p38 MAPK plays an important role in diabetes-induced inflammation in the retina, and inhibition of p38 MAPK offers a novel therapeutic approach for inhibiting the development of early stages of diabetic retinopathy and other complications of diabetes.
Abstract
Purpose.
p38 mitogen-activated protein kinase (MAPK) is known to play a regulatory role in inflammatory processes in disease. Inflammation has been linked also to the development of diabetic retinopathy in rodents. This study was conducted to evaluate the effect of a p38 MAPK inhibitor on the development of early stages of diabetic retinopathy in rats.
Methods.
Streptozotocin-diabetic rats were assigned to two groups—treated with the p38 MAPK inhibitor PHA666859 (Pfizer, New York, NY) and untreated—and compared with age-matched nondiabetic control animals.
Results.
At 2 months of diabetes, insulin-deficient diabetic control rats exhibited significant increases in retinal superoxide, nitric oxide (NO), cyclooxygenase (COX)-2, and leukostasis within retinal microvessels. All these abnormalities were significantly inhibited by the p38 MAPK inhibitor (25 mg/kgBW/d). At 10 months of diabetes, significant increases in the number of degenerate (acellular) capillaries and pericyte ghosts were measured in control diabetic rats versus those in nondiabetic control animals, and pharmacologic inhibition of p38 MAPK significantly inhibited all these abnormalities (all P < 0.05). This therapy also had beneficial effects outside the eye in diabetes, as evidenced by the inhibition of a diabetes-induced hypersensitivity of peripheral nerves to light touch (tactile allodynia).
Conclusions.
p38 MAPK plays an important role in diabetes-induced inflammation in the retina, and inhibition of p38 MAPK offers a novel therapeutic approach to inhibiting the development of early stages of diabetic retinopathy and other complications of diabetes.
Hyperglycemia causes metabolic and physiologic abnormalities in the retina that can lead eventually to degeneration or dysfunction of the retinal blood vessels and neurons. Diabetes-induced molecular and physiologic alterations consistent with inflammation observed in retinal tissue or cells include upregulation of iNOS, COX-2, ICAM-1, caspase 1, VEGF, and NF-κB; increased production of nitric oxide, prostaglandin E2, IL-1β, and cytokines; and increased permeability and leukostasis.1,2 Inhibition of this inflammatory cascade at any of its multiple steps can inhibit the histopathology characteristic of the early stages of diabetic retinopathy in animals.1,3 These findings have suggested a role of inflammation in development of retinopathy.1
A large body of evidence in preclinical studies indicates a crucial role of p38 MAPKα in inflammation, and p38 MAPK inhibitors have been shown to block production of IL-1, TNF, and IL-6 in vitro and in vivo in other diseases. The p38 MAPK pathway is also involved in the induction of other inflammatory molecules, such as COX-2 and iNOS,4–6 and p38-dependent phosphorylation of histone H3 has been shown to regulate NF-κB, also resulting in increased expression of inflammatory cytokines and chemokines.7 Suppression of p38 MAPK has been found to inhibit acute lung inflammation,8,9 ischemia/reperfusion,10 rheumatoid arthritis, Alzheimer's disease, and inflammatory bowel disease,11–13 as well as in ocular ischemia–reperfusion injury.14
The demonstrated role of p38 MAPK in the development of inflammation in a variety of diseases makes this protein an attractive target for pharmacologic intervention. Thus, we used the potent and bioavailable p38 MAPK inhibitor PHA666859 (Pfizer, New York, NY) to investigate the possibility that p38 MAPK also plays an important role in the pathogenesis of early stages of diabetic retinopathy in a rat model. In the same animals, we also investigated the possibility that p38 MAPK plays a role in the hypersensitivity to light touch (tactile allodynia) that causes some diabetic patients pain and discomfort.15
Materials and Methods
Animals
Male Lewis rats (200–225 g) were randomly assigned to become diabetic or remain nondiabetic, with two study durations: 2 months and 10 months after diabetes induction. Diabetes was induced by intraperitoneal injection of a freshly prepared solution of streptozotocin in citrate buffer (pH 4.5) at 55 mg/kg of body weight. Diabetic animals (nonfasting blood glucose >275 mg/dL 2 weeks after injection of streptozotocin) were assigned randomly to receive the p38 MAPK inhibitor or to remain untreated as diabetic control subjects. Drug administration was not begun until 2 weeks after streptozotocin, to ensure that all animals were satisfactorily diabetic and that the drug did not influence the severity of diabetes achieved. All animals were fed a powdered diet (Teklad 7004; Harlan Teklad, Madison, WI), with or without inhibitor added to the diet. Body weight was measured weekly. Insulin was given as needed to achieve gradual weight gain without preventing hyperglycemia and glucosuria (0–2 units SC of NPH insulin, 0–3 times per week). Thus, diabetic rats were insulin deficient but not grossly catabolic. All animals had free access to food and water and were maintained under a 14 hours on/10 hours off light cycle. Glycated hemoglobin (GHb; an estimate of the average level of hyperglycemia over the previous 2 months) was measured by an HPLC method (BioRad Variant, Hercules, CA) every 2 to 3 months, and all values from each animal were used to calculate a mean value for that animal. Data presented in Table 1 are for the duration of the 10-month experiment except as noted; data in the 2-month experiment were similar. Treatment of animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, as well as to specific institutional guidelines. Final group sizes for all measurements was n = 6–9, except as noted.
