The authors have found that antimicrobial peptide LL-37 partially restores high glucose–delayed wound healing in cultured pig corneas. This study suggests that LL-37, both antimicrobial and regenerative, is a potential therapeutic agent for hastening epithelial wound healing and preventing infection in patients with diabetic keratopathy.
Abstract
Purpose.
Patients with diabetes are at higher risk for delayed corneal reepithelialization and infection. Previous studies indicated that high glucose (HG) impairs epidermal growth factor receptor (EGFR) signaling and attenuates ex vivo corneal epithelial wound healing. The authors investigated the effects of antimicrobial peptide LL-37 on HG-attenuated corneal epithelial EGFR signaling and wound closure.
Methods.
Human corneal epithelial cells (HCECs) were stimulated with LL-37. Heparin-binding EGF-like growth factor (HB-EGF) shedding was assessed by measuring the release of alkaline phosphatase (AP) in a stable HCEC line expressing HB-EGF-AP. Activation of EGFR, phosphoinositide 3-kinase (PI3K), and extracellular signal-regulated kinases 1/2 (ERK1/2) was determined by Western blot analysis. Corneal epithelial wound closure was assessed in cultured HCECs and porcine corneas. LL-37 expression was determined by immune dot blot.
Results.
LL-37 induced HB-EGF-AP release and EGFR activation in a dose-dependent manner. LL-37 prolonged EGFR signaling in response to wounding. LL-37 enhanced the closure of a scratch wound in cultured HCECs and partially rescued HG-attenuated wound healing in an EGFR- and a PI3K-dependent manner and restored HG-impaired EGFR signaling in cultured porcine corneas. HG attenuated wounding-induced LL-37 expression in cultured HCECs.
Conclusions.
LL-37 is a tonic factor promoting EGFR signaling and enhancing epithelial wound healing in normal and high glucose conditions. With both antimicrobial and regenerative capabilities, LL-37 may be a potential therapeutic for diabetic keratopathy.
With rapid increases in the prevalence of diabetes mellitus (DM) worldwide, ocular complications have become a leading cause of blindness in the world.1 In addition to abnormalities of the retina (diabetic retinopathy) and the lens (cataract), various types of corneal epithelial disorders are also relatively common in persons with DM.2 Abnormalities of the cornea include defects in epithelium-basement membrane adhesion3–8 and altered epithelial functions such as basal cell degeneration,9 superficial punctate keratitis,10 breakdown of barrier function,11 fragility,12 recurrent erosions, and persistent epithelial defects.13 Furthermore, a considerable delay in corneal reepithelialization is often observed in patients with diabetes who undergo vitrectomy.2,13–15 Delayed healing of the epithelial defect may also result in sight-threatening complications, such as stromal opacification, surface irregularity, and microbial keratitis.15 Hence, accelerating epithelial healing is of great importance for effectively treating these complications.
We previously demonstrated that epithelial wounding induces the ectodomain shedding of heparin-binding EGF-like growth factor (HB-EGF), subsequent activation of epidermal growth factor receptor (EGFR), and its two major downstream effectors, phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK).16 Wound-induced EGFR activation plays a critical role in corneal epithelial wound healing.16–18 More recently, we discovered that, consistent with attenuating epithelial wound closure, high glucose also suppresses the EGFR-PI3K-AKT signaling pathway ex vivo.19 Importantly, hyperglycemia was found to disturb the expression and distribution of phospho-AKT in diabetic human corneas, suggesting an essential role of EGFR signaling in maintaining a healthy cornea.19 Echoing this concept is the finding that anti-EGFR cancer treatments resulted in diffuse punctuate keratitis and corneal erosion in patients.20,21 Thus, maintaining a proper level of EGFR signaling is critical for the physiologic state of an epithelium in tissue, and long-term exposure to hyperglycemia may inevitably affect the EGFR signaling apparatus, leading to cell dysfunction, including compromised barrier function and delayed wound healing.
LL-37, an epithelium and immune cell–secreted polypeptide from gene cathelicidin, belongs to a group of antimicrobial peptides that play an important role in host defense against infection.22,23 LL-37 plays a key role in innate immunity against a broad spectrum of microbes in a number of tissues, including the cornea,24 urinary tract,25 and skin.26,27 In addition to acting as a natural antibiotic for innate immune defense, emerging evidence suggests that LL-37 is an important cell-signaling molecule, especially during tissue damage.28–30 Moreover, LL-37 has been documented to interact with the G protein-coupled receptor and to transactivate EGFR, leading to keratinocyte migration.31 In human corneas, LL-37 peptide was detected throughout the epithelium, albeit at a low level, and its expression was upregulated in regrown human CECs.24 Wound-induced cathelicidin expression was also observed in B6 mice (Kumar A, Yu FS, unpublished data, 2009). To date, the process and release of LL-37 from cathelicidin in HCECs remains elusive. A recent report30 revealed that adenoviral transfer of LL-37 effectively promotes reepithelialization of excisional skin wounds in diabetic mice.
In the present study, we reported that LL-37 induces HB-EGF shedding, EGFR activation, and corneal epithelial wound closure in vitro. Moreover, we demonstrated that LL-37 partially restores high glucose–delayed wound healing ex vivo via EGFR signaling. Our results indicate that LL-37, both antimicrobial and regenerative, is a potential therapeutic for hastening epithelial wound healing and preventing infection in diabetic keratopathy.
