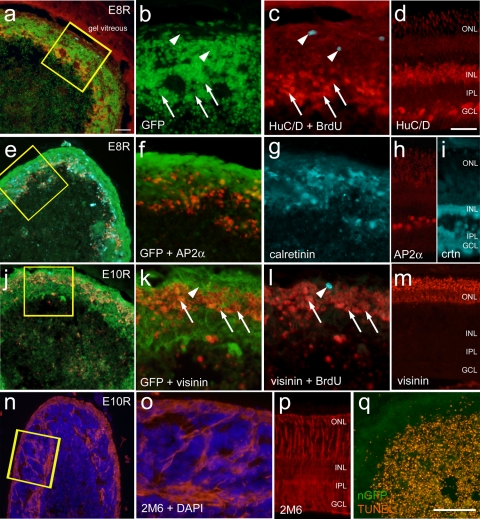
Many of the transplanted cells that remained within the VCM expressed neuronal markers. However, BrdU labeling was not found within VCM cells that expressed HuC/D (n = 1805) or visinin (n = 2407), suggesting that the VCM cells underwent terminal mitosis before BrdU exposure (Figs. 4c, 4l). On no occasion did BrdU labeling colocalize with neuronal markers. The VCM formed an outer layer of semi-laminated cells that expressed a variety of different neuronal markers (Fig. 4). Distinct from the outer layers of the VCM, the central core contained cellular debris and dying cells (Fig. 4). The VCM are formed from a reaggregation of transplanted cells because the acutely dissociated embryonic cells did not include clusters of more than 5 to 10 cells (not shown). Cells within the cortex expressed neuronal markers such as HuC/D (amacrine cells; Fig. 4c), AP2α (amacrine cells; Fig. 4f), calretinin (amacrine and horizontal cells; Fig. 4g), and visinin (photoreceptors; Fig. 4l). The lamination in the VCM cortex approximated that of the intact retina, with the photoreceptors residing in the outermost strata and the interneurons residing in deeper layers (compare Figs. 4a–m). The progenitor marker transitin, the avian homologue of mammalian nestin, was not detected in the VCM (data not shown). By comparison, Sox2 was detected in the nuclei of cells scattered across cortical regions of the VCMs (data not shown). Sox2 is known to be expressed by retinal progenitors and mature Müller glia in the chick.22 None of the Sox2+ cells were labeled for BrdU (data not shown), suggesting that these cells were differentiated, postmitotic Müller glia. Consistent with the hypothesis that Müller glia are found among the transplanted cells, the VCM contained 2M6+ cells that spanned the outer cortex (Figs. 4n, 4o). 2M6 is a monoclonal antibody known to label Müller glia in the chick retina.22 The laminar distribution of retinal neurons within the cortex of VCM was observed for transplants derived from all stages of development that we tested. All aggregates of transplanted cells where composed of outer layers of cells expressing neuronal and glial markers, and the inner core included cellular debris and numerous dying cells that were TUNEL positive (Fig. 4q). TUNEL-labeled cells were not observed in the outer cortex of cells, where differentiated neurons and glial cells were observed. The VCM appeared similar to the retinal spheroids described by Willbold et al.23 that form from nonadherent cultures of embryonic retinal cells.
Figure 4.
Transplanted embryonic cells form a reaggregated, semilaminated masses of cells within the vitreous. (a–c) VCM resulting from E8 retinal transplanted cells. Transverse sections through VCM were labeled with antibodies to HuC/D (red), BrdU (turquoise), and GFP (green). (d) Micrograph of colchicine-injured retina from an eye that received a transplant, illustrating the distribution of HuC/D. (e–i) VCM resulting from an E8 retinal transplant labeled with antibodies to AP2-α (red), calretinin (turquoise), and GFP (green). (h, i) Micrographs of colchicine-injured retina illustrating the distribution of AP2-α (h) and calretinin (i). (j–l) VCM resulting from an E10 retinal transplant labeled with antibodies to visinin (red), BrdU (turquoise), and GFP (green). (m) Micrograph of colchicine-injured retina illustrating the distribution of visinin. (n, o) Micrographs of an E10 retinal VCM labeled with antibodies to 2M6 (red) and DAPI (blue). (p) Micrograph of colchicine-injured retina illustrating the normal distribution of 2M6. DNA strand breaks were detected using the TUNEL (red) reaction on VCM sections from E10 retinal transplants (q). (c, l, arrows) Neuronal marker-positive cells. Arrowheads: BrdU+, neuronal marker-negative nuclei. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars: (d; also applies to h, i, m, p) 50 μm; (q; also applies to a, e, j, n) 100 μm.