Table 1.
Effect of PHA66859 on Diabetes Severity over the 10 Months of the Study
| Duration (mo) | Final BW (g) | 24-h Urine Volume (mL) | Average GHb* (%) | |
|---|---|---|---|---|
| Nondiabetic (N) | 10 | 643 ± 43 | <5 | 3.0 ± 0.1 |
| Diabetic (D) | 10 | 286 ± 26† | 79 ± 19† | 11.7 ± 1.6† |
| D+PHA666859 | 10 | 293 ± 19† | 89 ± 32‡ | 11.0 ± 1.1† |
Average in each animal over the entire duration of the study.
P < 0.05 compared with N control.
P < 0.05 compared with D control.
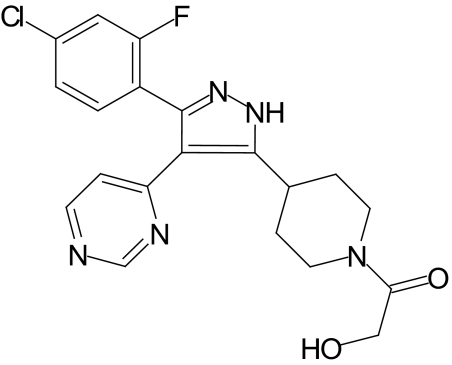
PHA666859 (Fig. 1), a pyrimidinyl pyrazole compound, is a potent, competitive, and reversible inhibitor of p38α MAPK with a p38α Ki = 53 nM. It has moderate selectivity over p38β MAP kinase (12-fold) and c-Jun NH2-terminal kinase (JNK) isoforms (30–250-fold). It did not inhibit p38γ or δ MAPK or extracellular receptor-activated kinase (ERK)-2 along with other kinase and nucleotide binding proteins (Table 2). Methods used for the potency and selectivity studies are described elsewhere.16 The compound is well tolerated at high doses in rats and has an oral bioavailability of 83%. The drug was administered in two experiments. The first was a 2-month experiment to determine how it should be administered (gavage versus in diet) and what concentration to use. The drug dose and route of administration selected from those studies was then used in the second, long-term study.
Figure 1.
Structure of PHA666859.
Table 2.
Potency and Selectivity of PHA666859
| Kinase | PHA666859 (IC50, μM) |
|---|---|
| P38α MAPK | 0.21 ± 0.09 |
| P38β MAPK | 2.45 ± 0.54 |
| P38γ MAPK | >200 |
| P38δ MAPK | >200 |
| JNK1 | 49.4 ± 18.7 |
| JNK2 | 6.02 ± 3.7 |
| JNK3 | 12.3 ± 4.4 |
| P38α MAPK | Ki = 53 ± 5 nM |
| JNK2 | Ki = 1780 ± 110 nM |
PHA666859 IC50 >200 μM: ERK2, PRAK, MSK, MK2, MK3, MNK, MKK6, CDK2, IKK1, and IKK2; PHA666859 IC50 >10 μM: PKCβ, PKA, NIM1, EGFR1, LCK, Met, FGFR1, Lyn, Ab1, CHK1, GSK3β, IGFR1, IRAK4, CDK2, and Aurora2.
Two-Month Outcome Measures
Superoxide Anion.
Fresh retinas from animals killed at 2 months of study were incubated in 0.5 mM lucigenin, and the luminescence was detected as reported previously.17 The superoxide scavenger tiron inhibits the diabetes-induced increase in luminescence by more than 90%,17 demonstrating that most of the luminescence induced in retinas from diabetic animals is due to superoxide production.
Western Blot Analysis.
Retinal homogenates were subjected to Western blot analysis, as reported by us previously.18,19 Antibodies against iNOS (mAb; 1:500 dilution; Transduction Laboratories, Lexington, KY), nitrotyrosine (polyclonal; 1:1000 dilution; Upstate Biotechnology, Lake Placid, NY), COX-2 (polyclonal; 1:500 dilution; Cayman Chemical, Ann Arbor, MI), ICAM-1 (1:250 dilution; R&D Systems, Minneapolis, MN) were used. Antibodies against PARS activity (1:400 dilution), p-p38 MAPK (threonine 180 and tyrosine 182; 1:1000 dilution), heat shock protein 27 (HSP27; polyclonal; 1:1500 dilution), p-HSP27 (ser82; 1:1000 dilution), BAD (1:1000 dilution), p-BAD (ser 112; 1:1000 dilution), MEK (monoclonal; 1:1000 dilution), p-MEK (ser 217 and ser 221; 1:1000 dilution), mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 (polyclonal; 1:1000 dilution), and p-MAPKAP kinase-2 (polyclonal; threonine 222; 1:1000 dilution) were all obtained from Cell Signaling Technology, Inc. (Danvers, MA).