Materials and Methods
Materials
Defined keratinocyte serum-free medium (SFM) and minimum essential medium (MEM) were purchased from Invitrogen (Grand Island, NY). Keratinocyte basal medium (KBM) was from BioWhittaker (Walkersville, MD). EGFR inhibitor AG1478 and PI3K inhibitor LY 294002 were from Calbiochem (La Jolla, CA). Antibodies against human EGFR, ERK2 (p42 MAPK), phosphorylated (P)-ERK1/2 (p42/p44), phosphorylated tyrosine (PY99), and protein A/G-agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against P-EGFR (tyrosine 845), P-AKT, and AKT were obtained from Cell Signaling (Beverly, MA). LL-37 was from AnaSpec (San Jose, CA). All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
THCE cells, an SV40-immortalized HCEC line,32 were grown in defined keratinocyte SFM in a humidified 5% CO2 incubator at 37°C and growth-factor starved in KBM for 16 hours before experiments. Primary HCECs were isolated from human donor corneas obtained from the Michigan Eye Bank. The epithelial sheet was separated from underlying stroma after overnight dispase treatment at 4°C. The dissected epithelial sheet was trypsinized, and cells were then collected by centrifugation. Primary HCECs were grown in defined keratinocyte SFM in a humidified 5% CO2 incubator at 37°C and then used at passage 3. All human tissue was handled in accordance to the tenets of the Declaration of Helsinki.
Measurement of HB-EGF-AP Shedding
A cell line expressing HB-EGF-AP fusion protein with alkaline phosphatase (AP) inserted into the heparin-binding region of HB-EGF was established by transfection of THCE cells with the expression plasmid pHB-EGF-AP.16 Growth factor–starved cells were challenged with LL-37 for 30 minutes. Culture medium was collected, and AP activity in collected media was measured using a chemiluminescence detection kit (Great EscAPe SEAP; BD Biosciences, Palo Alto, CA) according to the manufacturer's instruction. Briefly, 15 μL of the collected culture medium was heated with dilution buffer at 65°C for 30 minutes in a 96-well plate, followed by addition of assay buffer and substrate. Chemiluminescence was quantified on a fluorometer (GENios; Tecan, San Jose, CA). The readings, after subtracting background luminescence in KBM, were normalized against protein concentration and shown as fold increase over the control.
Determination of EGFR, AKT, and ERK Activation by Western Blot Analysis
To determine EGFR tyrosine phosphorylation, growth factor–starved THCE cells were stimulated with LL-37 or extensive wounding for the indicated time. Wounding was created by multiple linear scratches with a cut of 48-well shark tooth comb for DNA sequencing (Bio-Rad, Hercules, CA), going from one side of the dish to the other. The dish was then rotated, and scrapes were made the same way at 45°, 90°, and 135° to the original scrapes. Cells with no scrape wounds were used as the control. Damaged cells were washed away before the cells were fed with fresh KBM. The cells were lysed with RIPA buffer (150 mM NaCl, 100 mM Tris-HCl [pH 7.5], 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined using a protein assay kit (Micro BCA; Pierce, Rockford, IL). For each sample, 600 μg protein was immunoprecipitated with 10 μg antibody against EGFR followed by the addition of 20 μL protein A/G-agarose beads. Precipitants were immunoblotted with an antibody against PY99. The membrane was then stripped and reprobed with an antibody against EGFR to evaluate the total amount of EGFR precipitated. Phosphorylation of EGFR at tyrosine (Y) 845 was analyzed using specific antibody. Phosphorylation of ERK1/2 and AKT was determined using monoclonal antibodies against phospho-ERK1/2 and phospho-AKT; antibodies against ERK2 and AKT were used to detect equal protein loading of the respective phosphoproteins.
Wounding Assays
THCE cells were cultured to confluence and starved overnight in KBM. Cells were wounded by scratch with a sterile 0.1- to 10-μL pipet tip and allowed to heal for 24 hours in fresh KBM containing LL-37. The progress of migration was photographed immediately and again 24 hours after wounding at the same location along the wound edges with an inverted microscope equipped with a digital camera (SPOT; Diagnostic Instruments, Sterling Heights, MI). The extent of healing was defined as the ratio of the difference between the original and the remaining wound areas versus the original wound area.
