IL-33 signaling through ST2 polarizes macrophages to an M2 phenotype and ameliorates bacterial infection of the cornea.
Abstract
Purpose.
To determine the role of IL-33 in resistance to Pseudomonas aeruginosa keratitis.
Methods.
Corneal IL-33 mRNA and protein levels were tested in susceptible C57BL/6 (B6) and resistant BALB/c mice. B6 mice were injected with recombinant mouse IL-33 (rmIL-33) and disease severity, bacterial load, polymorphonuclear neutrophils (PMN) infiltrate, gene expression of inflammatory, and T-helper (Th)1/Th2 cytokines were tested by RT-PCR. IL-33 signaling and macrophage (Mφ) polarization also were examined.
Results.
IL-33 mRNA and protein were expressed constitutively in the normal corneas of both groups and were significantly elevated at 1 to 5 days after infection in BALB/c over B6 mice. rmIL-33–treated B6 mice showed less severe disease than did PBS controls and exhibited decreased bacterial load, PMN infiltrate, and corneal mRNA levels for IL-1β, MIP-2, and TNF-α. Th2-type cytokines (IL-4, -5, -10) also were significantly upregulated, and protein levels for TNF-α and IL-10 confirmed the mRNA data. To further investigate IL-33 in corneal inflammation, it was overexpressed in Mφ (RAW264.7 cells). This significantly increased IL-5 and IL-10, while it decreased IFN-γ and other pro-inflammatory cytokines. The role of the Mφ was further tested in infected rmIL-33 compared with PBS-injected mice. Immunostaining showed that rmIL-33 injection shifted Mφ polarization from NO synthase 2 to arginase production. Furthermore, peritoneally elicited cells (B6 mice) treated with lipopolysaccharide and rmIL-33 exhibited elevated ST2 levels and a shift from IL-12 to IL-10 mRNA production.
Conclusions.
These data provide evidence that IL-33 promotes a Th2-type immune response and reduces inflammation by polarizing the Mφ production of anti-inflammatory mediators in the cornea.
Keratitis caused by Pseudomonas aeruginosa is characterized by epithelial edema, stromal infiltrate, and corneal ulceration and can lead to vision loss.1 This sight-threatening infectious disease is, in large part, a consequence of the host inflammatory response,2 with previous studies showing that both innate and adaptive host immune responses are critical in its development.3,4 The innate host response to bacterial infection is primarily mediated by polymorphonuclear neutrophils (PMN) and macrophages (Mφ).5 The initial phase of host defense against many invading microbes such as P. aeruginosa also involves a family of proteins called Toll-like receptors (TLRs), which recognize microbial products and trigger an innate immune response,6,7 leading to the expression of various pro-inflammatory and anti-inflammatory cytokines/chemokines,8 including tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and macrophage inflammatory protein (MIP)-2, interleukin (IL)-1β, -4, -5, -6, -10, and -12. Inflammatory mediators may promote the elimination of bacteria, but, if they are unbalanced or uncontrolled, they may augment the inflammatory response, leading to tissue damage and corneal perforation. For example, gene expression profiling of Mφ has shown that Gram-negative bacteria induce transcriptional activation of a common host response that induces genes in Mφ, expressing an M1 program.9 M1 polarized cells, prototypical in Th1 responder strains of mice, such as B6,10 are characterized by the production of IL-12, TNF-α, MIP-2, and high levels of nitric oxide synthase 2 (NOS2).11,12 Excessive or prolonged M1 polarization can lead to tissue injury and contribute to pathogenesis. In contrast, Th2 responder mice (BALB/c) have a higher population of alternatively activated Mφ, designated M2 cells, that produce anti-inflammatory mediators such as IL-10, IL-1ra, and type II IL-1 decoy receptor,12 upregulate the production of arginase 1 (Arg1),10 and are critical to disease resolution. A subset of the latter cells can be induced by agonists of TLRs, including LPS.9 Thus, negative regulation of TLR signaling may be critical to avoid a detrimental and inappropriate inflammatory response.13 In this regard, the soluble TLRs (sTLR2 and sTLR4) act as decoy receptors by binding to their ligands and competitively blocking TLR2 and TLR4 signaling,14 whereas the IL-1 receptor–related protein ST2 negatively regulates TLR signaling by sequestering the recruitment of adaptor molecules such as MyD88 and TIRAP.15
