Isolated and pressurized human retinal arterioles in vitro dilated to bradykinin, adenosine, and increased flow, in part through nitric oxide synthase activation, whereas Rho kinase activation mediated myogenic tone and endothelin-1–induced vasoconstriction. Similarities in vasoreactivity and underlying signaling mechanisms were demonstrated between human and porcine retinal arterioles, supporting the latter as a viable experimental model of the human retinal microcirculation.
Abstract
Purpose.
Although the arteriolar segment contributes to flow regulation, there is sparse information at the single microvessel level on how vasomotor function is regulated in the human retina. The authors have previously reported vasoreactivity and its underlying mechanisms in isolated porcine retinal arterioles. Herein, they studied human retinal arterioles for comparison.
Methods.
Retinal tissues were obtained from seven patients undergoing enucleation. Human and porcine retinal arterioles were isolated and pressurized to 55 cm H2O luminal pressure for vasoreactivity study using videomicroscopic techniques.
Results.
Isolated human and porcine retinal arterioles developed myogenic tone and dilated dose dependently to bradykinin, adenosine, and sodium nitroprusside. Stepwise increases in luminal flow produced graded dilation with approximately 60% dilation at the highest flow tested. Nitric oxide (NO) synthase inhibitor L-NAME nearly abolished dilations to bradykinin and flow and attenuated the adenosine-induced dilation without altering the response to nitroprusside. Endothelin-1 caused dose-dependent constriction. Rho kinase (ROCK) inhibitor H-1152 blocked both myogenic tone and endothelin-1–induced constriction. Responses of retinal arterioles to all agonists and increased flow were similar between pigs and humans.
Conclusions.
Isolated human retinal arterioles dilate to bradykinin and increased flow in an NO-dependent manner. NO contributes, in part, to adenosine-induced vasodilation. Conversely, ROCK activation mediates myogenic tone and endothelin-1–induced vasoconstriction. Similarities in these vasoactive responses and the underlying mechanisms between human and porcine retinal arterioles support the latter as a viable experimental model of the human retinal microcirculation.
The retinal circulation is relatively unique in that it lacks direct innervation, and thus its regulation has been proposed to be controlled by a mechanism in association with changes in local hemodynamics and released substances (i.e., bradykinin and adenosine)1–5 as a function of oxygen supply and tissue metabolism. Abnormal vasomotor function can initiate or participate in the pathogenesis of cardiovascular complications, including hypertension6 and atherosclerosis,7 and in retinal diseases such as diabetic retinopathy. Several clinical studies have shown a reduction of retinal vasodilator function and retinal blood flow in patients with diabetes8–12 and hypertension.13,14 However, the vascular signaling mechanisms regulating retinal arteriolar tone in humans remain unknown. Because endothelial dysfunction in terms of deficient release of vasodilator nitric oxide (NO) and excessive release of vasoconstrictor endothelin-1 (ET-1) are regarded as key events for triggering numerous systemic and ocular vascular-related diseases,15–18 understanding the roles of endothelial NO and ET-1 in the regulation of vasomotor tone in human retinal arterioles has clinical implications. Although ET-1 can elicit potent vasoconstriction by activating protein kinases such as Rho kinase (ROCK) in large conduit arteries in other vascular beds,19,20 the detailed mechanisms by which ET-1 causes constriction of human small retinal resistance arterioles remain unclear. In the present study, we examined the response and its underlying mechanism elicited by physical shear stress and various endogenous vasoactive substances (bradykinin, adenosine, ET-1) in human retinal arterioles isolated from eyes donated by patients undergoing enucleation. We also compared the findings from human vessels with results from the pig, a potentially relevant animal model for the study of the retinal microcirculation.
Subjects and Methods
Human Subjects Study
Retinal tissues were obtained from seven patients (three men, four women; age, 58 ± 5 years [mean ± SD]; range, 36–70 years) undergoing enucleation after informed consent with approval of the Scott and White Institutional Review Board. The research followed the tenets of the Declaration of Helsinki. Eyes were enucleated from six patients who had ocular melanoma and from one patient with a severe chemical burn of the ocular surface. Two patients had been diagnosed with neovascular glaucoma, and one had diabetic retinopathy. Immediately after enucleation, the ocular tissue was examined grossly and sectioned for histopathologic examination, and the remaining eye tissue was transferred to a moist chamber on ice for microvessel isolation.
Animal Preparation
All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Scott and White Institutional Animal Care and Use Committee. Domestic pigs (8–12 weeks old of either sex; 7 to 10 kg) purchased from Barfield Farms (Rogers, TX) were sedated with tiletamine hydrochloride/zolazepam hydrochloride (4.4 mg/kg, intramuscularly; Telazol, Wyeth, Madison, NJ) and xylazine (2.2 mg/kg, intramuscularly) and were anesthetized with 2% to 4% isoflurane. Heparin (1000 U/kg) was administered into the marginal ear vein to prevent clotting. The eyes were enucleated and immediately placed in a moist chamber on ice.