Porcine Corneal Organ Culture
Porcine eyes were obtained from a local abattoir, transported to the laboratory on ice in a moist chamber, and processed for culture within 24 hours. An epithelial wound was made by demarcating an area on the central cornea with a trephine 5 mm in diameter and then removing the epithelium within the circle with a surgical scalpel, leaving an intact basement membrane.33 Corneas were processed for organ culture as previously described.34 Briefly, corneal-scleral rims, with the presence of approximately 4 mm of the limbal conjunctiva, were excised and cultured in MEM, leaving the epithelium exposed to air. Corneas were then cultured in a humidified 5% CO2 incubator at 37°C in MEM containing various reagents. MEM contained 5 mM d-glucose, and culture with MEM only was considered normal glucose (NG) condition. To mimic hyperglycemia, 20 mM glucose (high glucose [HG]; final glucose concentration, 25 mM) or 20 mM D-mannitol (high mannitol as an osmotic control) was added to MEM with or without LL-37 or inhibitors. Forty-eight hours after wounding, the corneas were stained with Richardson staining35 to mark the remaining wound area and then photographed under a dissecting microscope (Zeiss, Thornwood, NY) and a camera (MDS290; Kodak, Rochester, NY). Remaining denuded areas were quantified with the use of graphics editing software (Photoshop; Adobe, Mountain View, CA) and expressed in pixels. To assess the effects of high glucose and LL-37 on cell signaling ex vivo, epithelium was scraped off porcine corneas after organ culture, lysed in RIPA buffer, and processed for Western blot analysis. Band intensities were quantified with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Immunoblot Analysis of LL-37 Expression
THCE cells were cultured in normal or high glucose conditions for 7 days before they were extensively wounded with multiple linear scratches, as described. Cells were rinsed with PBS, re-fed with defined keratinocyte SFM containing NG or HG, and cultured for another 48 hours. Culture media were collected and centrifuged to remove cell debris. Cells were lysed in RIPA buffer, and protein concentration was determined. Equal amounts of culture media or cell lysates were applied to a nitrocellulose membrane (0.2 μm; Bio-Rad) by vacuum using a dot-blot apparatus (Bio-Rad). The membrane was fixed with 10% formalin and blocked in Tris-buffered saline containing 5% nonfat milk at room temperature. The membrane was then incubated overnight at 4°C with rabbit anti–human LL-37 antibody (PANATecs, Tübingen, Germany). After washing, the membrane was incubated for 1 hour at room temperature with goat anti–rabbit IgG conjugated to horseradish peroxidase. Immunoreactivity was visualized with reagents (Supersignal; Pierce, Rockford, IL). Culture media with or without LL-37 peptide served as positive and negative controls.
Statistical Analysis
Results are mean ± SEM. Statistical parameters were ascertained by statistical analysis software (SigmaStat; SyStat, San Jose, CA) with Student's t-test between two groups. P < 0.05 indicates a significant difference.
Results
Effect of LL-37 on HB-EGF Shedding
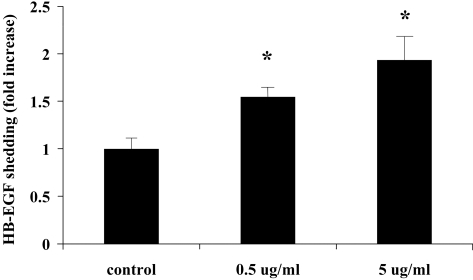
We previously showed that wounding elicited HB-EGF ectodomain shedding, and the released HB-EGF acted as an endogenous agonist for EGFR activation in an autocrine/paracrine manner.16 To determine whether LL-37 causes HB-EGF shedding, we stimulated the cell line expressing HB-EGF-AP with the peptide and measured AP activity in culture media. As shown in Figure 1, 0.5 μg/mL and 5 μg /mL LL-37 induced 1.5- and 2-fold increases in AP activity, respectively, suggesting that LL-37 stimulates pro-HB-EGF shedding in HCECs.
Figure 1.
LL-37 induces HB-EGF release in THCE cells expressing HB-EGF-AP. Growth factor–starved HB-EGFT-AP cells were treated with 0.5 or 5 μg/mL LL-37 for 30 minutes. HB-EGF shedding was measured and was expressed as mean ± SEM (n = 3) in fold increase. *P < 0.05. Results are representative of four independent experiments.
Activation of EGFR Signaling Pathways by LL-37
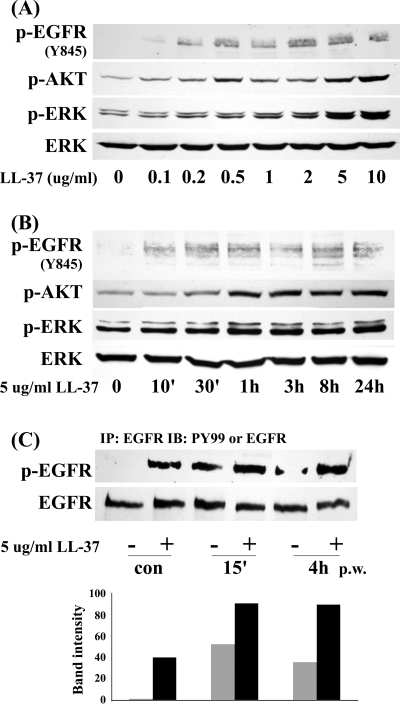
We next investigated the effects of LL-37 on EGFR signaling using cultured HCECs. Dose-response study (Fig. 2A) showed that LL-37 started activating EGFR and its downstream effectors AKT and ERK at 0.2 μg/mL and reached maximum effects at 5 and 10 μg/mL. Dosages higher than 10 μg/mL were shown to be cytotoxic to cultured HCECs.24 Time-course study (Fig. 2B) revealed that LL-37 at 5 μg/mL induced rapid (10 minutes, earliest time point test) and sustained (up to 24 hours) activation of EGFR signaling. We then sought to determine the combined effects of wounding and LL-37 in HCECs (Fig. 2C). Consistent with a previous study,16 extensive wounding resulted in rapid yet transient EGFR phosphorylation, prominent at 15 minutes postwounding (p.w.) and gradually declining at 4 hours p.w. The presence of LL-37 (5 μg/mL) increased wounding-induced EGFR phosphorylation at 15 minutes p.w. and maintained relatively higher levels of phospho-EGFR at 4 hours p.w., suggesting that LL-37 may potentially prolong EGFR signaling in response to wounding.