Embryo-Derived Retinal Cells and the Survival of Ganglion Cells In Vivo
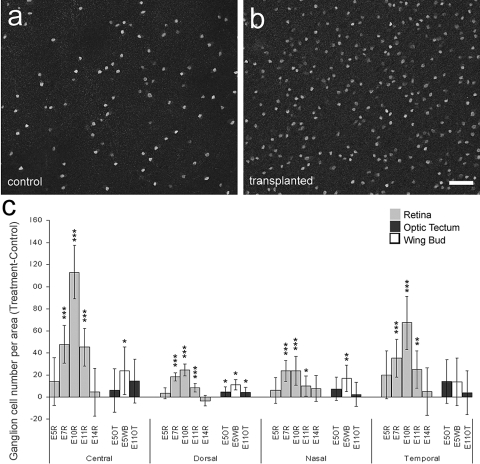
To test whether transplanted embryonic cells influence the survival of damaged ganglion cells in vivo, retinas from control eyes and those that received transplants were labeled with antibodies to Brn3a. Brn3a is expressed by approximately 98% of the ganglion cells.24–26 We assayed for surviving ganglion cells at 10 days after colchicine-treatment, 8 days after transplantation, when the damaging effects of colchicine have subsided and numbers of surviving ganglion cells have stabilized (Fig. 2). In these studies the transplanted cells were acutely dissociated cells from GFP-transgenic embryos. Using this transplantation paradigm, we never observed the migration of donor cells into the host retina unless cells were delivered to the subretinal space (Figs. 3, 4; Supplementary Fig. S3). In all regions of the retina there were significantly greater numbers of ganglion cells surviving in eyes that received transplantations of E7, E10, and E11 retinal cells compared with survival in vehicle-treated retinas (Figs. 5a–c). The most prominent survival-promoting effects were observed for the transplantation of E10 retinal cells (Figs. 5a–c). By comparison, numbers of surviving ganglion cells were not significantly affected by transplanted retinal cells from early or late stages of embryonic development. Transplanted retinal cells obtained from E5 or E14 embryos failed to significantly increase the numbers of surviving ganglion cells (Fig. 5c). Similarly, transplanted cells obtained from OT or from WB had little effect on the survival of ganglion cells in colchicine-damaged retinas (Fig. 5c). For example, transplanted tectal cells obtained from E5 or E10 embryos resulted in modest, but significant, increases in ganglion cell survival in dorsal regions of the retina, whereas the survival of ganglion cells in central, nasal, and temporal regions of the retina was not affected (Fig. 5c). Similarly, transplanted E5 WB cells resulted in modest, but significant, increases in the ganglion cell survival in central, dorsal, and nasal regions of the retina (Fig. 5c). The survival-supporting effects of embryonic WB and tectal cells were much smaller than the maximum effects resulting from transplanted embryonic retinal cells.
Figure 5.