Leukostasis.
Leukostasis was measured at 2 months, by using published methods.19–21 Briefly, anesthetized rats had a 20-gauge perfusion needle inserted into the base of the aortic arch, making sure that the needle did not obstruct the carotid arteries. The right atrium was cut to allow outflow, and PBS (60 mL) was perfused into the aorta at the normal cardiac output rate for a rat (60 mL/min) to clear erythrocytes and nonadherent leukocytes. Fluorescein isothiocyanate-coupled Concanavalin A lectin (20 μg/mL in PBS [pH 7.4], total concentration 5 mg/kg body weight; Vector Laboratories, Burlingame, CA) then was perfused to stain adherent leukocytes and vascular endothelium, followed by another wash with PBS (60 mL) at the same perfusion rate to remove excess Concanavalin A. The retina was flatmounted on a microscope slide, covered with antifade medium and a coverslip, and imaged via fluorescence microscopy. The total number of adherent leukocytes per retina was counted.
Ten-Month Outcome Measures
Quantitation of Retinal Disease.
The retinal vasculature was isolated from formalin-fixed tissue using the trypsin digest technique.19,22,23 After the purified vessel network was dried onto a glass slide, the preparations were stained with hematoxylin and periodic acid-Schiff, dehydrated, and coverslipped. Acellular capillaries were quantitated in four to seven field areas in the mid retina (200× magnification) in a masked manner. Acellular capillaries were identified as capillary-sized vessel tubes having no nuclei anywhere along their length and were reported per square millimeter of retinal area. Pericyte ghosts were estimated from the prevalence of protruding “bumps” in the capillary basement membranes from which pericytes had disappeared. At least 1000 capillary cells (endothelial cells and pericytes) in five field areas in the mid retina (400× magnification) were examined in a masked manner. Ghosts on any already acellular vessel were excluded.
Neurodegeneration.
Optic nerves were fixed in 2% glutaraldehyde to count the number of axons in the optic nerve (as a way to quantitate all ganglion cells in the retina). The fixation of this heavily myelinated tissue was not adequate, and as a result, we were not able to count axons in the center of the nerve. Thus, this measurement was regarded as unsuccessful (data not shown).
Tactile Allodynia.
A standardized testing regimen was performed to measure tactile allodynia,24 as reported previously by us.25 Von Frey filaments (Stoelting, Wood Dale, IL) were used to determine the 50% mechanical threshold for foot withdrawal. Lifting of the paw was recorded as a positive response, and the next lightest filament was chosen for the next measurement. Absence of a response after 5 seconds prompted the use of the next filament of increasing weight. All measurements were performed by an investigator who was unaware of the treatment group of individual animals.
Quantitation of PHA666859
The drug concentration was quantified by rat plasma using high-performance liquid chromatography–tandem mass spectroscopy (LC/MS/MS). Standard stock solutions of PHA666859 were prepared in acetonitrile and diluted to the working standard solutions (in acetonitrile). The internal standard used was buspirone, which was prepared in methanol (50 ng/mL). All standard and QC stocks were stored at −20°C.
Proteins were precipitated with acetonitrile/methanol (4:1, vol:vol) containing 0.1% formic acid. The LC-MS/MS system consisted of pumps (model LC10AD; Shimadzu, Columbia, MD), with an autosampler (CTC-PAL; LEAP Technologies, Carrboro, NC) and a cool stack and triple quadrupole mass spectrometer (Sciex API 4000; Applied Biosystems, Inc. [ABI], Foster City, CA). A gradient elution method was used to perform chromatography on a reversed-phase column (XBridge C18, 3.5 μm, 50 × 2.1 mm; Waters, Waltham, MA) at a flow rate of 500 μL/min (mobile phase A consisted of 100% HPLC grade water with 0.1% formic acid, and mobile phase B contained 100% acetonitrile with 0.1% formic acid). The mass spectrometer was operated under the following conditions: positive ion turbo-ionspray mode, ionspray potential, 5.0 kV; interface temperature, 400°C; curtain gas, 20; CAD gas, 6; GS1, 70; and GS2, 30. The conditions for PHA666859 were as follows: MS/MS transition, m/z 416.16→340.94; declustering potential, 91; and collision energy, 41. The conditions for buspirone (internal standard) were as follows: MS/MS transition, m/z 386.3→122.1; declustering potential, 80; and collision energy, 40. Peak areas were then determined (Analyst software, ver. 1.4.1; ABI). Quantitation was performed by linear regression with a 1/x2 weighting. The lower limit of quantitation for the assay was 1 ng/mL. The upper limit of quantitation for the assay was 5000 ng/mL.
Statistical Analysis
Data are expressed as the mean ± SD. Statistical analysis was performed with ANOVA, followed by Fisher's test. P < 0.05 was considered statistically significant.