Figure 2.
LL-37 activates and prolongs EGFR signaling in HCECs. (A) Growth factor–starved THCE cells were stimulated with different concentrations of LL-37 (0.1 μg/mL to 10 μg/mL) for 1 hour. (B) Growth factor–starved primary HCECs were stimulated with 5 μg/mL LL-37 for various time points (10 minutes to 24 hours). Cells were then lysed and subjected to Western blot analysis with anti–phospho-EGFR (Y845), phospho-AKT1/2 (p-AKT), phospho-ERK1/2 (p-ERK), and ERK2 (as a loading control) antibodies. (C) THCE cells were stimulated with extensive wounding, 5 μg/mL LL-37, or both for 15 minutes or 4 hours before they were lysed. THCE lysates were immunoprecipitated with EGFR antibody, immunoblotted with anti–PY99 antibody (p-EGFR), and reprobed with EGFR antibody (EGFR) to access the amount of EGFR precipitated. Bar graph represents the average band densities of p-EGFR normalized against total EGFR from two independent experiments.
Promotion of Spontaneous Wound Healing by LL-37 In Vitro
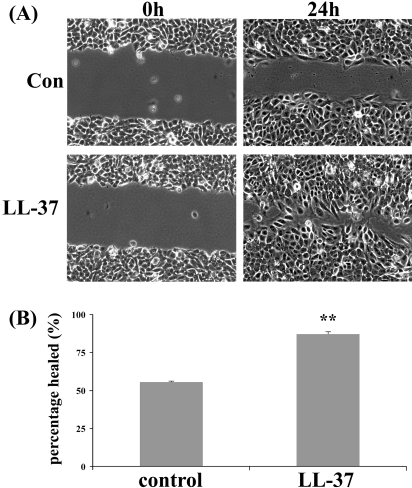
A previous study by Huang et al.24 showed that LL-37 promotes HCEC migration but not proliferation. To evaluate the effect of LL-37 on wound healing, we first tested its effects on the healing of a scrape wound made with a pipet tip in the monolayer of HCECs. As shown in Figure 3, though control cells cultured in KBM healed 55% after 24 hours, LL-37 (2 μg/mL)-treated ones healed 87%, indicating that LL-37 promotes the closure of scratch wounds in cultured HCECs.
Figure 3.
LL-37 enhances epithelial wound closure in a THCE cell monolayer. Growth factor–starved THCE cells were wounded with a pipet tip and allowed to heal in KBM containing 2 μg/mL LL-37. Wound closure was photographed immediately after wounding (0h) or 24 hours p.w. (24h). (A) Micrographs represent 1 of 3 samples performed each time. (B) Represents the statistical analysis of healing extent. Values are expressed as mean ± SEM (n = 3). **P < 0.01.
Acceleration of High Glucose–Delayed Corneal Epithelial Wound Healing by LL-37 Ex Vivo
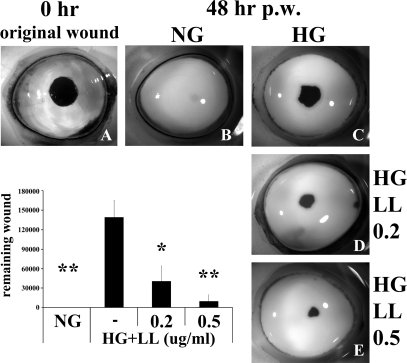
We previously used cultured porcine corneas as an ex vivo model to study epithelial wound healing, and this air/liquid corneal organ culture model has been shown to preserve the structural integrity and the responsiveness of epithelial cells to wounding and other stimulations.16,17,34,36 Similar to cultured cells, LL-37 was found to stimulate epithelial wound closure in cultured porcine corneas (data not shown). Recently, using this model, we demonstrated that epithelial wound closure was significantly impaired when porcine corneas were cultured in a high glucose condition (25 mM glucose) compared with those cultured in normal glucose (5 mM glucose) or in high mannitol (5 mM glucose plus 20 mM D-mannitol, serving as an osmotic control).19 Having demonstrated that LL-37 promotes wound closure in cultured HCECs, we next investigated whether it rescues HG-delayed wound healing (Fig. 4) in cultured porcine corneas. Compared with the corneas cultured in NG, which completely healed a 5-mm wound within 48 hours, HG-cultured corneas displayed significantly retarded wound closure. LL-37 at 0.2 and 0.5 μg/mL significantly accelerated the HG-delayed wound healing. Relatively lower concentrations of LL-37 were used because we observed that organ cultured corneas are more sensitive than cell cultures in response to various stimuli.19
Figure 4.
LL-37 reduces high glucose–mediated delay in epithelial wound healing in porcine corneal organ culture. Epithelial wound of 5-mm diameter was made (A, original wound) in the center of porcine corneas and allowed to heal for 48 hours in MEM containing NG (B; 5 mM d-glucose), or high glucose (C; 25 mM d-glucose) in the presence of 0.2 μg/mL (D) or 0.5 μg/mL (E) LL-37. Organ-cultured corneas were stained with Richardson staining solution to show the remaining wounds. Micrographs represent 1 of 3 samples performed each time. Bar graph represents the statistical analysis of the extent of healing. Values are expressed as mean ± SEM. *P < 0.05 and **P < 0.01 (Student's t-test) compared with HG. Results are representative of five independent experiments.