Transplanted embryonic retinal cells promote the survival of ganglion cells in colchicine-treated eyes. (a, b) Representative confocal micrographs of Brn3a+ ganglion cells in central regions of colchicine-treated retinas from eyes that received vehicle (a) or transplants of E10 retinal cells (b). (c) Histogram illustrating the difference (mean ± SD for treated − control) in the numbers of ganglion cells per 0.45 mm2 of retinas from eyes that received control injections and those that received transplants. The source of the transplanted cells included the embryonic retina (gray bars), OT (black bars), and WB (white bars). The developmental stage at which the donor cells were harvested and the region of the retina in which cells were counted are indicated along the x-axis. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Discussion
We found that the transplantation of embryonic retinal cells from mid to late stages of development spared significant numbers of ganglion cells from colchicine-mediated cell death. By comparison, embryonic retinal cells from earlier or later stages in development did not significantly influence the survival of colchicine-damaged ganglion cells. Although colchicine-mediated damage is different from the damage to ganglion cells in glaucomatous eyes, mechanisms that promote the survival of ganglion cells may be effective in different models of damage. Thus, our findings suggest that embryo-derived retinal cells may be a promising source of cells for prosurvival-based therapies aimed at attenuating the loss of ganglion cells in glaucomatous retinas. Glaucoma is a prevalent disease of the retina that involves the slow, progressive loss of ganglion cells, which begins in peripheral regions of the retina. As the ganglion cells die, vision is lost because the retina is unable to send visual information to the brain. Neuron replacement strategies for glaucomatous retinas have not been widely pursued. Instead, different neuroprotection strategies have been used to promote the survival of ganglion cells in different animal models of glaucoma. These strategies have included blocking excitotoxic signaling, enhancing the reuptake of excitatory transmitters,27 maintaining appropriate levels of purinergic signaling,28 regulating intraocular pressure, maintaining neurotrophic support,29 and attenuating microglial activation before ganglion cell loss.30 There are few reports of cell-based protection strategies in models of glaucoma. In a study from Yu et al.,31 GFP transgenic bone marrow-derived stromal cells (BMSCs) were transplanted into the vitreous of the eyes of Wistar rats with elevated intraocular pressure. In eyes that received transplanted BMSCs, there were elevated levels of FGF2, brain-derived neurotrophic factor (BDNF), and ciliary neurotrophic factor (CNTF).31 Furthermore, significantly more ganglion cells survived in the eyes that contained transplanted BMSCs than in those that received injections of vehicle.
We found that the proliferation of embryo-derived cells varies greatly, depending on the environment in which they are maintained. For example, the proliferation of embryonic retinal progenitors continues under culture conditions, beyond the normal developmental time-course of proliferation for cells in the intact retina. By contrast, the proliferation of embryonic retinal cells does not continue in the posthatch environment; we detected little BrdU incorporation when the embryo-derived cells were transplanted into the vitreous chamber of the posthatch eye. By contrast, the abundance of proliferating cells derived from embryonic WB and OT cells was not significantly different in culture compared with transplanted cells in the vitreous of the mature eye. Similarly, Ehrlich et al. 21 reported that the proliferation of OT cells continued when transplanted into the pecten of the postnatal eye. The pulse of BrdU that we used to label proliferating cells identified numbers of cells that continued to proliferate at the end of the experiment. Different administration paradigms for BrdU would be required to determine whether progenitors continued to divide within the vitreous of the mature eye immediately after transplantation. It is possible that cues produced by the mature retina inhibit proliferation. For example, a study by Close et al.32 indicated that TGFβ2 is produced by amacrine cells to inhibit the proliferation of late-stage retinal progenitors and complete the process of cell genesis. Alternatively, the factors and proteoglycans within the vitreous33 of the mature eye may inhibit the proliferation of embryo-derived retinal progenitors.
Although we detected the expression of different neuronal markers within the VCM, these cells might not have been able to fully differentiate and, thus, formed disorganized laminae in the outer VCM layers. Interactions of retinal cells with extraretinal cells and the establishment of intact basal laminae may be required to fully differentiate. For example, the intimate interaction between the photoreceptor precursors and the RPE is required for proper outer segment differentiation.34–36 Although cells within the VCMs express visinin, a gene that is normally expressed during early stages of photoreceptor differentiation, these cells fail to express interphotoreceptor retinoid binding protein or red/green opsin, markers of mature photoreceptors (data not shown). These findings suggest that the environment within the vitreous does not support the full differentiation of embryo-derived retinal cells. In addition, the destruction of basal laminae with the dissociation of donor cells for transplantation likely interferes with numerous developmental processes. For example, ganglion cell death and severe retinal dysplasia occurs when the inner limiting membrane is disrupted in developing chicks37,38 and mice.39,40 Thus, it is not surprising that lamination within the VCM was abnormal.