Results
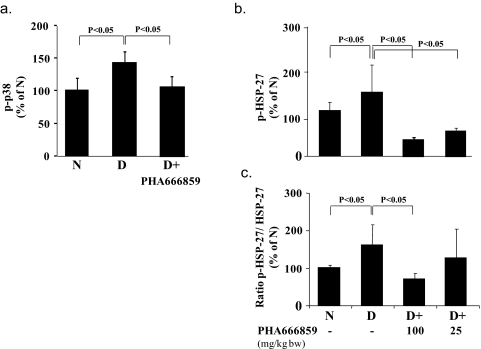
To select an effective dose and route of administration of PHA666859, we administered the drug in initial studies via gavage (3 mg/kg body weight[BW]/d) or in the diet (at 2.5, 25, or 135 mg/kgBW/d) for 2 months to diabetic animals. Administration of the lowest dose of PHA666859 had no effect on the expression or phosphorylation of p-HSP27 (a downstream biomarker of p38 activity) whether by gavage or in the food and so will not be discussed further.
Phosphorylation of p38 MAPK (p-p38) is known to activate the enzyme, and we therefore measured p-p38 at threonine 180/tyrosine 182. Retinas from control rats having insulin-deficient diabetes that was not treated with the p38 MAPK inhibitor showed a significant increase in p-p38, and administration of PHA666859 at a dose of 25 mg/kgBW/d significantly inhibited this change (Fig. 2a).
Figure 2.
Effects of 2 months' diabetes and p38 MAPK inhibition on (a) p-p38 MAPK, (b) p-HSP-27, and (c) p-HSP-27 as a percentage of total HSP27 in retinal homogenates from nondiabetic (N), diabetic control (D), and diabetic treated with PHA666859 groups. The inhibitor was administered at a dose of 25 mg/kgBW/d in (a–c) and also at a dose of 100 mg/kgBW/d in (b) and (c). n = 6–10 in all groups.
Since phosphorylation of HSP27 is known to be dependent on p38 MAPK activity,26 we measured the ability of the drug to inhibit the diabetes-induced increase in phosphorylation of HSP27 as a biomarker of p38 MAPK activity. Total expression of HSP27 was also measured. Both of the two higher doses of the orally administered drug significantly inhibited phosphorylation of HSP27 (Figs. 2b, 2c). Based on these findings, we administered PHA666859 in the diet to diabetic animals for long-term studies at a dose intended to be equivalent to 25 mg/kgBW/d. Although the data indicate that PHA666859 is an effective inhibitor of p38 MAPK activity in vivo, the data demonstrated inhibition of p38 activation as well (Fig. 2a). Although several classes of p38 inhibitors have been shown to block both activation and activity of p38MAPK directly through their interaction with this kinase,27,28 the possibility cannot be ruled out that PHA666859 modulates a target upstream of p38 MAPK in addition to its inhibitory effect on the target.
Diabetic rats in the long-term study were hyperglycemic and failed to gain weight at a normal rate. The severity of hyperglycemia was assessed every 2 to 3 months by determining glycated hemoglobin (GHb). GHb levels in the two diabetic groups (diabetic control and diabetic treated with PHA666859 in the diet) were comparable throughout the duration of the experiment and were significantly greater than normal (Table 1). GHb and random blood glucose values were similar in the 2- and 10-month studies. Over the 10 months of study, the actual intake of PHA666859 was determined to average 27 mg/kgBW/d. Blood levels of drug at this dose remained elevated throughout the day, and averaged 355 ± 88 ng/mL at 8 AM and 266 ± 56 at 4 PM (control animals had undetectable levels in the blood).
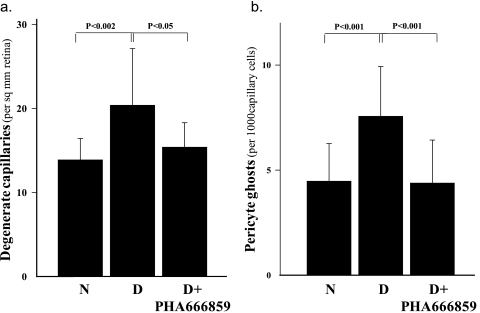
The retinal vessels of control rats with 10 months' duration of diabetes exhibited the expected capillary degeneration and pericyte ghosts compared with the nondiabetic control animals (Fig. 3). Daily consumption of PHA666859 from the onset of diabetes significantly inhibited each of these diabetes-induced lesions.
Figure 3.
Administration of PHA666859 inhibited the diabetes-induced increase in (a) degenerate, acellular capillaries and (b) pericyte ghosts in the retina, caused by diabetes of 10 months' duration. The inhibitor was administered at a dose of 25 mg/kgBW/d for the duration of the study. n = >8 in all groups.