Isolation and Cannulation of Microvessels
After enucleation, the anterior segments and vitreous bodies of the eyes were removed carefully under a dissection microscope.21 The posterior segment was placed in a cooled dissection chamber (∼8°C) containing a physiological salt solution (PSS) with 1% albumin (USB, Cleveland, OH). Single retinal arterioles (0.6–1.0 mm in length) were carefully dissected out using a pair of microdissection forceps (Dumont; Fine Science Tools, Foster City, CA) with the aid of a stereomicroscope (model SZX12; Olympus, Melville, NY). After careful removal of any remaining neural/connective tissues, the arteriole was then transferred for cannulation to a poly(methyl) methacrylate (Lucite, Beaumont, TX) vessel chamber containing PSS-albumin solution equilibrated with room air at ambient temperature. One end of the arteriole was cannulated using a glass micropipette filled with PSS-1% albumin solution, and the outside of the arteriole was securely tied to the pipette with 11–0 ophthalmic suture (Alcon, Fort Worth, TX). The other end of the vessel was cannulated with a second micropipette and also secured with suture. After cannulation, the vessel and pipettes were transferred to the stage of an inverted microscope (model CKX41; Olympus, Tokyo, Japan) coupled to a video camera (Sony DXC-190; Labtek, Campbell, CA), video micrometer (Cardiovascular Research Institute, Texas A&M Health Science Center, College Station, TX), and data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO) for continuous measurement and recording of the internal diameter throughout the experiment (Fig. 1). The cannulating pipettes were connected to independent pressure reservoirs. By adjusting the height of the reservoirs, the vessel was pressurized to 55 cm H2O (40 mm Hg) intraluminal pressure without flow. This level of pressure was used based on pressure ranges that have been documented in retinal arterioles in vivo and in the isolated, perfused retinal microcirculation22 and was consistent with the estimated ocular perfusion pressure in humans, as reported previously.23 Arterioles with side branches and leaks were excluded from further study, and all arterioles developed basal tone.
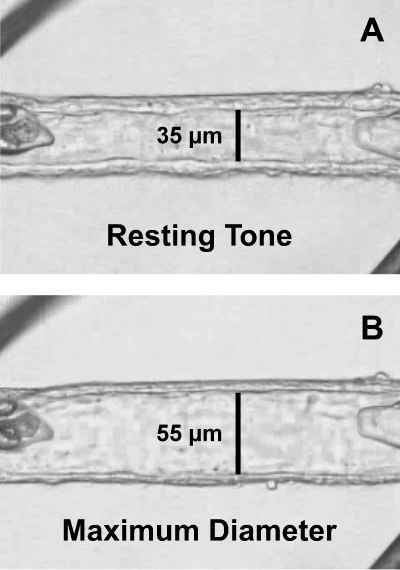
Figure 1.
Image of an isolated human retinal arteriole cannulated with glass micropipettes and secured with ophthalmic sutures. (A) The vessel was transferred to the stage of an inverted microscope and was allowed to develop resting basal tone (35-μm internal diameter) at 55 cm H2O intraluminal pressure. (B) Maximum diameter (55-μm internal diameter) of the vessel was established in Ca2+-free solution containing 0.1 mM sodium nitroprusside. The images were taken through a video port of an inverted microscope.
Experimental Protocols
Cannulated arterioles were bathed in PSS-1% albumin at 36°C to 37°C. After vessels developed a stable basal tone (∼60 minutes), dose-dependent responses to bradykinin, adenosine, sodium nitroprusside, and ET-1 were established. Vessels were exposed to each concentration of agonist for 4 to 5 minutes until a stable diameter was maintained. Vascular response to increased flow was studied under constant intraluminal pressure using dual-reservoir techniques, as described previously.24 In brief, the luminal flow was produced by simultaneously moving the pressure reservoirs in opposite directions of the same magnitude, which generates a pressure gradient (ΔP; range, 10–60 cm H2O) across the length of the vessel without changing intraluminal pressure.24 We have previously demonstrated that the luminal flow is increased linearly with increasing ΔP and the range of mean volumetric flows for ΔP from 0 to 60 cm H2O is 0 to 34.8 nL/s (0–2.1 μL/min),24,25 corresponding to the range reported in retinal arterioles in vivo.26 Vasomotor responses were also examined in the presence of NO synthase inhibitor L-NAME (10 μM)27 and ROCK inhibitor H-1152 (10 μM).28
Chemicals
All drugs were obtained from Sigma-Aldrich (St. Louis, MO). ET-1 was dissolved in distilled water, whereas all other drugs were dissolved in PSS. Subsequent concentrations of ET-1 were diluted in PSS.
Statistical Analysis
At the end of each functional experiment, the vessel was relaxed with 0.1 mM sodium nitroprusside in EDTA (1 mM)-Ca2+-free PSS to obtain its maximum diameter at 55 cm H2O intraluminal pressure.21 Diameter changes in response to vasodilator agonists were normalized to this maximum vasodilation and expressed as percentage of maximum dilation. Diameter changes in response to the ET-1 were normalized to the resting diameter and were expressed as percentage change in resting diameter. The median effective concentration (EC50) value for the vasodilator responses was calculated using statistical software (Prism; GraphPad, San Diego, CA). Data are reported as mean ± SEM, and n represents the number of vessels (2–3 per patient and 1 per pig) studied. Student's t-test or ANOVA followed by Bonferroni multiple-range test was used to determine the significance of experimental interventions, as appropriate. P < 0.05 was considered significant.
Results
Clinical Characteristics
In this study, three patients had hypercholesterolemia, three patients had hypertension, two patients had obesity with one concurrent type 2 diabetes, and two patients were currently smoking. Medications that were taken for these cardiovascular disease risk factors included statins (four patients), angiotensin II type 1 (AT1) receptor antagonist (two patients), non-steroidal anti-inflammatory drugs (NSAIDs; two patients), insulin (one patient), and Ca2+ channel blocker (one patient). One patient was a healthy, 45-year-old male nonsmoker who was not taking any medications.