Embryonic retinal cells that were cultured and then transplanted frequently migrated into NMDA-damaged retinas but failed to differentiate as neurons. It remains possible that the transplanted cells remain in an undifferentiated state because the mature eye lacks the cues to drive differentiation. Consistent with this hypothesis, it has previously been shown that differentiation of retinal neurons is greatly slowed during late stages of embryonic and early postnatal development.41 For example, the differentiation of photoreceptors in far peripheral regions of the retina requires at least 20 additional days compared with the time needed for photoreceptors to fully differentiate during earlier stages of development in central regions of retina.41 The failure of naive progenitors to properly differentiate within the mature retina has been demonstrated in different damage and transplantation paradigms. For example, recent reports in the rodent have demonstrated widespread integration of neural stem cells into damaged retinas, with widespread failure of these cells to differentiate into retinal cells.42,43 Similarly, retinal progenitors that are transplanted into the subretinal space of RCS rats differentiate into glial cells but not neuronal cells, suggesting that the cues for neuronal differentiation are lacking in the mature, degenerating retina.44 Further, the integration, survival, and differentiation of brain-derived progenitors in the developing retinas of opossums are attenuated as the age of the host increases.45 There is some evidence in the rodent retina that the transplantation of retinal progenitors into degenerating retinas leads to the incorporation of donor cells that express some neuronal proteins, but few of these cells acquire neuronal morphology.46,47 These findings suggest that mature and damaged retinas do not provide a microenvironment that promotes the differentiation of naive neural progenitors. Alternatively, the signals that drive that maturation and differentiation of retinal neurons are absent, or are at very low levels, in the postnatal chick eye.
It remains uncertain why greater numbers of transplanted cells migrated into NMDA-damaged retinas than into colchicine-damaged retinas. It is possible that differences in the types of damage influenced the ability of donor cells to migrate. Additionally, it is possible that the different damage paradigms influenced the survival of donor cells within the retina. For example, colchicine-damaged retinas may not support the survival of transplanted cells that migrate into the retina. However, it seems most likely that the preparation of the embryo-derived cells before transplantation influenced the ability of these cells to migrate into the retina. In the NMDA-damage paradigm, donor cells were maintained in culture for 4 to 24 days before transplantation, whereas in the colchicine-damage paradigm, the donor cells were transplanted immediately after dissection and dissociation. It is possible that culture conditions enhance the ability of cells to migrate. In support of this notion, Akagi et al.48 showed increased migration for cells maintained in culture for 10 passages when compared with cells passaged only three times.
The efficacy of neuroprotection varied with the developmental stage at which the transplanted retinal cells were obtained. Interestingly, the most protective source of transplanted cells was from a developmental stage (E10) when the overproduction of ganglion cells is pruned through programmed cell death.49 From E7 to E12, the retinal ganglion cell axons reach their target, the OT, and begin to compete for proper synaptic partners and target-derived support. Our data suggest that secreted factors from the tissue of origin are produced to support the survival of ganglion cells. Some evidence has suggested that growth hormone is protective to embryonic ganglion cells.50–54 In addition, factors that inhibit TGFβ and BMP signaling may be neuroprotective because these factors inhibit the death of ganglion cells in the embryonic retina.55 Similarly, BDNF has been shown to be necessary for ganglion cell survival in the developing retina.33,56,57 Interestingly, embryonic tectal cells did not potently support the survival of mature ganglion cells. This finding suggests that the trophic support provided by embryonic tectal cells may not be freely diffusible, may be abolished during the dissociation and transplantation procedures, or may not stimulate the survival of mature ganglion cells.
The survival-promoting factors that are produced by the embryo-derived cells are likely to be secreted and diffusible. Our in vivo studies indicate that the protective effects of the embryo-derived cells are mediated by diffusible factors because, in nearly all instances, the transplanted cells did not contact the retina. It remains uncertain whether the protective effects of the embryo-derived cells acted directly on the ganglion cells or indirectly by influencing the activities of retinal glia. For example, we have recently reported that the neuroprotective effects of FGF2 are likely mediated through changes in the activity of the Müller glia, not through signaling directly at the retinal neurons.22 Similarly, previous studies have demonstrated that exogenous glial cell line-derived neurotrophic factor, BDNF, and CNTF are neuroprotective to different types of retinal neurons,33,57–61 and the receptors or downstream second messengers are expressed primarily by the Müller glia.62–65 Consistent with our observations, coculture and in vivo transplantation studies using neural stem/progenitor cells provide elevated levels of CNTF, vascular endothelial growth factor, and superoxide dismutase 2 to striatal neurons, leading to greater neuronal survival.66 However, in our studies and those of others, it remains uncertain whether factors from the transplanted cells stimulate the survival of neurons in the host tissues directly or indirectly by modifying glial functions.