ST2 is a novel member of the TLR superfamily with unique anti-inflammatory properties. ST2 mRNAs were expressed in all human tissues examined, induced by cytokines and phorbol esters. Three species of mRNAs were observed in different human cells and tissues. In contrast, only two species of ST2 mRNAs were observed in BALB/c-3T3 cells, and ST2 mRNA was absent in most tissues of normal mice.16 Higher expression levels on the surfaces of fibroblasts,17 mast cells,18 and Th2 cells19,20 have been reported. IL-33 is a recently identified member of the IL-1 family that signals through ST2. The interaction between IL-33 and ST2 mediate its biological effects by recruiting the adaptor molecules MyD88, IRAK1, IRAK4, and TRAF6, activating mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-κB, leading to the production of Th2-associated cytokines such as IL-4, -5, and -13. IL-33 is most closely related structurally to the IL-1 family, including IL-1β and IL-18, and has important functions in host defense, immune regulation, and inflammation.21 However, unlike IL-1β and IL-18, which promote pro-inflammatory and Th1-associated responses, IL-33 predominantly induces the production of Th2 cytokines and increases levels of serum immunoglobulin.21 In vitro, IL-33 enhanced IL-5 and IL-13 production by polarized Th2, but not Th1, cell lines.22 In addition, the in vivo administration of exogenous IL-33 into naive mice provoked type 2 responses, production of Th2 cytokines and IgE, eosinophilia, and some pathologic changes in mucosal tissues.21
Although IL-33 was detected in epithelial cells from the bronchus and small airways, fibroblasts, and smooth muscle cells, which suggested a possible role in the regulation of mucosal inflammation,23,24 the expression and functional role of IL-33 (as a specific ligand of ST2) in bacterial keratitis remains unknown. In the present study, we tested the expression of IL-33 in the cornea of B6 and BALB/c mice before and after P. aeruginosa infection. Our data provide direct evidence that IL-33 is constitutively expressed in the cornea (epithelium) and is significantly elevated in BALB/c over B6 mice after infection. Furthermore, B6 mice treated with rmIL-33 protein had less severe corneal disease after P. aeruginosa infection. Mechanistically, our data support the tenets that treatment reduced corneal infection and inflammation by negatively regulating pro-inflammatory cytokines, polarizing corneal Mφ to produce anti-inflammatory mediators such as Arg1, and upregulating type 2 and anti-inflammatory cytokine production.
Materials and Methods
Corneal Infection
Eight-week-old female C57BL/6 (B6) and BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized with ether and placed beneath a stereoscopic microscope at 40× magnification, and the cornea (left eye) was wounded with three 1-mm incisions using a sterile 25-gauge needle. Then a bacterial suspension (5 μL) containing 1 × 106 colony-forming units (CFU)/μL of P. aeruginosa (ATCC strain 19660), prepared as described previously,25,26 was applied. Corneas were examined at 1, 3, and 5 days postinfection (dpi) to monitor disease. Animals were treated humanely and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ocular Response to Infection
Corneal disease was graded in rmIL-33– compared with PBS-treated mice using a scale described previously27: 0, clear or slight opacity partially or fully covering the pupil; +1, slight opacity fully covering the anterior segment; +2, dense opacity partially or fully covering the pupil; +3, dense opacity covering the entire anterior segment; and +4, corneal perforation or phthisis. A clinical score was calculated for each group of mice (n = 5/group/treatment/experiment) to express disease severity, and slit lamp photography (5 dpi) was used to illustrate disease.
Bacterial Plate Counts
Corneas from rmIL-33 and control PBS-treated B6 mice were collected (n = 5/group/time/experiment) at 1 and 5 dpi, and the number of viable bacteria were quantitated. Individual corneas were homogenized in sterile saline (0.85% NaCl) containing 0.25% bovine serum albumin (BSA). Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar (Difco Laboratories, Detroit, MI) in triplicate, and plates were incubated overnight at 37°C. Results are reported as 105 CFU per cornea ± SEM.