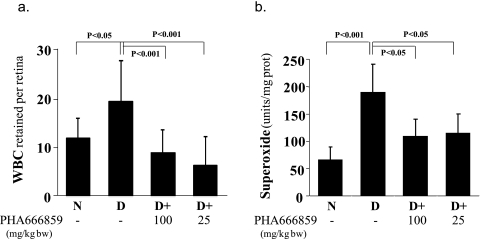
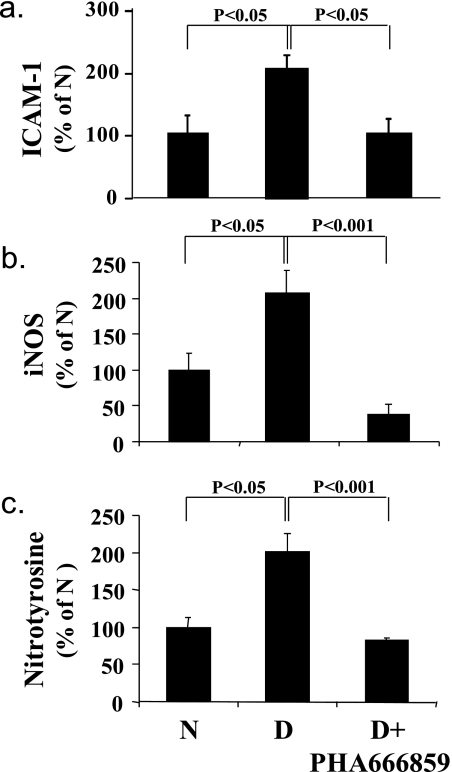
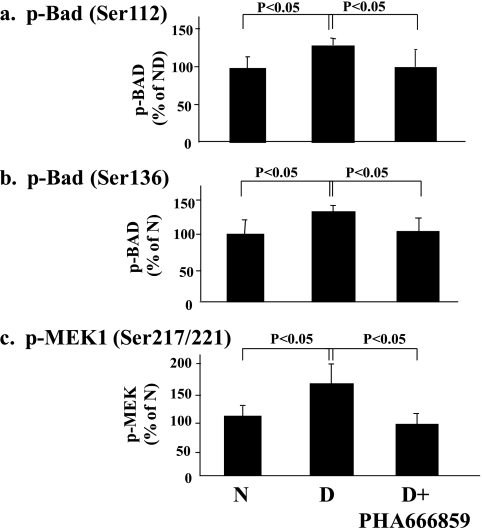
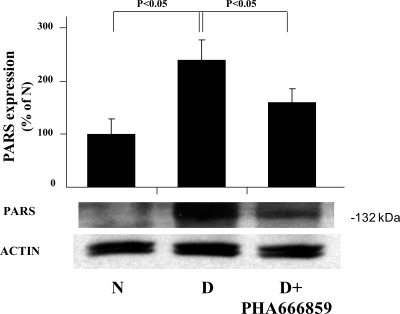
In an effort to gain insight pertaining to how the beneficial effects of PHA666859 on retinopathy were mediated, we also measured other abnormalities that have been postulated to be important in the pathogenesis of retinopathy. Diabetes caused a significant increase in leukostasis and generation of superoxide (Fig. 4), expression of ICAM-1 and iNOS, nitration of retinal proteins (nitrotyrosine; Fig. 5), and phosphorylation of BAD at serines 112 and 136 and MEK at serines 217/221 (Fig. 6). Oral administration of PHA666859 soon after the onset of diabetes inhibited each of these abnormalities. The effect of drug on the diabetes-induced increase in poly(ADP ribosyl)ation of retinal proteins was mixed, with the inhibitor significantly inhibiting the poly(ADP-ribosyl)ation of retinal protein at approximately 132 kDa (Fig. 7), but this effect was not apparent at other molecular weights (not shown). The p38 MAPK inhibition did not have an effect on the diabetes-induced upregulation of COX-2 or MAPK-activated protein kinase 2 (not shown).
Figure 4.
Diabetes (2-month duration) results in increased (a) adherence of white blood cells (WBCs) to the retinal vasculature and (b) generation of superoxide by the isolated retina. PHA666859 administered at a dose of at least 25 mg/kgBW/d inhibited both of these abnormalities. n = 6–10 in all groups.
Figure 5.
Effects of diabetes and p38 MAPK inhibition on retinal expression of (a) ICAM-1, (b) iNOS, and (c) modification of retinal proteins by nitrotyrosine. The inhibitor was administered at a dose of 25 mg/kgBW/d for 10 months. n = 6–10 in all groups.
Figure 6.
Effects of diabetes and p38 MAPK inhibition on phosphorylation of BAD at (a) ser112 and (b) ser136 and of MEK at (c) ser217/221. The inhibitor was administered at a dose of 25 mg/kgBW/d for 10 months. n = 6–10 in all groups.
Figure 7.
Effects of diabetes and p38 MAPK inhibition on poly(ADP-ribosyl)ation (PARS) of retinal protein of approximately 132 kDa. The amount of poly(ADP-ribosyl)ation of other retinal proteins (not shown) was little influenced by p38 MAPK inhibition. The inhibitor was administered at a dose of 25 mg/kgBW/d for 10 months n = 6–10 in all groups.
