Abstract
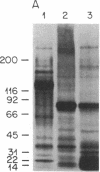
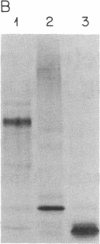
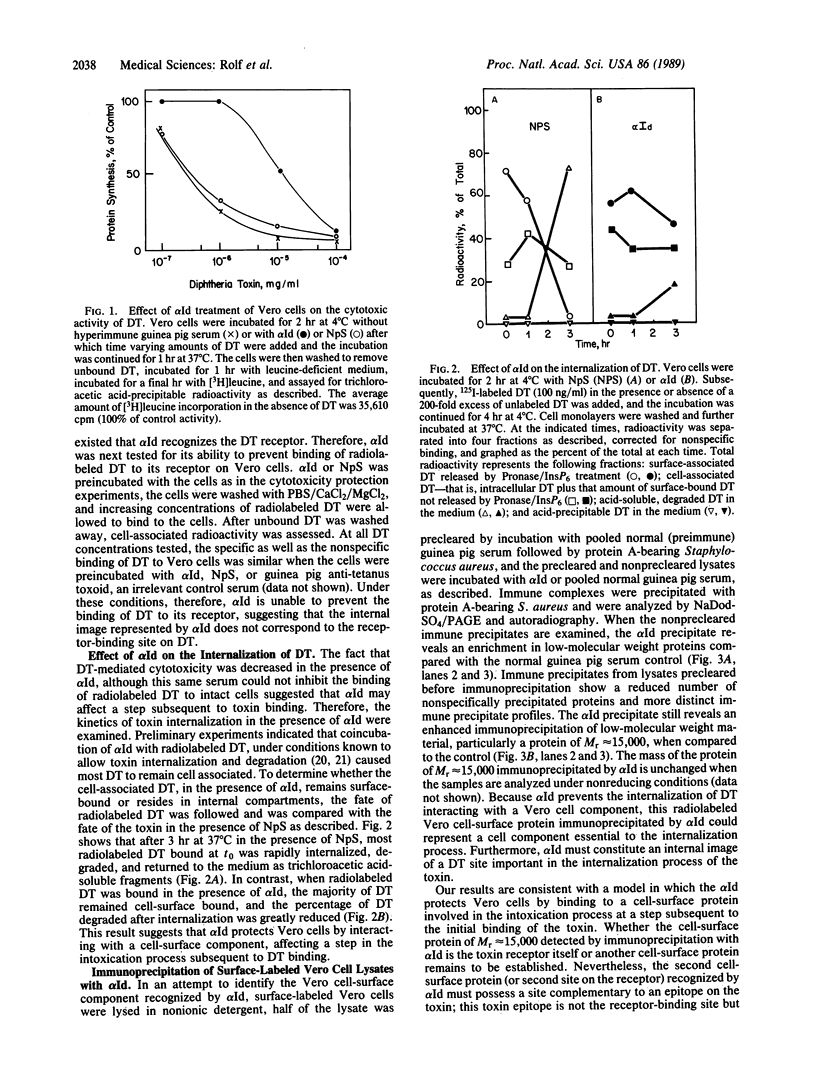
An anti-idiotypic serum prepared against the combining site (idiotype) of specific anti-diphtheria toxoid antibodies was characterized with respect to its interaction with highly diphtheria toxin-sensitive Vero cells. Although the anti-idiotypic serum protected Vero cells against the cytotoxic action of diphtheria toxin, it did not prevent the binding of 125I-labeled diphtheria toxin to the cells but did inhibit the internalization and degradation of 125I-labeled toxin. This anti-idiotypic serum immunoprecipitated a cell-surface protein from radiolabeled Vero cells with an apparent Mr of approximately 15,000. These results are consistent with the hypothesis that the anti-idiotypic serum contains antibodies that carry an internal image of an internalization site on the toxin and that a cell-surface protein involved in toxin internalization possesses a complementary site recognized by both the toxin and the anti-idiotypic antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cieplak W., Gaudin H. M., Eidels L. Diphtheria toxin receptor. Identification of specific diphtheria toxin-binding proteins on the surface of Vero and BS-C-1 cells. J Biol Chem. 1987 Sep 25;262(27):13246–13253. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J. R., Moehring J. M., Moehring T. J. Binding and uptake of diphtheria toxin by toxin-resistant Chinese hamster ovary and mouse cells. Mol Cell Biol. 1983 Jul;3(7):1283–1294. doi: 10.1128/mcb.3.7.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Eidels L., Hart D. A. Effect of polymers of L-lysine on the cytotoxic action of diphtheria toxin. Infect Immun. 1982 Sep;37(3):1054–1058. doi: 10.1128/iai.37.3.1054-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Ross L. L., Hart D. A. Diphtheria toxin-receptor interaction: a polyphosphate-insensitive diphtheria toxin-binding domain. Biochem Biophys Res Commun. 1982 Nov 30;109(2):493–499. doi: 10.1016/0006-291x(82)91748-x. [DOI] [PubMed] [Google Scholar]
- Falmagne P., Capiau C., Lambotte P., Zanen J., Cabiaux V., Ruysschaert J. M. The complete amino acid sequence of diphtheria toxin fragment B. Correlation with its lipid-binding properties. Biochim Biophys Acta. 1985 Jan 21;827(1):45–50. doi: 10.1016/0167-4838(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa S., Uchida T., Mekada E., Moynihan M. R., Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983 Apr 10;258(7):4311–4317. [PubMed] [Google Scholar]
- Islam M. N., Pepper B. M., Briones-Urbina R., Farid N. R. Biological activity of anti-thyrotropin anti-idiotypic antibody. Eur J Immunol. 1983 Jan;13(1):57–63. doi: 10.1002/eji.1830130113. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Maxfield F. R., Hardegree M. C., Habig W. H. Receptor-mediated endocytosis of diphtheria toxin by cells in culture. Proc Natl Acad Sci U S A. 1982 May;79(9):2912–2916. doi: 10.1073/pnas.79.9.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Ross G. D. Characterization of the lymphocyte membrane receptor for factor H (beta 1H-globulin) with an antibody to anti-factor H idiotype. J Exp Med. 1982 May 1;155(5):1400–1411. doi: 10.1084/jem.155.5.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada E., Okada Y., Uchida T. Identification of diphtheria toxin receptor and a nonproteinous diphtheria toxin-binding molecule in Vero cell membrane. J Cell Biol. 1988 Aug;107(2):511–519. doi: 10.1083/jcb.107.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain D., Kanwar Y. S., Blobel G. Identification of a receptor for protein import into chloroplasts and its localization to envelope contact zones. Nature. 1988 Jan 21;331(6153):232–237. doi: 10.1038/331232a0. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Proia R. L., Eidels L., Hart D. A. Diphtheria toxin:receptor interaction. Characterization of the receptor interaction with the nucleotide-free toxin, the nucleotide-bound toxin, and the B-fragment of the toxin. J Biol Chem. 1981 May 25;256(10):4991–4997. [PubMed] [Google Scholar]
- Schreiber A. B., Couraud P. O., Andre C., Vray B., Strosberg A. D. Anti-alprenolol anti-idiotypic antibodies bind to beta-adrenergic receptors and modulate catecholamine-sensitive adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7385–7389. doi: 10.1073/pnas.77.12.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Maron R., Elias D., Cohen I. R. Autoantibodies to insulin receptor spontaneously develop as anti-idiotypes in mice immunized with insulin. Science. 1982 Apr 30;216(4545):542–545. doi: 10.1126/science.7041258. [DOI] [PubMed] [Google Scholar]