Myeloperoxidase Assay
A myeloperoxidase (MPO) assay was used to quantitate PMN in the cornea of rmIL-33– and control PBS-treated B6 mice. Corneas (n = 5/group/time/experiment) were excised at 1 and 5 dpi and homogenized in 1.0 mL of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St. Louis, MO). Samples were freeze-thawed four times and centrifuged at 13,000 rpm for 10 minutes. A 0.1-mL aliquot of the supernatant was added to 2.9 mL of 50 mM phosphate buffer containing o-dianisidine dihydrochloride (16.7 mg/100 mL) and hydrogen peroxide (0.0005%). The change in absorbance at 460 nm was monitored for 5 minutes at 30-second intervals, and the results were expressed as units of MPO per cornea, where 1 U activity is equivalent to 2 × 105 PMN.28
IL-33 Protein Treatment
B6 mice (n = 5/group/treatment/experiment) were injected subconjunctivally with 1 μg rmIL-33 protein (Axxora LLC, San Diego, CA) or PBS 1 day before infection. One and 3 dpi, each mouse was injected intraperitoneally with 1 μg rmIL-33 diluted in 100 μL PBS. Control mice similarly received an equal volume of PBS.
Macrophage Depletion
Liposomes, composed of phospholipid bilayers and containing dichloromethylene diphosphonate (clodronate) or PBS (control liposomes), were prepared as described.29 Clodronate was provided by Roche Diagnostics GmbH (Mannheim, Germany). Clodronate-containing liposome suspension (8 μL) was injected subconjunctivally in B6 mice using a 50-μL Hamilton syringe with a 30-gauge needle, as generally described. This route of injection has been shown previously to deplete Mφ in the bulbar conjunctiva.30,31 On day −4 (day 0 = day of infection), clodronate-containing liposomes (5 μL/mouse/group) were injected; on day −2, 3 μL more were injected similarly. On day −1, 5 μL rmIL-33 or PBS was injected as described; 1 μg also was injected intraperitoneally on 1 and 3 dpi. Corneas were infected (day 0), as described. Control mice for each mouse strain received similar injections of PBS liposomes.
Cell Culture and IL-33 Overexpression
RAW264.7 (ATCC; TIB-71) cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen Life Technologies, Carlsbad, CA), l-glutamine (4 μM), penicillin (100 U/mL), and streptomycin (100 μg/mL; Invitrogen) at 37°C and 5% CO2. After lipopolysaccharide (LPS, P. aeruginosa serotype 10; Sigma) (1 μg/mL) stimulation for 2, 4, 6, and 8 hours, ST2 mRNA expression was determined by real-time RT-PCR. To further investigate the immunoregulatory role of IL-33 in the host inflammatory response, another series of experiments was performed to test the effect of IL-33/ST2 signaling on LPS stimulation. In accordance with the manufacturer's protocol, a six-well plate of RAW cells (5 × 105 cells/well) was transiently transfected (18 hours) with a mouse IL-33 expression plasmid (pORF-mIL-33; InvivoGen, San Diego, CA) or empty vector (InvivoGen) control using transfectamine-2000 (Invitrogen). IL-33 transfected RAW cells were stimulated with LPS (1 μg/mL) for 6 hours, and cells were collected and processed for total RNA extraction as described. Real-time RT-PCR detected mRNA levels of pro-inflammatory cytokines (IL-1β, MIP-2, IL-6, and TNF-α), Th1-related (IFN-γ and IL-12) and Th2-type cytokines (IL-5 and IL-10).
Real-time RT-PCR
Peritoneal Mφ, RAW cells, or whole corneas32,33 harvested from normal and infected (1, 3, and 5 dpi) B6 and BALB/c and rmIL-33 protein or PBS-treated B6 mice (1 and 5 dpi) (n = 5 mice/group/time/experiment) were collected and homogenized in RNA STAT-60 (Tel-Test, Friendsville, TX), and total RNA was isolated according to the manufacturer's instruction. Then 1 μg total RNA was reverse transcribed to produce a cDNA template for PCR reaction. For real-time RT-PCR amplification, 1 μL each cDNA sample was used per 20 μL PCR reaction. Sequences of primer sets for real-time RT-PCR are shown in Table 1. RT-PCR measurements were analyzed in duplicate in three independent runs using a real-time detection system (Bio-Rad Laboratories, Hercules, CA). Relative mRNA levels of IL-33, IL-1β, MIP-2, TNF-α, IFN-γ, IL-4, -5, -6, -10, -12, and ST2 were calculated after normalizing to β-actin, as described.4,34,35
Table 1.