Vascular Reactivity
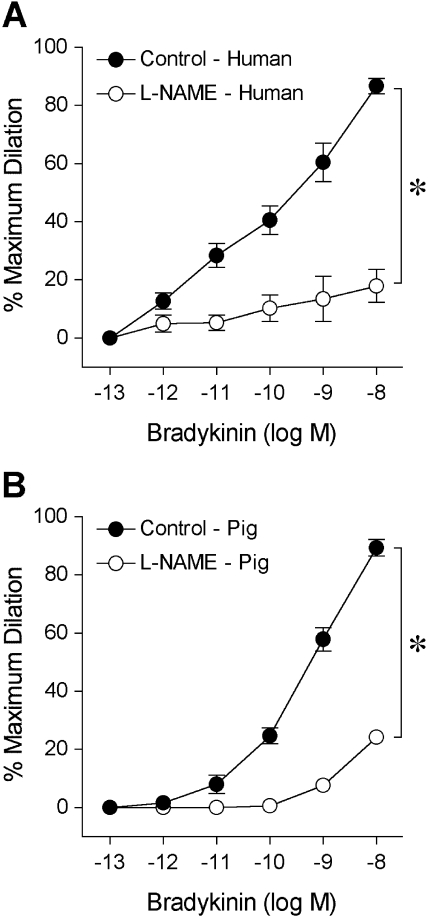
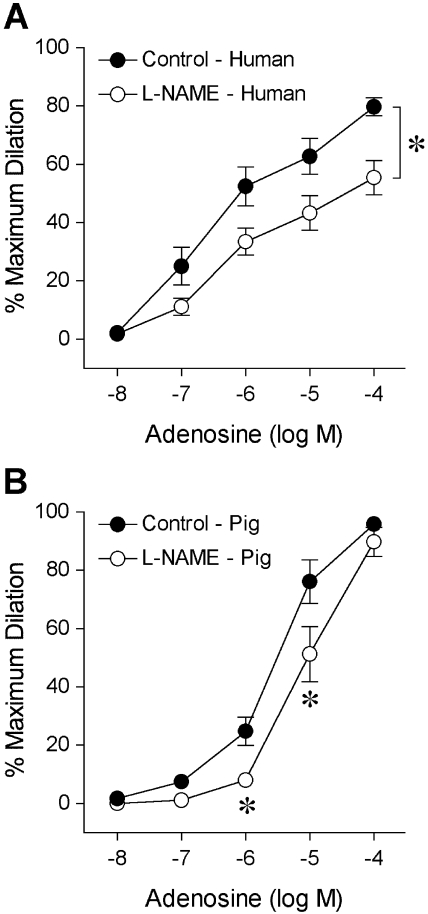
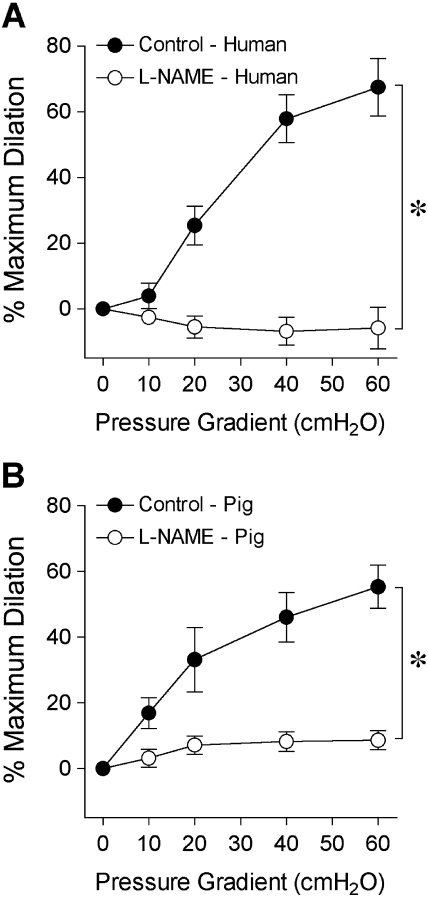
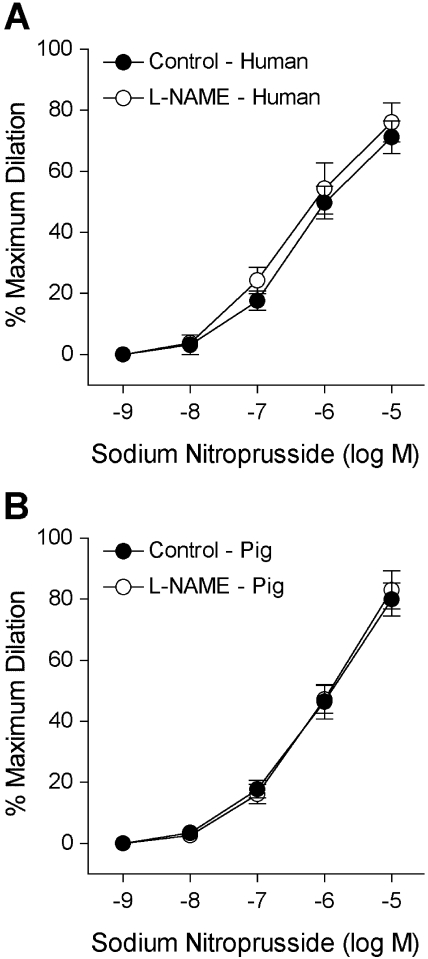
The isolated human retinal arterioles (n = 17) developed stable basal tone (i.e., constricted to 69% ± 1% of their maximum diameter) at 36°C to 37°C bath temperature with 55 cm H2O intraluminal pressure. The average resting and maximum diameters of the vessels were 43 ± 2 μm (range, 32–64 μm) and 63 ± 3 μm (range, 50–89 μm), respectively. In the pig study, the average resting and maximum diameters were 52 ± 2 μm and 85 ± 2 μm (n = 26), respectively. Both human (Fig. 2A) and porcine (Fig. 2B) retinal arterioles dilated to bradykinin in a dose-dependent manner with threshold responses at 1 pM and 10 pM, respectively. Bradykinin exhibited comparable potency (human EC50 = 0.15 nM; porcine EC50 = 0.50 nM; P = 0.06) and elicited nearly 90% maximum dilation at 10 nM in both vessel types (Fig. 2). Subsequent administration of L-NAME abolished these vasodilator responses, except at the last concentration (10 nM), where nearly 20% dilation remained (Fig. 2). Adenosine also caused dose-dependent dilation of human (Fig. 3A) and porcine (Fig. 3B) retinal arterioles, with 80% to 90% maximum dilation at 0.1 mM. However, adenosine exhibited greater potency (human EC50 = 0.26 μM; porcine EC50 = 3.6 μM; P < 0.05) in human vessels. Exposure to L-NAME consistently reduced the dilation of human vessels to adenosine in a significant manner (Fig. 3), whereas the response of porcine vessels was significantly reduced at 1 μM and 10 μM adenosine. Figure 4 displays graded vasodilation of both human and porcine retinal arterioles when the pressure gradient, and thus luminal flow, was increased in a stepwise manner. Under control conditions, the highest flow elicited nearly 50% to 60% of maximum dilation in retinal arterioles from both species; but in the presence of L-NAME, the responses were abolished (zero flow vs. all steps of flow; one-way ANOVA, P > 0.05). Furthermore, both human (Fig. 5A) and porcine (Fig. 5B) retinal arterioles dilated dose dependently to the endothelium-independent NO donor sodium nitroprusside with threshold response at 10 nM, comparable potency (human EC50 = 0.42 μM; porcine EC50 = 0.87 nM; P = 0.10) and maximum dilation of approximately 75% to 80% at 10 μM. L-NAME did not alter this vasodilator response (Fig. 5).