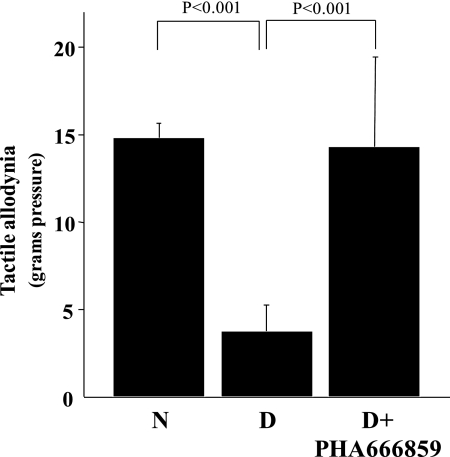
Beneficial effects of p38 MAPK inhibition in diabetes were not limited to the retina. Diabetes enhanced sensitivity of peripheral nerves to light touch in the rats (tactile allodynia; Fig. 8), and administration of the p38 MAPK inhibitor significantly suppressed this diabetes-induced hypersensitivity of sensory function. The mechanism of this action was not explored further.
Figure 8.
Effects of diabetes and p38 MAPK inhibition on tactile allodynia in hind paws of mice. The inhibitor was administered at a dose of 25 mg/kgBW/d, and the duration of diabetes and drug administration was 10 months. n = 6–10 in all groups.
Discussion
p38 MAPK is one of three major MAPK signaling pathways triggered by a variety of extracellular ligands and stresses, including proinflammatory cytokines, reactive oxygen species, growth factors, osmotic stress, and UV irradiation.29 p38α is the major isoform of p38 MAPK associated with inflammation, and inhibition of p38 MAPK activity blocks upregulation of a variety of proinflammatory mediators, including monocyte chemoattractant protein-1, granulocyte-macrophage colony-stimulating factor, vascular endothelial growth factor, COX-2, and iNOS, matrix metalloproteinases, leukotrienes, and adhesion molecules required for leukostasis and leukocyte emigration.30 Likewise, NADPH oxidase and the resulting superoxide generation are under the control of p38 MAPK.31–33
Diabetes has been found to activate p38 MAPK in a variety of tissues, including kidney, nerve, vasculature, and heart.34–39 In vitro studies likewise have shown that high glucose can activate a p38 MAPK signaling pathway in aortic, renal (mesangial), and pancreatic β cells.34,40,41
The present study demonstrates that p38 MAPK activation plays a role in the long-term vascular histopathology of diabetic retinopathy. Inhibition of p38 MAPK activation in diabetes inhibited the diabetes-induced death of endothelial cells and pericytes and significantly inhibited degeneration of retinal capillaries. This finding is important, because capillary nonperfusion and degeneration are believed to play a major role in the eventual development of retina ischemia and subsequent development of retinal neovascularization.
Accumulating evidence suggests that inflammation plays a critical role in the development of the early vascular lesions of diabetic retinopathy.1,2 Inhibition of this inflammatory cascade at any of multiple steps can inhibit the histopathology characteristic of early stages of diabetic retinopathy in animals,1,3 thus providing strong evidence of an important role of inflammation in the pathogenesis of diabetic retinopathy. p38 MAPK is known to regulate gene expression of inflammatory proteins via effects on multiple transcription factors, but it also is able to regulate NF-κB by controlling the phosphorylation of histone H3 in chromatin at NF-κB-binding sites of inflammatory genes, including IL-8 and MCP-1,7,42 and the stimulation of NF-κB DNA protein binding at the promoter for iNOS.43 Inhibition of p38 was found to inhibit the generation of iNOS and nitrotyrosine33,44 and to partially inhibit the death of retinal pigment epithelial cells cultured in high glucose.44 Our finding that inhibition of p38 MAPK in diabetic animals inhibited the increase in leukostasis and expression of retinal iNOS suggests that p38 MAPK activation in the retina is proinflammatory in diabetes and that inhibition of retinopathy by p38 MAPK inhibition is most likely due in part to inhibition of those inflammatory processes.
The therapy also may act via other mechanisms. p38 MAPK activation is involved in superoxide production, as illustrated by the inhibition of angiotensin II-dependent hypertension, organ damage, and superoxide anion production after inhibition of p38 MAPK.32 p38 MAPK has been reported to activate NADPH oxidase by enhancing phosphorylation and assembly of NADPH oxidase subunits,31,45 and inhibition of p38 MAPK inhibits activation of NADPH oxidase.46 In our study, treatment with a p38 MAPK inhibitor suppressed the diabetes-induced increase in production or accumulation of superoxide by the retina, possibly through direct effects on NADPH oxidase or transcriptional regulation of inflammatory proteins and cytokines.
p38 MAPK lies upstream of HSP27 and activates the heat shock protein via phosphorylation.47,48 Inhibition of p38 MAPK in our study inhibited the diabetes-induced increase in phosphorylation of HSP27, and this effect was associated with an inhibition of the development of vascular disease. Phosphorylation of HSP27 has been shown to impair its chaperone function, and a mutant having a tertiary structure that mimics phosphorylated Hsp27 is less able to protect against oxidative stress than is the wild-type molecule.49 Likewise, phosphorylation of BAD is known to increase apoptosis in cells.50 Thus, inhibition of phosphorylation of HSP27 or BAD after inhibition of p38 MAPK may have contributed to the beneficial effects of PHA666859 therapy.