It remains possible that neuroprotection may be supported by different types of cells. Most transplantation studies have focused on replacement strategies by which the sources of transplanted cells have been derived from embryonic, undifferentiated cells of the tissue that is diseased. Given that survival and cell death are processes that occur normally during development, it is not surprising that embryonic cells produce trophic factors that influence the survival of mature cells in the same tissue. It remains possible that nonneural or heterologous neural and glial cell types produce neuroprotective factors. For example, human mesenchymal cells cocultured with microglia have been shown to promote the survival of dopaminergic neurons in culture paradigms and in an experimental model for Parkinson's disease.67 However, our data indicate that immature retinal cells better promote the survival of mature retinal neurons than cells obtained from embryonic tectum or wing bud. The identity of the cells that provide trophic support to the ganglion cells remains uncertain. We assume that either the late-stage retinal progenitors or immature Müller glia are the cellular sources of trophic support. However, we cannot exclude the possibility that immature retinal neurons are a source of neuroprotective factors.
Conclusions
We conclude that the microenvironment provided by the postnatal eye does not support the proliferation and differentiation of embryo-derived retinal cells. Furthermore, we conclude that, at particular stages of development, embryonic retinal cells effectively promote the survival of ganglion cells; the survival-promoting effects of embryonic tectal and wing bud cells are modest by comparison. We propose that embryo-derived retinal cells produce diffusible factors that promote the survival of mature ganglion cells. We further propose that cell transplantation is a viable strategy not only for cell replacement but also to promote cell survival in diseased and degenerating retinas.
Supplementary Material
Acknowledgments
The AP2-α, BrdU, vimentin, and visinin antibodies developed by Trevor Williams, Stephen J. Kaufman, Joshua R. Sanes, and Constance Cepko, respectively, were obtained from the Developmental Studies Hybridoma Bank under auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences. The authors thank Paul Mozdiak of the NCSU Department of Poultry Sciences for kindly providing eGFP transgenic eggs and Paul Linser of the University of Florida, for kindly providing the 2M6 antibody. The Ad5-eGFP virus and RCAS-eGFP plasmid were provided by K. Reed Clark (Columbus Children's Hospital) and Teri Belecky-Adams (Indiana University-Purdue University), respectively. Confocal microscopy was performed at the Hunt-Curtis Imaging Facility at the Department of Neuroscience of The Ohio State University. Statistical analyses were performed with help from Chris Holloman at The Ohio State University Statistical Consulting Service. Additionally, the authors thank Megan Cermak, Sarada Eleswarpu, and Christopher Zelinka for providing technical support.
Footnotes
Supported by National Institutes of Health, National Eye Institute Grant EY016043-03 (AJF), the OSU Graduate School Presidential Fellowship (2008; JJS), and the Prevent Blindness Ohio Young Investigators Student Fellowship Award for Female Scholars in Vision Research (2006, 2008; JJS)
Disclosure: J.J. Stanke, None; A.J. Fischer, None
References
- 1.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006;444:203–207 [DOI] [PubMed] [Google Scholar]
- 2.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 2009;4:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiler MJ, Aramant RB. Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function. Semin Ophthalmol 2005;20:31–42 [DOI] [PubMed] [Google Scholar]
- 4.Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res 2004;23:475–494 [DOI] [PubMed] [Google Scholar]
- 5.Arai S, Thomas BB, Seiler MJ, et al. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp Eye Res 2004;79:331–341 [DOI] [PubMed] [Google Scholar]
- 6.Mohand-Said S, Hicks D, Simonutti M, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res 1997;29:290–297 [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Shan Q, Ma P, et al. Differentiation potential of bone marrow mesenchymal stem cells into retina in normal and laser-injured rat eye. Sci China C Life Sci 2004;47:241–250 [DOI] [PubMed] [Google Scholar]