Figure 2.
Vasodilator response of isolated and pressurized human (A) and porcine (B) retinal arterioles to bradykinin. The control human (resting diameter, 44 ± 3 μm; maximum diameter, 64 ± 3 μm; n = 12) and porcine (resting diameter, 49 ± 4 μm; maximum diameter, 77 ± 4 μm; n = 6) retinal arterioles dilated dose dependently to bradykinin. In the presence of NO synthase inhibitor L-NAME (10 μM), the bradykinin-induced dilation of both human (resting diameter, 46 ± 4 μm; maximum diameter, 67 ± 4 μm; n = 8) and porcine (resting diameter, 49 ± 4 μm; maximum diameter, 77 ± 4 μm; n = 6) vessels was significantly attenuated. *P < 0.05 versus control.
Figure 3.
Vasodilator response of isolated and pressurized human (A) and porcine (B) retinal arterioles to adenosine. The control human (resting diameter, 44 ± 4 μm; maximum diameter, 65 ± 4 μm; n = 9) and porcine (resting diameter, 45 ± 4 μm; maximum diameter, 82 ± 5 μm; n = 8) retinal arterioles dilated dose dependently to adenosine. In the presence of L-NAME (10 μM), the adenosine-induced dilation of both human (resting diameter, 43 ± 4 μm; maximum diameter, 65 ± 4 μm; n = 9) and porcine (resting diameter, 44 ± 4 μm; maximum diameter, 82 ± 5 μm; n = 8) vessels was significantly attenuated. *P < 0.05 versus control.
Figure 4.
Vasodilator response of isolated and pressurized human (A) and porcine (B) retinal arterioles to increased flow. The control human (resting diameter, 41 ± 3 μm; maximal diameter, 61 ± 3 μm; n = 6) and porcine (resting diameter, 61 ± 3 μm; maximal diameter, 99 ± 1 μm; n = 6) retinal arterioles dilated to a stepwise increase in pressure gradient (i.e., flow). In the presence of L-NAME (10 μM), the flow-induced dilation of both human (resting diameter, 40 ± 2 μm; maximum diameter, 61 ± 3 μm; n = 6) and porcine (resting diameter, 57 ± 3 μm; maximum diameter, 99 ± 1 μm; n = 6) vessels was abolished. *P < 0.05 versus control
Figure 5.
Vasodilator response of isolated and pressurized human (A) and porcine (B) retinal arterioles to sodium nitroprusside. The control human (resting diameter, 43 ± 6 μm; maximal diameter, 68 ± 6 μm; n = 6) and porcine (resting diameter, 49 ± 4 μm; maximal diameter, 78 ± 4 μm; n = 5) retinal arterioles dilated dose dependently to sodium nitroprusside. The sodium nitroprusside-induced vasodilation of both human (resting diameter, 45 ± 8 μm; maximum diameter, 70 ± 7 μm; n = 5) and porcine (resting diameter, 47 ± 3 μm; maximum diameter, 78 ± 4 μm; n = 5) retinal vessels was not altered by L-NAME (10 μM).
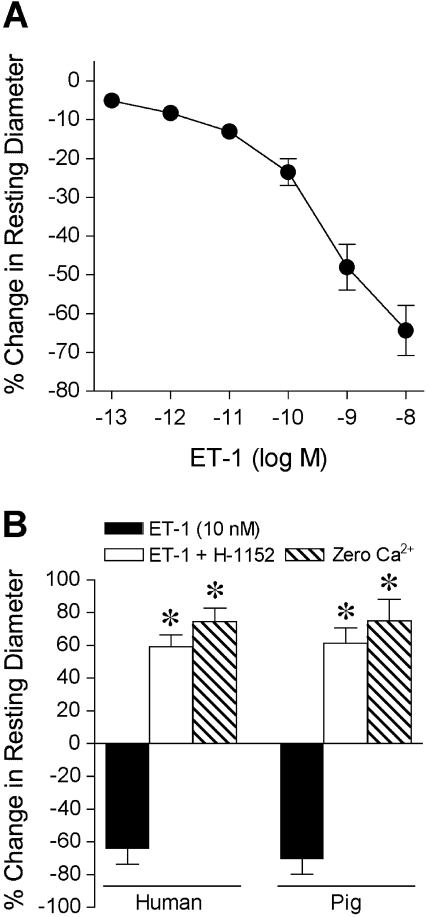
Administration of ET-1 to the vessel bath caused a rapid dose-dependent constriction of the human retinal arterioles (Fig. 6A) that stabilized within 5 minutes at each concentration. A relatively low concentration of ET-1 at 1 pM produced a threshold 5% to 10% constriction of human retinal arterioles with 41 ± 8 μm resting diameter. At 10 nM, the highest ET-1 concentration tested, the retinal arterioles constricted by 62% ± 9% of their resting diameter (Fig. 6A). A similar vasoconstrictor response to ET-1 was also observed in porcine retinal arterioles (data not shown). Administration of specific ROCK inhibitor H-1152 to human retinal arterioles not only fully reversed vasoconstriction evoked by 10 nM ET-1, but also caused a significant loss of myogenic tone by a 59% ± 7% increase in resting diameter toward its maximum level (i.e., in 0.1 mM sodium nitroprusside and Ca2+-free solution), as shown in Figure 6B. In a similar manner, constriction of porcine retinal arterioles to 10 nM ET-1 was reversed during exposure to H-1152, and loss of nearly 80% of myogenic tone was obtained (Fig. 6B). These results suggest a role for the ROCK-signaling pathway leading to the vasoconstriction of retinal arterioles in response to ET-1 stimulation and in the development of myogenic tone.