Diabetic rats also develop a hypersensitivity to light touch compared with nondiabetic control animals,24,51 similar to what develops in some diabetic patients.15 Inhibition of p38 MAPK has beneficial effects related to nerve function, including regulation of inflammatory heat hyperalgesia and mechanical allodynia.52,53 In other diseases, p38 MAPK is known to play an important role in pain responses mediated via the central nervous system through expression of COX-2 and other mediators.53,54 The present studies provide evidence that p38 MAPK activation also plays a role in the tactile allodynia that develops in diabetes.
Acknowledgments
The authors thank Terri Quenzer for bioanalytical work.
Footnotes
Supported by a grant from Pfizer Pharmaceutical, Public Health Service Grant EY00300, and a grant from the Medical Research Service of the Department of Veteran Affairs. Support services were provided by the CWRU Visual Science Research Center Core Facilities supported by National Institutes of Health Grant P30EY11373.
Disclosure: Y. Du, Pfizer (F); J. Tang, Pfizer (F); G. Li, Pfizer (F); L. Berti-Mattera, Pfizer (F); C.A. Lee, Pfizer (F); D. Bartkowski, Pfizer (F, E); D. Gale, Pfizer (F, E); J. Monahan, Pfizer (F, E); M.R. Niesman, Pfizer (F, E); G. Alton, Pfizer (F, E); T.S. Kern, Pfizer (F)
References
- 1.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007;2007:95103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol 2008;30:65–84 [DOI] [PubMed] [Google Scholar]
- 3.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 4.Badger AM, Roshak AK, Cook MN, et al. Differential effects of SB 242235, a selective p38 mitogen-activated protein kinase inhibitor, on IL-1 treated bovine and human cartilage/chondrocyte cultures. Osteoarthritis Cartilage 2000;8:434–443 [DOI] [PubMed] [Google Scholar]
- 5.Guan Z, Buckman SY, Pentland AP, Templeton DJ, Morrison AR. Induction of cyclooxygenase-2 by the activated MEKK1→SEK1/MKK4→p38 mitogen-activated protein kinase pathway. J Biol Chem 1998;273:12901–12908 [DOI] [PubMed] [Google Scholar]
- 6.Guan Z, Buckman SY, Springer LD, Morrison AR. Regulation of cyclooxygenase-2 by the activated p38 MAPK signaling pathway. Adv Exp Med Biol 1999;469:9–15 [DOI] [PubMed] [Google Scholar]
- 7.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol 2002;3:69–75 [DOI] [PubMed] [Google Scholar]
- 8.Nick JA, Young SK, Brown KK, et al. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol 2000;164:2151–2159 [DOI] [PubMed] [Google Scholar]
- 9.Haddad EB, Birrell M, McCluskie K, et al. Role of p38 MAP kinase in LPS-induced airway inflammation in the rat. Br J Pharmacol 2001;132:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem 2006;281:6760–6767 [DOI] [PubMed] [Google Scholar]
- 11.Perregaux DG, Dean D, Cronan M, Connelly P, Gabel CA. Inhibition of interleukin-1 beta production by SKF86002: evidence of two sites of in vitro activity and of a time and system dependence. Mol Pharmacol 1995;48:433–442 [PubMed] [Google Scholar]
- 12.Johnson GV, Bailey CD. The p38 MAP kinase signaling pathway in Alzheimer's disease. Exp Neurol 2003;183:263–268 [DOI] [PubMed] [Google Scholar]
- 13.Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J 2004;18:1550–1552 [DOI] [PubMed] [Google Scholar]
- 14.Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci 2003;44:5383–5395 [DOI] [PubMed] [Google Scholar]
- 15.Otto M, Bak S, Bach FW, Jensen TS, Sindrup SH. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain 2003;101:187–192 [DOI] [PubMed] [Google Scholar]
- 16.Burnette BL, Selness S, Devraj R, et al. SD0006: a potent, selective and orally available inhibitor of p38 kinase. Pharmacology 2009;84:42–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 2003;35:1491–1499 [DOI] [PubMed] [Google Scholar]
- 18.Du Y, Sarthy V, Kern T. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol 2004;287:R735–R741 [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 2007;56:337–345 [DOI] [PubMed] [Google Scholar]
- 20.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 2002;16:438–440 [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 2004;53:2960–2967 [DOI] [PubMed] [Google Scholar]
- 22.Kern TS, Tang J, Mizutani M, et al. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci 2000;41:3972–3978 [PubMed] [Google Scholar]
- 23.Kern TS, Engerman RL. Pharmacologic inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes 2001;50:1636–16342 [DOI] [PubMed] [Google Scholar]
- 24.Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain 1996;68:293–299 [DOI] [PubMed] [Google Scholar]
- 25.Berti-Mattera LN, Kern TS, Siegel RE, Nemet I, Mitchell R. Sulfasalazine blocks the development of tactile allodynia in experimentally diabetic rats. Diabetes 2008;57(10):2801–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rousseau S, Dolado I, Beardmore V, et al. CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal 2006;18:1897–1905 [DOI] [PubMed] [Google Scholar]