Primer Sequences for Real-time PCR
| Gene | Primer Sequences |
|---|---|
| IL-33 | Forward CCT CCC TGA GTA CAT ACA ATG ACC |
| Reverse GTA GTA GCA CCT GGT CTT GCT CTT | |
| IL-1β | Forward CGC AGC AGC ACA TCA ACA AGA GC |
| Reverse TGT CCT CAT CCT GGA AGG TCC ACG | |
| MIP-2 | Forward TGT CAA TGC CTG AAG ACC CTG CC |
| Reverse AAC TTT TTG ACC GCC CTT GAG AGT GG | |
| TNF-α | Forward GCA AGC TTC GCT CTT CTG TCT ACT GAA CTT |
| Reverse GCT CTA GAA TGA GAT AGC AAA TCG GCT GAC | |
| IFN-γ | Forward GTT ACT GCC ACG GCA CAG TCA TTG |
| Reverse ACC ATC CTT TTG CCA GTT CCT CCA G | |
| IL-4 | Forward GAA GAA CAC CAC AGA GAG TGA GC |
| Reverse CTT TCA GTG ATG TGG ACT TGG AC | |
| IL-5 | Forward AAA GAG AAG TGT GGC GAG GAG AGA C |
| Reverse CCT TCC ATT GCC CAC TCT GTA CTC ATC | |
| IL-6 | Forward CAC AAG TCC GGA GAG GAG AC |
| Reverse CAG AAT TGC CAT TGC ACA AC | |
| IL-10 | Forward TGC TAA CCG ACT CCT TAA TGC AGG AC |
| Reverse CCT TGA TTT CTG GGC CAT GCT TCT C | |
| IL-12 | Forward GGT CAC ACT GGA CCA AAG GGA CTA TG |
| Reverse ATT CTG CTG CCG TGC TTC CAA C | |
| ST2 | Forward TGA CGG CCA CCA GAT CAT TCA CAG |
| Reverse GCC AAA GCA AGC TGA ACA GGC AAT AC | |
| β-Actin | Forward GAT TAC TGC TCT GGC TCC TAG C |
| Reverse GAC TCA TCG TAC TCC TGC TTG C |
ELISA Analysis
Protein levels for IL-33 and cytokines/chemokines were quantitated using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN), as described.35 Normal uninfected corneas and infected corneas of B6 and BALB/c mice at 1 and 5 dpi (n = 5/group/time/experiment) were collected and homogenized with a glass pestle (Fischer, Itasca, IL) in 500 μL PBS containing 0.1% Tween 20, a protease inhibitor cocktail (Complete; Roche) was centrifuged, and a 50-μL aliquot of the supernatant was assayed for IL-33 protein according to the manufacturer's instructions.
Infected corneas of B6 mice (n = 5/group/time/experiment) treated with rmIL-33 or PBS were collected at 3 and 5 dpi and tested for TNF-α and IL-10 protein levels. Individual samples were homogenized as described in 1 mL PBS containing 0.1% Tween 20 and centrifuged, and 50 μL each supernatant was assayed according to the manufacturer's instructions. The reported sensitivities of these assays are <6.5 pg/mL for IL-33, <5.1 pg/mL for TNF-α, and <4 pg/mL for IL-10.
Immunostaining
Normal uninfected and infected eyes were enucleated (n = 3/group/time) at 1 dpi from B6 and BALB/c mice, immersed in PBS, embedded in OCT compound (Tissue-Tek; Miles, Elkhart, IN), and frozen in liquid nitrogen. Ten-micrometer sections were cut, mounted to polylysine-coated glass slides, incubated at 37°C overnight, and fixed in acetone. After blocking with 0.01 M phosphate buffer containing 2.5% BSA and donkey IgG (1:100) for 30 minutes at room temperature, sections were incubated with primary antibody and goat anti-mouse IL-33 (10 μg/mL; R&D Systems) for 1 hour, followed by Alexa Fluor 660-conjugated donkey anti-goat antibody (1:1500) for 1 hour. Sections were then incubated for 2 minutes with nuclear acid stain (SYTOX Green, 1:15,000; Lonza, Walkersville, MD). Controls were similarly treated with either omission of the primary antibody or replacement of it with species-specific IgG. Sections were visualized, and digital images were captured with a confocal laser scanning microscope (TSC SP2; Leica Microsystems, Exton, PA).