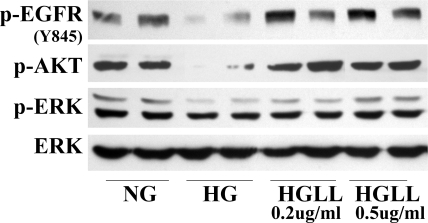
Reversal of High Glucose–Impaired EGFR Signaling by LL-37 Ex Vivo
Given that the effects of LL-37 on ex vivo corneal epithelial wound healing were dramatic and highly reproducible, we sought to determine the underlying mechanisms by assessing EGFR signaling of organ-cultured porcine corneal epithelium (Fig. 5). Consistent with our previous results,19 the activation of EGFR and AKT was significantly decreased in HG-cultured corneas. ERK activation was also attenuated, but to a much lesser extent. LL-37, at both concentrations tested (0.2 and 0.5 μg/mL), upregulated the phosphorylation levels of EGFR and AKT to the control level, and higher concentrations of LL-37 also restored ERK activation, suggesting that LL-37 may prevent HG-targeted impairment of EGFR signaling ex vivo.
Figure 5.
LL-37 restores high glucose–impaired EGFR signaling in cultured porcine corneas. Epithelial cells were scraped from the porcine corneas (see Fig. 4) and lysed for Western blot analysis with antibodies against p-EGFR (Y845), p-AKT, p-ERK, and ERK. Two representative corneas are shown.
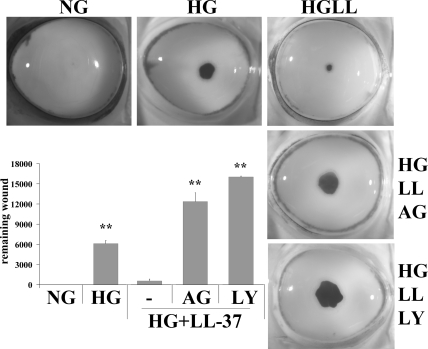
Involvement of EGFR Signaling in LL-37–Enhanced Epithelial Wound Healing
Having demonstrated that LL-37 restores EGFR signaling in high glucose condition, particularly EGFR and PI3K/AKT, we sought to determine the effects of EGFR and PI3K inhibitors on LL-37–rescued epithelial wound closure of porcine corneas cultured in high glucose (Fig. 6). The presence of AG1478 (EGFR inhibitor) or LY294002 (PI3K inhibitor) abolished LL-37–accelerated wound closure, resulting in a greatly retarded healing rate that was worse than that of corneas cultured in HG, suggesting an essential role of the EGFR-PI3K-AKT pathway in the regenerative functions of LL-37 in CECs.
Figure 6.
LL-37-enhanced epithelial wound closure in high glucose occurs via EGFR signaling. Corneal epithelial wound of 5-mm diameter was made and allowed to heal for 48 hours in MEM containing (5 mM d-glucose) or HG (25 mM d-glucose) in the presence of LL-37 (0.5 μg/mL) with AG1478 (1 μM) or LY294002 (40 μM). Wounded corneas were stained with Richardson staining solution to show the remaining wounds. Micrographs represent 1 of 3 samples performed each time. Bar graph represents the statistical analysis of the extent of healing. Values are expressed as mean ± SEM. **P < 0.01 (Student's t-test, compared with HG+LL-37). Results are representative of two independent experiments.
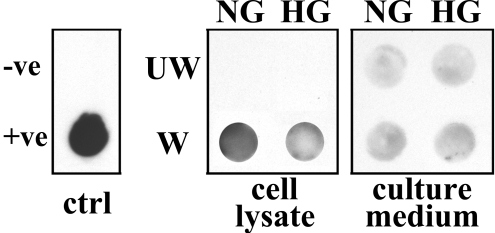
Effect of High Glucose on Wounding-Induced LL-37 Expression in Cultured HCECs
To determine the effects of high glucose on the expression and secretion of LL-37 in THCE cells, immune-dot blot analysis was performed (Fig. 7). A significant increase in LL-37 expression was detected in extensive injured THCE cells, consistent with an earlier report by Huang et al.24 that LL-37 level was upregulated in regrown corneal epithelial samples. This wounding-induced production of LL-37, however, was somewhat attenuated in cells continually cultured in high glucose. Accumulation of LL-37 in culture media was slightly upregulated after injury but was unaffected by HG.
Figure 7.
LL-37 expression is downregulated in high-glucose culture. Lysates or culture media from unwounded (UW) or wounded (W) THCE cells cultured in NG or HG were subjected to immune-dot blot analysis with LL-37 antibody. Culture media with or without LL-37 peptide served as positive (+ve) and negative (−ve) controls (ctrl).
Discussion
In this study, we investigated the effects of the antimicrobial peptide LL-37 on corneal epithelial wound healing in vitro and ex vivo. We demonstrated that LL-37 induced HB-EGF shedding, activated EGFR signaling including PI3K/AKT and ERK pathways, and promoted wound healing in cultured human CECs. Importantly, we showed that high glucose suppressed the wounding-induced upregulation of LL-37 and exogenously added LL-37, partially restored high glucose-impaired wound healing, and disrupted EGFR signaling in cultured porcine corneas. Taken together, we conclude that LL-37 promotes corneal epithelial wound healing via the HB-EGF→EGFR→PI3K→AKT signaling pathway and that topical application of LL-37, alone or in combination with other tonic factors, may be used as a remedy to accelerate delayed wound healing observed in diabetic corneas.