- 27.Mikkelsen SS, Jensen SB, Chiliveru S, et al. RIG-I-mediated activation of p38 MAPK is essential for viral induction of interferon and activation of dendritic cells: dependence on TRAF2 and TAK1. J Biol Chem 2009;284:10774–10782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan JE, Holdgate GA, Campbell D, et al. Prevention of MKK6-dependent activation by binding to p38alpha MAP kinase. Biochemistry 2005;44:16475–16490 [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001;81:807–869 [DOI] [PubMed] [Google Scholar]
- 30.Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol 2004;4:372–377 [DOI] [PubMed] [Google Scholar]
- 31.Lal AS, Clifton AD, Rouse J, Segal AW, Cohen P. Activation of the neutrophil NADPH oxidase is inhibited by SB 203580, a specific inhibitor of SAPK2/p38. Biochem Biophys Res Commun 1999;259:465–470 [DOI] [PubMed] [Google Scholar]
- 32.Bao W, Behm DJ, Nerurkar SS, et al. Effects of p38 MAPK inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol 2007;49:362–368 [DOI] [PubMed] [Google Scholar]
- 33.Yoo BK, Choi JW, Shin CY, et al. Activation of p38 MAPK induced peroxynitrite generation in LPS plus IFN-gamma-stimulated rat primary astrocytes via activation of iNOS and NADPH oxidase. Neurochem Int 2008;52:1188–1197 [DOI] [PubMed] [Google Scholar]
- 34.Igarashi M, Wakasaki H, Takahara N, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest 1999;103:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adhikary L, Chow F, Nikolic-Paterson DJ, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 2004;47:1210–1222 [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc Diabetol 2005;4:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komers R, Lindsley JN, Oyama TT, Cohen DM, Anderson S. Renal p38 MAP kinase activity in experimental diabetes. Lab Invest 2007;87:548–558 [DOI] [PubMed] [Google Scholar]
- 38.Igarashi M, Hirata A, Yamaguchi H, et al. Characterization of activation of MAP kinase superfamily in vasculature from diabetic rats. J Atheroscler Thromb 2007;14:235–244 [DOI] [PubMed] [Google Scholar]
- 39.Agthong S, Tomlinson DR. Inhibition of p38 MAP kinase corrects biochemical and neurological deficits in experimental diabetic neuropathy. Ann N Y Acad Sci 2002;973:359–362 [DOI] [PubMed] [Google Scholar]
- 40.Macfarlane WM, Smith SB, James RF, et al. The p38/reactivating kinase mitogen-activated protein kinase cascade mediates the activation of the transcription factor insulin upstream factor 1 and insulin gene transcription by high glucose in pancreatic beta-cells. J Biol Chem 1997;272:20936–20944 [DOI] [PubMed] [Google Scholar]
- 41.Wilmer WA, Dixon CL, Hebert C. Chronic exposure of human mesangial cells to high glucose environments activates the p38 MAPK pathway. Kidney Int 2001;60:858–871 [DOI] [PubMed] [Google Scholar]
- 42.Soloaga A, Thomson S, Wiggin GR, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 2003;22:2788–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 1999;97:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan Z, Feng W, Hong J, Zheng Q, Shuai J, Ge Y. p38MAPK and ERK promote nitric oxide production in cultured human retinal pigmented epithelial cells induced by high concentration glucose. Nitric Oxide 2009;20:9–15 [DOI] [PubMed] [Google Scholar]
- 45.Parinandi NL, Kleinberg MA, Usatyuk PV, et al. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L26–L38 [DOI] [PubMed] [Google Scholar]
- 46.Yamamori T, Inanami O, Nagahata H, Cui Y, Kuwabara M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett 2000;467:253–258 [DOI] [PubMed] [Google Scholar]
- 47.Wong JW, Shi B, Farboud B, et al. Ultraviolet B-mediated phosphorylation of the small heat shock protein HSP27 in human keratinocytes. J Invest Dermatol 2000;115:427–434 [DOI] [PubMed] [Google Scholar]
- 48.Garmyn M, Mammone T, Pupe A, Gan D, Declercq L, Maes D. Human keratinocytes respond to osmotic stress by p38 map kinase regulated induction of HSP70 and HSP27. J Invest Dermatol 2001;117:1290–1295 [DOI] [PubMed] [Google Scholar]
- 49.Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem 1999;274:18947–18956 [DOI] [PubMed] [Google Scholar]
- 50.Chen XQ, Fung YW, Yu AC. Association of 14–3-3gamma and phosphorylated bad attenuates injury in ischemic astrocytes. J Cereb Blood Flow Metab 2005;25:338–347 [DOI] [PubMed] [Google Scholar]
- 51.Calcutt NA. Experimental models of painful diabetic neuropathy. J Neurol Sci 2004;220:137–139 [DOI] [PubMed] [Google Scholar]
- 52.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 2003;23:4017–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 2003;23:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 2003;521:1–21 [PubMed] [Google Scholar]