Normal uninfected and infected eyes were enucleated (n = 3/group/time) at 1, 3, and 5 dpi from PBS and rmIL-33–treated B6 mice and were prepared for immunostaining, as described. After blocking with 0.01 M phosphate buffer containing 2.5% BSA and goat IgG (1:100) for 3 minutes at room temperature, sections were incubated with primary antibodies, rat anti-mouse Mφ (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-mouse NOS2 (1:100; Santa Cruz Biotechnology), rabbit anti-mouse Arg1 (1:100; Santa Cruz Biotechnology), or rabbit anti-mouse ST2 (2 μg/mL; ProSci Incorporated, Poway, CA) for 1 hour, followed by Alexa Fluor 546-conjugated goat anti-rat antibody (1:1500; Invitrogen) or Alexa Fluor 633-conjugated goat anti-rabbit antibody (1:1500; Invitrogen) for another hour. Controls were run with species-specific IgG replacing the primary antibody. SYTOX nuclear label, confocal visualization, and image capture were performed as described.
Mφ Isolation and Stimulation to Detect ST2 by RT-PCR
Mφ were isolated from B6 mice as described.2,33,36 Briefly, Mφ were induced into the peritoneal cavity by intraperitoneal injection of 1 mL of 3% Brewer's thioglycollate medium (BD Biosciences, Sparks, MD) 5 days before kill. Cells were collected by peritoneal lavage with DMEM containing 10% FBS and stained with 0.4% trypan blue, and viable cells (>95%) were counted with a hemacytometer. Mφ were seeded into 24-well tissue culture plates at a density of 6 × 106 cells/well and were incubated for 4 hours. Then nonadherent cells were removed, and fresh media containing PBS (negative control) or LPS (1 μg/mL) with or without 0.5 μg/mL rmIL-33 was added. Cells were stimulated for 18 hours at 37°C and were processed for RT-PCR to detect mRNA levels of ST2, IL-10, and IL-12, as described.
Statistical Analysis
The difference in clinical score between two groups at each time point was tested by the Mann-Whitney U test. An unpaired, two-tailed Student's t-test was used to determine statistical significance for RT-PCR, MPO, bacterial plate count, and ELISA analyses, and data were considered significant at P < 0.05. All experiments were performed at least twice to ensure reproducibility, and data from both experiments are shown.
Results
IL-33 Expression in Cornea
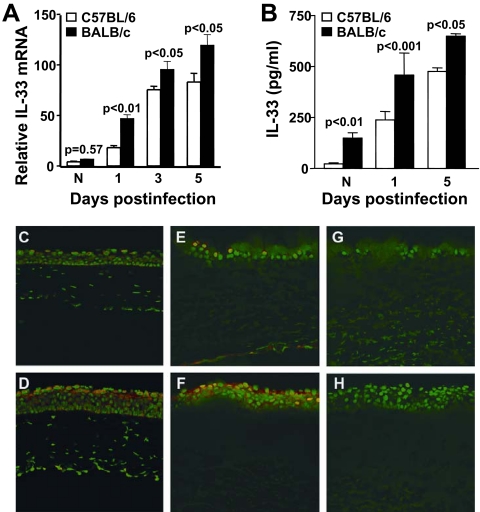
IL-33 mRNA levels were constitutively expressed similarly in the uninfected normal corneas of both mouse groups. However, after infection, IL-33 mRNA levels were significantly upregulated in BALB/c over B6 mouse corneas at 1 (P < 0.01), 3 (P < 0.05), and 5 (P < 0.05) dpi (Fig. 1A). IL-33 protein in the normal BALB/c mouse was greater than in B6 mice (P < 0.01), but in the infected corneas, protein correlated with gene expression levels in that significantly more IL-33 was detected at 1 (P < 0.001) and 5 dpi (P < 0.05) (Fig. 1B) in BALB/c compared with B6 mice. Immunostaining for IL-33 in the normal uninfected cornea of B6 (Fig. 1C) compared with BALB/c (Fig. 1D) mice showed qualitatively more staining in the epithelium of BALB/c mice. This pattern continued at 1 dpi in BALB/c (Fig. 1F) compared with B6 mouse cornea (Fig. 1E). Negative controls (primary antibody substituted with species-specific IgG) showed no positive IL-33 staining in normal cornea (data not shown) or at 1 dpi (Figs. 1G, 1H).
Figure 1.