Figure 6.
Vasoconstrictor response of isolated and pressurized retinal arterioles to ET-1. (A) ET-1 elicited dose-dependent constriction of human retinal arterioles (resting diameter, 41 ± 8 μm; maximum diameter, 65 ± 7 μm; n = 5). (B) ET-1 (10 nM) caused comparable constriction of human (resting diameter, 33 ± 2 μm; maximum diameter, 58 ± 1 μm; n = 4) and porcine (resting diameter, 50 ± 4 μm; maximum diameter, 82 ± 4 μm; n = 4) retinal arterioles. Subsequent administration of H-1152 (10 μM) reversed the ET-1–induced constriction and reduced the myogenic tone of the vessels from both species by increasing the diameter toward maximum. Maximum diameter of vessels was established in Ca2+-free solution containing 0.1 mM sodium nitroprusside (Zero Ca2+). *P < 0.05 versus ET-1 alone.
Discussion
The salient findings of the present study are that arterioles isolated from human retina exhibit myogenic tone and dilate to endogenous chemicals such as adenosine and bradykinin and to physical (increases in shear stress/flow) stimuli in an NO synthase-mediated fashion. The development of myogenic tone and vasoconstriction to ET-1 rely on the activation of the ROCK signaling pathway. Similar results for these vasomotor responses were obtained from isolated porcine retinal arterioles. A fundamental understanding of vasomotor regulation mechanisms of human retinal arterioles is essential because the loss of precision and sensitivity of arteriolar blood flow regulation in the retina are known to be involved in the development of many retinal diseases.29,30
Clinical evidence suggests that NO can influence retinal vascular tone and regulate retinal blood flow in humans.13,14,31,32 Stimulation of metabolic activity in the retina with diffuse flickering light has been shown to increase retinal artery diameter32,33 and retinal blood flow33 in healthy human subjects, which is reduced by NO synthase blockade.32 However, the relative contribution of neural or vascular sources of NO remains unclear, and the associated confounding influences from local hemodynamic changes cannot be excluded because of the inherent limitations of in vivo preparation with multiple tissue types. Furthermore, the role of endothelial NO in signaling vasodilation to putative metabolic vasodilators has not been addressed. Therefore, in the present study, we used isolated vessel techniques to characterize the vasomotor response of single retinal arterioles and to explore the possible pathways involved. The purine metabolite adenosine has been proposed to play a significant role in the local metabolic regulation of retinal blood flow,1–4 whereas bradykinin may influence retinal arteriolar tone because the tissue kallikrein-kinin system, including bradykinin receptors, has been identified in the human retina.5 Our findings support a direct vasoactive role for both substances because human retinal arterioles exhibited robust dilations to bradykinin and adenosine. The vasodilator capacity of human retinal arterioles in response to bradykinin and adenosine was comparable to the responses of porcine retinal arterioles, as reported in the present study. There was a slight difference in the threshold response to bradykinin and to adenosine between the species with higher sensitivity in human vessels. However, the potency of adenosine but not bradykinin was significantly greater in human vessels. At the mechanistic level, NO synthase blockade nearly abolished the dilation of human retinal arterioles to bradykinin and slightly reduced the dilation to adenosine. The relative role of endothelial NO in exerting bradykinin and adenosine responses was also observed in the porcine arterioles, suggesting considerable similarity of vasomotor regulation and the underlying signaling mechanism of human and porcine retinal arterioles in response to the stimulation of the putative metabolic vasodilators adenosine and bradykinin. The ability of the smooth muscle from both species to respond to NO/guanylyl cyclase signaling also appeared equivalent because human and porcine retinal arterioles21 dilated in a comparable manner to NO donor sodium nitroprusside. In concert, our findings provide the first direct evidence for a prominent vascular contribution of NO synthase activation in the dilation of human retinal arterioles to putative endogenous regulators of retinal arteriolar tone.
Mechanical influences, such as an increase in shear stress due to luminal flow, have been shown to elicit endothelium-dependent, NO-mediated dilation in other microvascular beds, including coronary,24 mesenteric,34 and skeletal muscle arterioles.35 This flow-mediated vasodilator response is thought to contribute to flow regulation by recruiting blood flow to the tissue when metabolic demand is increased (e.g., functional hyperemia) or oxygen supply to the tissue is inadequate (e.g., reactive hyperemia and hypoxia).36 Flow-mediated dilation of the brachial artery by ultrasound measurement after transient forearm ischemia has been widely used as an index to assess endothelial function in patients.37,38 Although physiological corroboration of this vascular phenomenon in the retina is lacking, Nagaoka et al.39 have shown that hypoxia in cats elicits a delayed dilation of retinal arterioles after increases in luminal blood flow, velocity, and wall shear rate. Because NO synthase blockade prevented delayed vasodilation without affecting the initial increase in wall shear rate, an index of shear stress, the authors speculated that the activation of endothelial NO production by a shear stress-sensitive mechanism contributed to the delayed vasodilator response. Our present results showing the first direct evidence of the existence of a flow-induced dilation mechanism in human, as well as porcine, retinal arterioles support this contention. This vasoactive response appears to be mediated solely by an NO signaling cascade, because NO synthase blockade abolished the dilation of vessels from both species. Taken together, our findings demonstrate that human and porcine retinal arterioles exhibit the ability to actively respond to increases in luminal flow and elicit NO-mediated vasodilation, but the in vivo significance of the flow-mediated response in the human retinal microcirculation remains to be elucidated.