In addition to its acting as a natural antibiotic, emerging evidence suggests that LL-37 is an important cell-signaling molecule that stimulates the recruitment of circulating immune effector cells23,24,37–40 and the migration of keratinocytes and CECs in vitro.30,31 An earlier study28 revealed that LL-37 was strongly expressed in healing epithelium but was lacking in chronic ulcer epithelium in the skin. Consistent with a study24 showing that injury caused the upregulation of cathelicidin in cultured HCECs, we showed that cellular production of LL-37 was greatly upregulated. However, the levels of LL-37 in the culture media, if any, appear to be low and only slightly upregulated by wounding, suggesting that additional stimulation, such as pathogen challenge, may be required for cathelicidin to be processed and released as an antimicrobial peptide. Moreover, the wounding-induced upregulation of LL-37 was dampened by high glucose in cultured human CECs, suggesting that the lack of proper expression of antimicrobial peptides in response to wounding may contribute to impaired innate defense in patients with diabetes. To date, little is known about antimicrobial peptide expression in diabetes.41 Functionally, LL-37 was found to induce keratinocytes and lung epithelial proliferation and migration through a G protein–coupled receptor (GPCR) and EGFR.29–31 Similar to that shown for immune cells,42 the GPCR for LL-37 in keratinocytes and CECs is believed to be formyl peptide receptor-like 1 (FPRL1).24,30 In human CECs, LL-37 was reported to stimulate cell migration, but not proliferation, in an EGFR-dependent manner.24 Hence, LL-37 represents another group of factors with a regenerative role in epithelial cells via its ability to transactivate EGFR and its downstream effectors. The ligands involved in GPCR-mediated EGFR activation include mostly HB-EGF and transforming growth factor-alpha (TGF-α). Our study suggests a role for HB-EGF in LL-37-FPRL1-EGFR signaling pathway in CECs. Another known endogenous mediator for EGFR transactivation, TGF-α, may also be involved because its expression, like that of HB-EGF, is also upregulated in the healing corneal epithelium,43 and investigation of its role in corneal epithelial wound healing is warranted.
We previously showed that wounding triggers the rapid and transient phosphorylation of EGFR and the activation of its downstream signaling pathways, including ERK and PI3K pathways.16 The activation of EGFR pathways is a key event required for proper wound healing.16 We18 and others44 have shown that the release of adenosine triphosphate (ATP) from injured cells may act as an early cell damage signal, contributing at least partially to the wound-induced EGFR activation. As with ATP, we observed the rapid induction of LL-37 in mouse corneas in response to injury in vivo (Kumar A, Yu FS, unpublished results, 2009). Interestingly, the presence of LL-37, unlike that of extracellular ATP or EGFR ligands, resulted in elevated EGFR activation up to 24 hours. We propose that the prolonged activation of EGFR signaling may be an underlying mechanism for LL-37 to enhance cell migration, proliferation, or both, two key events leading to wound closure. In vivo, wounding of an epithelium is likely to first induce LL-37 expression in the cells surrounding the injury site. Its levels at the ocular surface are expected to rise, when polymorphonuclear leukocytes are infiltrated to the site of injury, providing an additional and likely a sustained source of LL-37, leading to a prolonged activation of EGFR during the healing process. Thus, LL-37, by acting as a multifunctional molecule, plays a role in protecting the ocular surface from injury and injury-associated infection.
Studies in both humans and animal models of types 1 and 2 diabetes revealed that delayed wound healing is a major corneal complication in diabetes.5,6,19,45–61 However, the limitation in the tissue size of diseased animals has hampered the biochemical analysis of hyperglycemia-induced alterations in the cornea. Organ culture represents a relevant experimental approach to study the influence of a high-glucose environment on the cornea.6,62,63 Kabosova et al.6 showed that organ-cultured human corneas from diabetic retinopathy patients exhibited much delayed epithelial wound healing, in complete accordance with clinical data in patients with diabetes. Moreover, patient corneas in organ culture preserved the same marked abnormalities and altered expression of proteinases and growth factors as in vivo.6 We used cultured porcine corneas as an ex vivo model to study epithelial wound healing and showed recently that high glucose impairs the EGFR-PI3K-AKT pathway in cultured corneas, a phenomenon that was confirmed by immunohistochemistry of the corneas from patients with diabetes.19 Hence, corneal organ culture may serve as an excellent model to identify reagents and treatments that may facilitate high glucose-impaired wound healing. The data presented here show that LL-37, at a nontoxic dosage, significantly but incompletely restored corneal epithelial wound healing delayed by high glucose in an ex vivo setting. Our study is in line with a recent skin wound healing study showing that adenoviral transfer of LL-37 significantly improved reepithelialization in an ob/ob mouse diabetes model.30 The use of adenoviral delivery limits clinic application and is also unable to distinguish whether the peptide LL-37 is actually present and responsible for the effects observed in vivo.64 The present study suggests that the topical application of LL-37 might be an effective way to treat corneal epithelial abrasions in patients with diabetes who are undergoing vitrectomy.