- 8.Gamm DM, Wang S, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE 2007;2:e338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Girman S, Lu B, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci 2008;49:3201–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull ND, Irvine KA, Franklin R, Martin K. Transplanted oligodendrocyte precursor cells reduce neurodegeneration in a model of glaucoma. Invest Ophthalmol Vis Sci 2009;50:4244–4253 [DOI] [PubMed] [Google Scholar]
- 11.Chapman SC, Lawson A, Macarthur WC, et al. Ubiquitous GFP expression in transgenic chickens using a lentiviral vector. Development 2005;132:935–940 [DOI] [PubMed] [Google Scholar]
- 12.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951;88:49–92 [PubMed] [Google Scholar]
- 13.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 1992;195:231–272 [DOI] [PubMed] [Google Scholar]
- 14.Fischer AJ, Skorupa D, Schonberg DL, Walton NA. Characterization of glucagon-expressing neurons in the chicken retina. J Comp Neurol 2006;496:479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 2001;4:247–252 [DOI] [PubMed] [Google Scholar]
- 16.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci 2002;22:9387–9398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia 2003;43:70–76 [DOI] [PubMed] [Google Scholar]
- 18.Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res 2005;24:161–182 [DOI] [PubMed] [Google Scholar]
- 19.Morgan IG. Intraocular colchicine selectively destroys immature ganglion cells in chicken retina. Neurosci Lett 1981;24:255–260 [DOI] [PubMed] [Google Scholar]
- 20.Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res 1999;39:685–697 [DOI] [PubMed] [Google Scholar]
- 21.Ehrlich D, Sattayasai J, Gurusinghe C, Zappia J. The avian pecten provides a potent substrate for growth and development of dissociated embryonic neural implants. Brain Res 1987;430:139–144 [DOI] [PubMed] [Google Scholar]
- 22.Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia 2009;57:166–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willbold E, Rothermel A, Tomlinson S, Layer PG. Muller glia cells reorganize reaggregating chicken retinal cells into correctly laminated in vitro retinas. Glia 2000;29:45–57 [PubMed] [Google Scholar]
- 24.Liu W, Khare SL, Liang X, et al. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development 2000;127:3237–3247 [DOI] [PubMed] [Google Scholar]
- 25.Xiang M, Zhou L, Macke JP, et al. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci 1995;15:4762–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron 1993;11:689–701 [DOI] [PubMed] [Google Scholar]
- 27.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res 2008;173:495–510 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell CH, Lu W, Hu H, Zhang X, Reigada D, Zhang M. The P2X(7) receptor in retinal ganglion cells: a neuronal model of pressure-induced damage and protection by a shifting purinergic balance. Purinergic Signal 2009;5:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper NG, Laabich A, Fan W, Wang X. The relationship between neurotrophic factors and CaMKII in the death and survival of retinal ganglion cells. Prog Brain Res 2008;173:521–540 [DOI] [PubMed] [Google Scholar]
- 30.Bosco A, Inman DM, Steele MR, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 2008;49:1437–1446 [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 2006;344:1071–1079 [DOI] [PubMed] [Google Scholar]
- 32.Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development 2005;132:3015–3026 [DOI] [PubMed] [Google Scholar]
- 33.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 1993;602:304–317 [DOI] [PubMed] [Google Scholar]
- 34.Hollyfield JG, Witkovsky P. Pigmented retinal epithelium involvement in photoreceptor development and function. J Exp Zool 1974;189:357–378 [DOI] [PubMed] [Google Scholar]
- 35.Bumsted KM, Rizzolo LJ, Barnstable CJ. Defects in the MITF(mi/mi) apical surface are associated with a failure of outer segment elongation. Exp Eye Res 2001;73:383–392 [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Nookala S, Narayanan C, et al. Proteomic analysis of the retina: removal of RPE alters outer segment assembly and retinal protein expression. Glia 2009;57:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halfter W. Disruption of the retinal basal lamina during early embryonic development leads to a retraction of vitreal end feet, an increased number of ganglion cells, and aberrant axonal outgrowth. J Comp Neurol 1998;397:89–104 [DOI] [PubMed] [Google Scholar]
- 38.Halfter W, Dong S, Balasubramani M, Bier ME. Temporary disruption of the retinal basal lamina and its effect on retinal histogenesis. Dev Biol 2001;238:79–96 [DOI] [PubMed] [Google Scholar]
- 39.Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest Ophthalmol Vis Sci 2005;46:1000–1009 [DOI] [PubMed] [Google Scholar]