ET-1, a 21-amino-acid peptide produced primarily by vascular endothelial cells through the endothelin-converting enzyme (ECE-1), has been shown to be the most potent endogenous vasoconstrictor.40 Further research shows that vascular smooth muscle cells41,42 and neuronal cells in the retina43 can also synthesize ET-1. Our recent results in pigs provide direct evidence that retinal arterioles possess endothelial/smooth muscle ECE-1 and that its activation is sufficient to elicit ET-1–mediated smooth muscle constriction through ET type A receptor activation.44 The concentration of ET-1 in the plasma of patients with ischemic types of retinal vein occlusion45,46 and progressive primary open-angle glaucoma47 and in the ocular fluid of patients with diabetic retinopathy48,49 has been reported to be elevated. Moreover, ET receptor blockade has been shown to improve retinal blood flow in patients with primary open-angle glaucoma and in healthy subjects.50 Therefore, it can be inferred that there is a connection between the ET-1 system and human retinal blood flow regulation under health and ocular disease states.
Although the pathophysiology of ET-1 has been implicated in some human ocular diseases, the direct characterization of the dose-dependent reaction of human retinal arterioles to ET-1 is lacking. A previous in vitro study reported that a single high dose of ET-1 (1 nM) caused constriction of cryopreserved human retinal arterioles in the absence of myogenic tone.51 The present study extends these earlier findings, demonstrating the dose-dependent constriction of freshly isolated (1-hour postenucleation) human retinal arterioles to ET-1 in the presence of myogenic tone. We found that ET-1 elicits vasoconstriction at a threshold concentration of 1 pM, which is comparable to that in porcine retinal arterioles.44 Notably, this concentration has been reported for vitreous endothelin levels (1.18 ± 0.05 pM) in patients without ocular or retinal diseases.49 From the ET-1 concentration-response curve shown in Figure 6A, the retinal arterioles exhibited a 10% to 20% constriction at 10 pM. This level of ET-1 is commonly found in the plasma of patients with ocular disease (7.8 ± 1.1 pM)49 or with ischemic retinal vascular occlusions (range, 7–12 pM).45 It should be emphasized that in the vascular walls, ET-1 levels can be approximately three to five times higher than those found in the plasma.52 Therefore, the concentrations of ET-1 used in the present study cover the physiological and pathophysiological ranges. Elevated levels of ET-1, depending on the severity of the disease, in the ocular circulation can potentially cause vessel spasm (focal arteriolar constriction) and lead to the reduction of blood flow and tissue ischemia in the human retina.
Previous studies have shown ET-1–induced constrictions of small bovine retinal arteries53 and human retinal arterioles51 are mediated by the opening of voltage-gated Ca2+ channels, but it remains unknown whether specific protein kinase signaling pathways downstream from Ca2+ are involved in this vasomotor response. ROCK has been shown to be a possible signaling molecule modulating contractile myofilament sensitivity to Ca2+, thus regulating the force of smooth muscle contraction.54 However, it is unclear whether ET-1 also uses this signaling molecule in the retinal arterioles to exert its contractile action. We found that specific pharmacological blockade of ROCK significantly reversed the ET-1–induced constriction, and inhibited the myogenic tone, of both human and porcine retinal arterioles (Fig. 6B), indicating the crucial role of the ROCK pathway in evoking retinal arteriolar constriction and maintaining resting vascular tone. Because accumulating evidence suggests that ROCK activation is closely associated with numerous vascular diseases,55 it is speculated that enhanced ET-1 release during ocular disease development may contribute not only to the increased basal tone (i.e., reduction in resting diameter) and enhanced vasoconstriction but also to the vascular pathology. Taken together, our findings show that human retinal arterioles can actively respond to ET-1 stimulation and that ROCK signaling plays a critical role in this contractile function.
A possible limitation of our study is the inability to determine whether vascular disease influences the results. Most of the patients were middle aged to elderly and had taken medications for cardiovascular disease (statins, AT1 receptor antagonists, NSAIDs, insulin) that could have influenced vascular function. However, one of the patients was middle aged and relatively healthy, and we found that the vasomotor activity in this patient was not notably different from that of patients with known vascular disease. Although advancing age and risk factors for cardiovascular disease (hypertension, hypercholesterolemia, diabetes) can trigger the early pathogenic event of endothelial dysfunction, it appears that as humans age, either the retinal arterioles maintain NO bioavailability for vasodilation or the medications used to treat vascular disease have the ability to restore endothelial function related to NO signaling. Interestingly, statins, which were taken by 4 of 7 donors in the present study for hypercholesterolemia, have been shown to exert pleiotropic effects independently of cholesterol lowering, such as improvement in endothelium-dependent, NO-mediated dilation in patients with cardiovascular disease.56,57 In healthy subjects, we have recently shown that simvastatin elicits an increase in retinal blood flow in association with elevated plasma levels of nitrite and nitrate, the major oxidative metabolites of NO.23 We also have demonstrated that simvastatin dilates isolated porcine retinal arterioles in part through endothelial-released NO.58 It is speculated that statins might have contributed to the restoration or maintenance of NO-dependent retinal vasomotor function in patients with cardiovascular risk in the present study. It is also worth noting that we only examined human retinal arterioles in the range of 30 to 60 μm in the present study. Although blood flow is known to be regulated at the microcirculatory level, the possible heterogeneity in vasomotor responses and signal transduction mechanisms of vessel sizes outside the present study cannot be excluded. Future studies with larger sample sizes at different arteriolar segments are needed to adequately address these questions and to pursue more detailed mechanistic analysis, especially because an imbalance of NO and ET-1 levels has been implicated in pathophysiological conditions such as retinal ischemic disease and diabetic retinopathy.59
In summary, we found that isolated human retinal arterioles develop a stable basal tone and dilate to bradykinin and adenosine, and to increased luminal flow, in an NO-dependent manner. We also demonstrated that ET-1, at in vivo pathophysiological concentrations, is a potent vasoconstrictor of human and porcine retinal arterioles. It appears that the activation of the ROCK signaling pathway is responsible for the vasoconstriction to ET-1 and for the maintenance of myogenic tone. Interestingly, our present study discloses similarities in vasoreactivity and its underlying signaling mechanisms between human and porcine retinal arterioles, which provides support for the pig as a relevant animal model for the study of the retinal microcirculation. Our results provide initial information toward our understanding of NO and ET-1 physiology and pathophysiology concerning the regulation of retinal arteriolar tone and may suggest important therapeutic targets for patients with ocular diseases related to retinal vascular dysfunction.