IL-33 expression in cornea. mRNA expression levels of IL-33 (A) were significantly higher in infected corneas of BALB/c than of B6 mice at 1, 3, and 5 dpi (P < 0.01, P < 0.05, and P < 0.05, respectively). No difference was detected between groups in normal uninfected mice. IL-33 protein levels (B) were significantly higher in the corneas of BALB/c than of B6 mice at 1 and 5 dpi (P < 0.001 and P < 0.05, respectively). Constitutive expression of IL-33 protein was significantly higher in the normal uninfected corneas of BALB/c than of B6 mice (P < 0.01). Data are the mean ± SEM and represent two similar experiments each, with 5 mice/group/time. Immunostaining to localize IL-33 in the cornea showed qualitatively more intense positive staining for IL-33 (red) in the normal uninfected corneal epithelium of BALB/c (D) compared with B6 (C) mice. At 1 dpi, BALB/c also exhibited a more robust expression of IL-33 (F) than did B6 (E) mice. Negative controls for B6 (G) and BALB/c (H) mice, in which the primary antibody was replaced with a species-specific IgG, showed no positive staining for IL-33. Original magnification, ×120.
IL-33 and Host Resistance
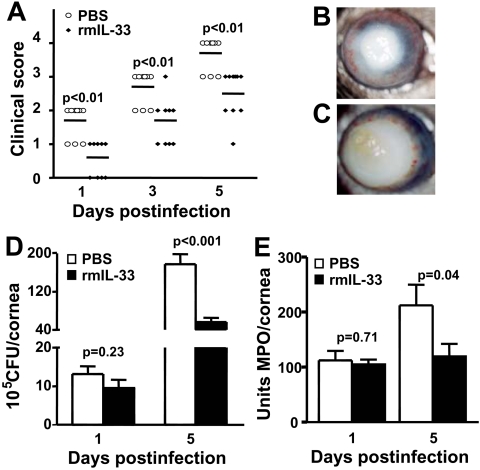
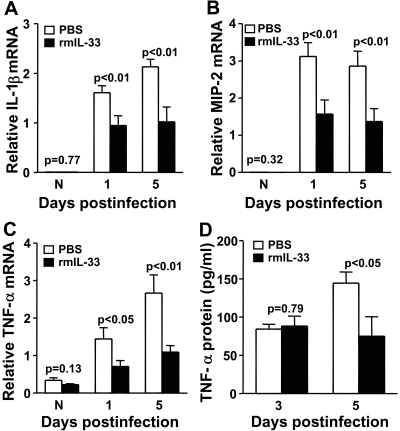
Because IL-33 mRNA expression was lower in the infected cornea of B6 compared with BALB/c mice, the next series of in vivo studies tested whether IL-33 (a specific ligand of ST2) was protective in bacterial keratitis. For this, B6 mice were injected with rmIL-33 protein. Clinical scores (Fig. 2A) showed that treated B6 mice exhibited significantly decreased corneal disease at 1, 3, and 5 dpi (each at P < 0.01) compared with PBS treatment. A representative photomicrograph taken with a slit lamp at 5 dpi also revealed less corneal opacity or disease in the rmIL-33 (Fig. 2B) compared with PBS (Fig. 2C) treated mice. We next investigated whether bacterial count or PMN number differed in the infected corneas of the two groups. At 1 dpi, no difference in bacterial count (Fig. 2D) or PMN (Fig. 2E) was detected, but at 5 dpi, both bacterial (P < 0.001) and PMN (P = 0.04) values were elevated in PBS compared with rmIL-33 treated mice. Treatment with rmIL-33 protein compared with PBS also significantly decreased mRNA levels of IL-1β (Fig. 3A; P < 0.01 at both 1 and 5 dpi), MIP-2 (Fig. 3B; P < 0.01 at both 1 and 5 dpi), and TNF-α (Fig. 3C; P < 0.05 and <0.01 at 1 and 5 dpi, respectively). To confirm the mRNA data, corneal protein levels for TNF-α were tested (Fig. 3D) and were significantly downregulated in rmIL-33 compared with PBS-treated B6 mice at 5 (P < 0.05), but not at 3 dpi (P = 0.79), paralleling, in part, the mRNA data.