- 40.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol 1998;8:983–986 [DOI] [PubMed] [Google Scholar]
- 41.Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res 2008;1192:76–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu B, Kwan T, Kurimoto Y, Shatos M, Lund RD, Young MJ. Transplantation of EGF-responsive neurospheres from GFP transgenic mice into the eyes of rd mice. Brain Res 2002;943:292–300 [DOI] [PubMed] [Google Scholar]
- 43.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci 2000;16:197–205 [DOI] [PubMed] [Google Scholar]
- 44.Yang P, Seiler MJ, Aramant RB, Whittemore SR. Differential lineage restriction of rat retinal progenitor cells in vitro and in vivo. J Neurosci Res 2002;69:466–476 [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi DS, Van Hoffelen SJ, Young MJ. Differentiation and morphological integration of neural progenitor cells transplanted into the developing mammalian eye. Ann N Y Acad Sci 2003;995:127–139 [DOI] [PubMed] [Google Scholar]
- 46.Kinouchi R, Takeda M, Yang L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci 2003;6:863–868 [DOI] [PubMed] [Google Scholar]
- 47.Chacko DM, Rogers JA, Turner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun 2000;268:842–846 [DOI] [PubMed] [Google Scholar]
- 48.Akagi T, Haruta M, Akita J, Nishida A, Honda Y, Takahashi M. Different characteristics of rat retinal progenitor cells from different culture periods. Neurosci Lett 2003;341:213–216 [DOI] [PubMed] [Google Scholar]
- 49.Mayordomo R, Valenciano AI, de la Rosa EJ, Hallbook F. Generation of retinal ganglion cells is modulated by caspase-dependent programmed cell death. Eur J Neurosci 2003;18:1744–1750 [DOI] [PubMed] [Google Scholar]
- 50.Sanders EJ, Parker E, Harvey S. Growth hormone-mediated survival of embryonic retinal ganglion cells: signaling mechanisms. Gen Comp Endocrinol 2008;156:613–621 [DOI] [PubMed] [Google Scholar]
- 51.Chavarria T, Valenciano AI, Mayordomo R, et al. Differential, age-dependent MEK-ERK and PI3K-Akt activation by insulin acting as a survival factor during embryonic retinal development. Dev Neurobiol 2007;67:1777–1788 [DOI] [PubMed] [Google Scholar]
- 52.Harvey S, Baudet ML, Sanders EJ. Growth hormone and cell survival in the neural retina: caspase dependence and independence. Neuroreport 2006;17:1715–1718 [DOI] [PubMed] [Google Scholar]
- 53.Sanders EJ, Parker E, Harvey S. Retinal ganglion cell survival in development: mechanisms of retinal growth hormone action. Exp Eye Res 2006;83:1205–1214 [DOI] [PubMed] [Google Scholar]
- 54.Sanders EJ, Parker E, Aramburo C, Harvey S. Retinal growth hormone is an anti-apoptotic factor in embryonic retinal ganglion cell differentiation. Exp Eye Res 2005;81:551–560 [DOI] [PubMed] [Google Scholar]
- 55.Beier M, Franke A, Paunel-Gorgulu AN, Scheerer N, Dunker N. Transforming growth factor beta mediates apoptosis in the ganglion cell layer during all programmed cell death periods of the developing murine retina. Neurosci Res 2006;56:193–203 [DOI] [PubMed] [Google Scholar]
- 56.Frade JM, Bovolenta P, Martínez-Morales JR, Arribas A, Barbas JA, Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development 1997;124:3313–3320 [DOI] [PubMed] [Google Scholar]
- 57.LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci 1998;39:592–602 [PubMed] [Google Scholar]
- 58.Chong NH, Alexander RA, Waters L, Barnett KC, Bird AC, Luthert PJ. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest Ophthalmol Vis Sci 1999;40:1298–1305 [PubMed] [Google Scholar]
- 59.Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci 2002;43:3319–3326 [PubMed] [Google Scholar]
- 60.Seiler MJ, Thomas BB, Chen Z, et al. BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function. Exp Eye Res 2008;86:92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci 2004;27:531–542 [DOI] [PubMed] [Google Scholar]
- 62.Kahn MA, Huang CJ, Caruso A, et al. Ciliary neurotrophic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acidic protein in glial cells. J Neurochem 1997;68:1413–1423 [DOI] [PubMed] [Google Scholar]
- 63.Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA.Hyde CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res 2009;88:1051–1064 [DOI] [PubMed] [Google Scholar]
- 64.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci 2000;20:4081–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci 2000;41:927–936 [PubMed] [Google Scholar]
- 66.Madhavan L, Ourednik V, Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells 2008;26:254–265 [DOI] [PubMed] [Google Scholar]
- 67.Kim YJ, Park HJ, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009;57:13–23 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.