Acknowledgments
The authors thank Glen O. Brindley, MD, and Jonathan H. Tsai, MD, for their contributions to patient enrollment and Wenjuan Xu and Hongfang Liu for their expert technical assistance.
Footnotes
Supported by Scott and White Research Foundation (TWH), Retina Research Foundation (TWH, LK), National Institutes of Health/National Eye Institute Grants R01EY018420 (TWH) and K08EY016143 (RHR), the Scott and White Research Foundation Ophthalmic Vascular Research Program (LK), and the Kruse Family Endowment Fund (LK).
Disclosure: T.W. Hein, None; R.H. Rosa, Jr, None; Z. Yuan, None; E. Roberts, None; L. Kuo, None
References
- 1.Braunagel SC, Xiao JG, Chiou GC. The potential role of adenosine in regulating blood flow in the eye. J Ocul Pharmacol 1988;4:61–73 [DOI] [PubMed] [Google Scholar]
- 2.Gidday JM, Park TS. Adenosine-mediated autoregulation of retinal arteriolar tone in the piglet. Invest Ophthalmol Vis Sci 1993;34:2713–2719 [PubMed] [Google Scholar]
- 3.Gidday JM, Maceren RG, Shah AR, Meier JA, Zhu Y. KATP channels mediate adenosine-induced hyperemia in retina. Invest Ophthalmol Vis Sci 1996;37:2624–2633 [PubMed] [Google Scholar]
- 4.Campochiaro PA, Sen HA. Adenosine and its agonists cause retinal vasodilation and hemorrhages: implications for ischemic retinopathies. Arch Ophthalmol 1989;107:412–416 [DOI] [PubMed] [Google Scholar]
- 5.Ma JX, Song Q, Hatcher HC, Crouch RK, Chao L, Chao J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res 1996;63:19–26 [DOI] [PubMed] [Google Scholar]
- 6.Feldstein C, Romero C. Role of endothelins in hypertension. Am J Ther 2007;14:147–153 [DOI] [PubMed] [Google Scholar]
- 7.Ihling C, Bohrmann B, Schaefer HE, Technau-Ihling K, Loeffler BM. Endothelin-1 and endothelin converting enzyme-1 in human atherosclerosis—novel targets for pharmacotherapy in atherosclerosis. Curr Vasc Pharmacol 2004;2:249–258 [DOI] [PubMed] [Google Scholar]
- 8.Pemp B, Garhofer G, Weigert G, et al. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci 2009;50:4029–4032 [DOI] [PubMed] [Google Scholar]
- 9.Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol 2008;246:763–769 [DOI] [PubMed] [Google Scholar]
- 10.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci 1996;37:886–897 [PubMed] [Google Scholar]
- 11.Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol 2004;88:887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandecka A, Dawczynski J, Blum M, et al. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care 2007;30:3048–3052 [DOI] [PubMed] [Google Scholar]
- 13.Michelson G, Warntges S, Harazny J, Oehmer S, Delles C, Schmieder RE. Effect of NOS inhibition on retinal arterial and capillary circulation in early arterial hypertension. Retina 2006;26:437–444 [DOI] [PubMed] [Google Scholar]
- 14.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 2004;35:1289–1293 [DOI] [PubMed] [Google Scholar]
- 15.Trepels T, Zeiher AM, Fichtlscherer S. The endothelium and inflammation. Endothelium 2006;13:423–429 [DOI] [PubMed] [Google Scholar]
- 16.Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res 2007;26:205–238 [DOI] [PubMed] [Google Scholar]
- 17.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst 2002;3:1–15 [DOI] [PubMed] [Google Scholar]
- 18.Lam HC, Lee JK, Lu CC, Chu CH, Chuang MJ, Wang MC. Role of endothelin in diabetic retinopathy. Curr Vasc Pharmacol 2003;1:243–250 [DOI] [PubMed] [Google Scholar]
- 19.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1–induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol 2002;283:H983–H989 [DOI] [PubMed] [Google Scholar]
- 20.Batchelor TJ, Sadaba JR, Ishola A, Pacaud P, Munsch CM, Beech DJ. Rho-kinase inhibitors prevent agonist-induced vasospasm in human internal mammary artery. Br J Pharmacol 2001;132:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hein TW, Yuan Z, Rosa RH, Jr, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci 2005;46:2113–2119 [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni P, Joshua IG, Roberts AM, Barnes G. A novel method to assess reactivities of retinal microcirculation. Microvasc Res 1994;48:39–49 [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka T, Takahashi A, Sato E, et al. Effect of systemic administration of simvastatin on retinal circulation. Arch Ophthalmol 2006;124:665–670 [DOI] [PubMed] [Google Scholar]
- 24.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol 1990;259:H1063–H1070 [DOI] [PubMed] [Google Scholar]
- 25.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 1995;92:518–525 [DOI] [PubMed] [Google Scholar]
- 26.Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci 1985;26:1124–1132 [PubMed] [Google Scholar]
- 27.Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci 2006;47:693–699 [DOI] [PubMed] [Google Scholar]
- 28.Mueed I, Tazzeo T, Liu C, et al. Isoprostanes constrict human radial artery by stimulation of thromboxane receptors, Ca2+ release, and RhoA activation. J Thorac Cardiovasc Surg 2008;135:131–138 [DOI] [PubMed] [Google Scholar]
- 29.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 2007;14:25–38 [DOI] [PubMed] [Google Scholar]
- 30.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 2008;27:284–330 [DOI] [PubMed] [Google Scholar]