Our recent study led us to propose that high glucose targets specifically the EGFR-PI3K-AKT pathways, leading to epithelial abnormalities and diabetic keratopathy. The role of EGFR in corneal homeostasis was further confirmed by the clinical observation that targeting EGFR with cetuximab (an EGFR monoclonal antibody) and gefitinib (an EGFR kinase inhibitor) for cancer treatments resulted in ocular abnormalities in patients, including diffuse punctate keratitis and corneal erosion.20,21 These phenotypes are commonly found in patients with diabetic keratopathy.10,13 Topical application of EGF improved cetuximab-caused persistent epithelial defects in a patient with advanced colorectal carcinoma.65 Hence, the ability of LL-37 to negate high glucose–induced corneal epithelial wound healing delay is likely related to its activity to induce EGFR transactivation. Indeed, our organ culture study revealed that LL-37 restored the high glucose–targeted phosphorylation of EGFR, AKT, and ERK. Furthermore, we demonstrated that LL-37–enhanced epithelial wound closure in high glucose was sensitive to EGFR and PI3K inhibition. Thus, treatments that enhance EGFR signaling may be used as therapeutic agents for diabetic complications and delayed wound healing in the cornea and in the skin. This will be tested in an animal model of diabetic corneal wound healing.57,66
In summary, we showed that LL-37 is a tonic for the corneal epithelium and that its topical application may become a mainstream therapeutic modality for healing postsurgical and persistent corneal epithelial defects and for the prevention of corneal infection associated with these defects in patients with diabetes.
Acknowledgments
The authors thank Jenny Huang for editorial assistance.
Footnotes
Supported by National Institutes of Health Grants R01EY10869 and EY17960 and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Wayne State University.
Disclosure: J. Yin, None; F.-S.X. Yu, None
References
- 1.Clark CM, Lee DA. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med 1995;332:1210–1217 [DOI] [PubMed] [Google Scholar]
- 2.Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol 2005;89:254–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend J, Ishii Y, Thoft RA. Corneal epithelial changes in diabetic rats. Ophthalmic Res 1982;14:269–278 [DOI] [PubMed] [Google Scholar]
- 4.Taylor HR, Kimsey RA. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci 1981;20:548–553 [PubMed] [Google Scholar]
- 5.Ljubimov AV, Huang Z-S, Huang GH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem 1998;46:1033–1042 [DOI] [PubMed] [Google Scholar]
- 6.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res 2003;77:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar DT, Spurr MSJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 1992;110:537–540 [DOI] [PubMed] [Google Scholar]
- 8.McDermott AM, Xiao TL, Kern TS, Murphy CJ. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry 2003;74:443–452 [PubMed] [Google Scholar]
- 9.Friend J, Ishii Y, Thoft RA. Corneal epithelial changes in diabetic rats. Ophthalmic Res 1982;14:269–278 [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Okugawa K, Amano S, et al. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye 2005;19:418–421 [DOI] [PubMed] [Google Scholar]
- 11.Rehany U, Ishii Y, Lahav M, Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea 2000;19:534–538 [DOI] [PubMed] [Google Scholar]
- 12.Saini J, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol 1995;30:142–146 [PubMed] [Google Scholar]
- 13.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc 1981;79:180–199 [PMC free article] [PubMed] [Google Scholar]
- 14.Brightbill FS, Myers FL, Bresnick GH. Postvitrectomy keratopathy. Am J Ophthalmol 1978;85:651–655 [DOI] [PubMed] [Google Scholar]
- 15.Pflugfelder SC. Is autologous serum a tonic for the ailing corneal epithelium? Am J Ophthalmol 2006;142:316–317 [DOI] [PubMed] [Google Scholar]
- 16.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci 2004;45:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci 2007;48:636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci 2007;120:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses EGFR-PI3K-AKT signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009;58:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NT, Kris MG, Pao W, et al. Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol 2005;23:165–174 [DOI] [PubMed] [Google Scholar]
- 21.Specenier P, Koppen C, Vermorken JB. Diffuse punctate keratitis in a patient treated with cetuximab as monotherapy. Ann Oncol 2007;18:961–962 [DOI] [PubMed] [Google Scholar]
- 22.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol 2002;110:823–831 [DOI] [PubMed] [Google Scholar]
- 23.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 2006;1758:1408–1425 [DOI] [PubMed] [Google Scholar]
- 24.Huang LC, Petkova TD, Reins RY, Proske RJ, McDermott AM. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci 2006;47:2369–2380 [DOI] [PubMed] [Google Scholar]
- 25.Smeianov V, Scott K, Reid G. Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Microbes Infect 2000;2:773–777 [DOI] [PubMed] [Google Scholar]
- 26.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol 2006;306:91–110 [DOI] [PubMed] [Google Scholar]
- 27.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun 2005;73:6771–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003;120:379–389 [DOI] [PubMed] [Google Scholar]
- 29.Shaykhiev R, Beisswenger C, Kandler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 2005;289:L842–L848 [DOI] [PubMed] [Google Scholar]
- 30.Carretero M, Escamez MJ, Garcia M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol 2008;128:223–236 [DOI] [PubMed] [Google Scholar]
- 31.Tokumaru S, Sayama K, Shirakata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 2005;175:4662–4668 [DOI] [PubMed] [Google Scholar]
- 32.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 1995;36:614–621 [PubMed] [Google Scholar]
- 33.Zieske J, Gipson I. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci 1986;27:1–7 [PubMed] [Google Scholar]
- 34.Xu KP, Li XF, Yu FS. Corneal organ culture model for assessing epithelial responses to surfactants. Toxicol Sci 2000;58:306–314 [DOI] [PubMed] [Google Scholar]
- 35.Richardson K, Jarett L, Finke E. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 1960;35:313–323 [DOI] [PubMed] [Google Scholar]
- 36.Yin J, Lu J, Yu FS. Role of small GTPase rho in regulating corneal epithelial wound healing. Invest Ophthalmol Vis Sci 2008;49:900–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Niyonsaba F, Ushio H, et al. Synergistic effect of antibacterial agents human [beta]-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci 2005;40:123–132 [DOI] [PubMed] [Google Scholar]
- 38.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol 2005;21:147–151 [DOI] [PubMed] [Google Scholar]
- 39.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 2006;12:636–641 [DOI] [PubMed] [Google Scholar]
- 40.Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chem Immunol Allergy 2005;86:55–71 [DOI] [PubMed] [Google Scholar]
- 41.Barnea M, Madar Z, Froy O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem Biophys Res Commun 2008;367:452–456 [DOI] [PubMed] [Google Scholar]
- 42.De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000;192:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci 2000;41:1346–1355 [PubMed] [Google Scholar]
- 44.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res 2007;85:130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushi S, Merola LO, Tanaka M, Datiles M, Kinoshita JH. Reepithelialization of denuded corneas in diabetic rats. Exp Eye Res 1980;31:611–621 [DOI] [PubMed] [Google Scholar]
- 46.Datiles MB, Kador PF, Fukui HN, Hu TS, Kinoshita JH. Corneal re-epithelialization in galactosemic rats. Invest Ophthalmol Vis Sci 1983;24:563–569 [PubMed] [Google Scholar]
- 47.Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant trolox. Res Commun Mol Pathol Pharmacol 1996;93:3–12 [PubMed] [Google Scholar]
- 48.McDermott AM, Kern TS, Murphy CJ. The effect of elevated extracellular glucose on migration, adhesion and proliferation of SV40 transformed human corneal epithelial cells. Curr Eye Res 1998;17:924–932 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Akiba K, Noguchi T, et al. Matrix metalloproteinase activity is enhanced during corneal wound repair in high glucose condition. Curr Eye Res 2000;21:608–615 [PubMed] [Google Scholar]
- 50.Fujita H, Morita I, Takase H, Ohno-Matsui K, Mochizuki M. Prolonged exposure to high glucose impaired cellular behavior of normal human corneal epithelial cells. Curr Eye Res 2003;27:197–203 [DOI] [PubMed] [Google Scholar]
- 51.Lu W, Ebihara N, Miyazaki K, Murakami A. Reduced expression of laminin-5 in corneal epithelial cells under high glucose condition. Cornea 2006;25:61–67 [DOI] [PubMed] [Google Scholar]
- 52.Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes 2006;55:1141–1147 [DOI] [PubMed] [Google Scholar]
- 53.Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J Ocul Pharmacol Ther 2007;23:89–102 [DOI] [PubMed] [Google Scholar]
- 54.Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea 2007;26:S75–S81 [DOI] [PubMed] [Google Scholar]
- 55.Wakuta M, Morishige N, Chikama T, Seki K, Nagano T, Nishida T. Delayed wound closure and phenotypic changes in corneal epithelium of the spontaneously diabetic Goto-Kakizaki rat. Invest Ophthalmol Vis Sci 2007;48:590–596 [DOI] [PubMed] [Google Scholar]
- 56.Zagon IS, Sassani JW, Myers RL, McLaughlin PJ. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res Bull 2007;72:18–24 [DOI] [PubMed] [Google Scholar]
- 57.Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol 2007;125:1082–1088 [DOI] [PubMed] [Google Scholar]
- 58.Yucel I, Yucel G, Akar Y, Demir N, Gurbuz N, Aslan M. Transmission electron microscopy and autofluorescence findings in the cornea of diabetic rats treated with aminoguanidine. Can J Ophthalmol 2006;41:60–66 [DOI] [PubMed] [Google Scholar]
- 59.Nakamura M, Sato N, Chikama TI, Hasegawa Y, Nishida T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res 1997;64:1043–1050 [DOI] [PubMed] [Google Scholar]
- 60.Nakamura M, Sato N, Chikama T, Hasegawa Y, Nishida T. Fibronectin facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res 1997;64:355–359 [DOI] [PubMed] [Google Scholar]
- 61.Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia 2003;46:839–842 [DOI] [PubMed] [Google Scholar]
- 62.Gareskog M, Cederberg J, Eriksson UJ, Wentzel P. Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol 2007;23:63–74 [DOI] [PubMed] [Google Scholar]
- 63.Argirova MD, Argirov OK. Region-specific pathophysiological alterations occurring in calf lenses in vitro during hyperglycemia. Graefes Arch Clin Exp Ophthalmol 2002;240:126–130 [DOI] [PubMed] [Google Scholar]
- 64.Gallo RL. Sounding the alarm: multiple functions of host defense peptides. J Invest Dermatol 2008;128:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foerster CG, Cursiefen C, Kruse FE. Persisting corneal erosion under cetuximab (Erbitux) treatment (epidermal growth factor receptor antibody). Cornea 2008;27:612–614 [DOI] [PubMed] [Google Scholar]
- 66.Zagon IS, Jenkins JB, Sassani JW, et al. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes 2002;51:3055–3062 [DOI] [PubMed] [Google Scholar]