- 31.Polak K, Dorner G, Kiss B, et al. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol 2000;84:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorner GT, Garhofer G, Kiss B, et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 2003;285:H631–H636 [DOI] [PubMed] [Google Scholar]
- 33.Garhofer G, Zawinka C, Resch H, Huemer KH, Dorner GT, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision Res 2004;44:833–838 [DOI] [PubMed] [Google Scholar]
- 34.Smiesko V, Lang DJ, Johnson PC. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. Am J Physiol 1989;257:H1958–H1965 [DOI] [PubMed] [Google Scholar]
- 35.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res 1994;74:416–421 [DOI] [PubMed] [Google Scholar]
- 36.Davis MJ, Hill M, Kuo L. Local regulation of blood flow. In: Tuma RF, Duran WN, Ley K. eds. Handbook of Physiology: The Cardiovascular System: Microcirculation Bethesda, MD: The American Physiological Society and Elsevier; 2008:159–284 [Google Scholar]
- 37.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111–1115 [DOI] [PubMed] [Google Scholar]
- 38.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol 1996;270:H1435–H1440 [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka T, Sakamoto T, Mori F, Sato E, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci 2002;43:3037–3044 [PubMed] [Google Scholar]
- 40.Yanagisawa M, Kurihara H, Kimura S, et al. A novel vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415 [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Davenport A. Secretion of endothelin-1 and endothelin-3 by human cultured vascular smooth muscle cells. Br J Pharmacol 1995;114:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire JJ, Johnson CM, Mockridge JW, Davenport AP. Endothelin converting enzyme (ECE) activity in human vascular smooth muscle. Br J Pharmacol 1997;122:1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ripodas A, de Juan J, Roldan-Pallares M, et al. Localisation of endothelin-1 mRNA expression and immunoreactivity in the retinal and optic nerve from human and porcine eye: evidence for endothelin-1 expression in astrocytes. Brain Res 2001;912:137–143 [DOI] [PubMed] [Google Scholar]
- 44.Hein TW, Ren Y, Yuan Z, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci 2009;50:3329–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iannaccone A, Letizia C, Pazzaglia S, Vingolo EM, Clemente G, Pannarale MR. Plasma endothelin-1 concentrations in patients with retinal vein occlusions. Br J Ophthalmol 1998;82:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haufschild T, Prunte C, Messerli J, Flammer J. Increased endothelin-1 plasma level in young adults with retinal vascular occlusive diseases. Klin Monatsbl Augenheilkd 2004;221:357–359 [DOI] [PubMed] [Google Scholar]
- 47.Emre M, Orgül S, Haufschild T, Shaw S, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol 2005;89:60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oku H, Kida T, Sugiyama T, Hamada J, Sato B, Ikeda T. Possible involvement of endothelin-1 and nitric oxide in the pathogenesis of proliferative diabetic retinopathy. Retina 2001;21:647–651 [DOI] [PubMed] [Google Scholar]
- 49.Roldan-Pallares M, Rollin R, Mediero A, et al. Immunoreactive ET-1 in the vitreous humor and epiretinal membranes of patients with proliferative vitreoretinopathy. Mol Vis 2005;11:461–471 [PubMed] [Google Scholar]
- 50.Resch H, Karl K, Weigert G, et al. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest Ophthalmol Vis Sci 2009;50:358–363 [DOI] [PubMed] [Google Scholar]
- 51.Yu DY, Su EN, Cringle SJ, Alder VA, Yu PK, Desantis L. Effect of betaxolol, timolol and nimodipine on human and pig retinal arterioles. Exp Eye Res 1998;67:73–81 [DOI] [PubMed] [Google Scholar]
- 52.Grantham JA, Schirger JA, Williamson EE, et al. Enhanced endothelin-converting enzyme immunoreactivity in early atherosclerosis. J Cardiovasc Pharmacol 1998;31:S22–S26 [DOI] [PubMed] [Google Scholar]
- 53.Nyborg N, Prieto D, Benedito S, Nielsen P. Endothelin-1–induced contraction of bovine retinal small arteries is reversible and abolished by nitrendipine. Invest Ophthalmol Vis Sci 1991;32:27–31 [PubMed] [Google Scholar]
- 54.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 2000;522:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 2006;290:C661–C668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masumoto A, Hirooka Y, Hironaga K, et al. Effect of pravastatin on endothelial function in patients with coronary artery disease (cholesterol-independent effect of pravastatin). Am J Cardiol 2001;88:1291–1294 [DOI] [PubMed] [Google Scholar]
- 57.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009;119:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagaoka T, Hein TW, Yoshida A, Kuo L. Simvastatin elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and mevalonate-rho kinase pathways. Invest Ophthalmol Vis Sci 2007;48:825–832 [DOI] [PubMed] [Google Scholar]
- 59.Haefliger IO, Flammer J, Beny JL, Luscher TF. Endothelium-dependent vasoactive modulation in the ophthalmic circulation. Prog Retin Eye Res 2001;20:209–225 [DOI] [PubMed] [Google Scholar]