Figure 2.
rmIL-33 protein treatment (A). Significantly lower clinical scores were seen at 1, 3, and 5 dpi in rmIL-33 compared with PBS mice (P < 0.01, for each). The experiment was performed twice with 5 mice/group/time, and data shown are individual scores from both experiments. Photographs taken with a slit lamp at 5 dpi show less severe disease after rmIL-33 treatment (B) compared with corneal thinning or perforation in PBS-treated controls (C). Plate counts to enumerate viable bacteria (D) and MPO assay to quantitate corneal PMN (E) showed fewer bacteria and PMN in the cornea of rmIL-33 than of PBS-treated mice at 5 dpi (P < 0.01 and P = 0.04, respectively), with no significant difference in either between the two groups at 1 dpi. Data for both are the mean ± SEM and represent two similar experiments, each with 5 mice/group/time.
Figure 3.
Pro-inflammatory and rmIL-33 mediators. Real-time RT-PCR showed rmIL-33 treatment significantly downregulated mRNA levels for IL-1β (P < 0.01 and P < 0.01; A), MIP-2 (P < 0.01 and P < 0.01; B), and TNF-α (P < 0.05 and P < 0.01; C) at 1 and 5 dpi, respectively. mRNA data were selectively confirmed by ELISA for TNF-α at 3 and 5 dpi (D) and showed a significant decrease in TNF-α protein at 5 dpi only (P < 0.05). Data are the mean ± SEM and represent the results of two experiments, each with 5 mice/group/time.
IL-33 Differentially Regulates Th1/Th2-type Immunity
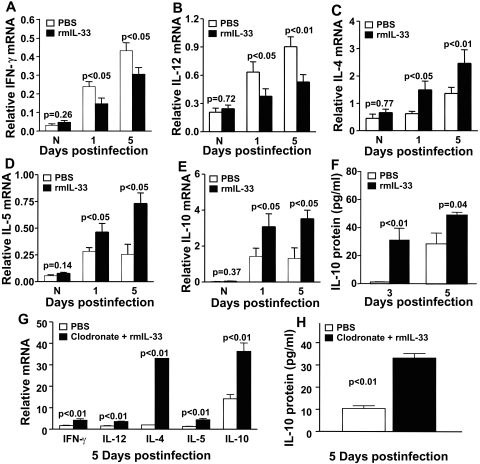
mRNA expression of type-1 cytokines IFN-γ (Fig. 4A; P < 0.05 at both 1 and 5 dpi) and IL-12 (Fig. 4B; P < 0.05 and <0.01 at 1 and 5 dpi) was significantly decreased in the cornea of rmIL-33– compared with PBS-treated mice. In contrast, mRNA expression of type-2 cytokines, including IL-4 (Fig. 4C; P < 0.05 and <0.01 at 1 and 5 dpi), IL-5 (Fig. 4D; P < 0.05 at both 1 and 5 dpi), and IL-10 (Fig. 4E; P < 0.05 at both 1 and 5 dpi) were significantly upregulated in rmIL-33– compared with PBS-treated B6 mice. ELISA showed that IL-10 protein levels (Fig. 4F) were upregulated at 3 and 5 dpi (P < 0.01 and P = 0.04). Mφ-depleted mice also were tested to determine whether rmIL-33 had a direct effect on T cells. Data show that in the absence of Mφ, Th2-type T-cell cytokines were upregulated, and increased mRNA levels of IL-4 (P < 0.01), IL-5 (P < 0.01), and IL-10 (P < 0.01) (and protein, P < 0.01, Fig. 4H) were detectable compared with control levels (Fig. 4G). In addition, Th1 cytokines IFN-γ (P < 0.01) and IL-12 (P < 0.01) also were modestly elevated in clodronate-treated mice compared with controls.
Figure 4.
Th1/Th2 regulation. Real-time RT-PCR showed that treatment with rmIL-33 significantly decreased mRNA levels for Th1 cytokines (A) IFN-γ (P < 0.05 and P < 0.05) and (B) IL-12 (P < 0.05 and P < 0.01) at 1 and 5 dpi Levels of mRNA for Th2 cytokines were significantly upregulated after rmIL-33 treatment compared with PBS controls at 1 and 5 dpi for (C) IL-4 (P < 0.05 and P < 0.01, respectively), (D) IL-5 (P < 0.05 for both), and (E) IL-10 (P < 0.05 for both). ELISA confirmed the mRNA data for IL-10 (F) at 3 and 5 dpi (P < 0.01 and P = 0.04) compared with PBS controls. After Mφ depletion, mRNA levels for Th2 cytokines were significantly upregulated after rmIL-33 treatment compared with PBS controls at 5 dpi (G) (P < 0.0.l for IL-4, -5 and -10 and -10 protein; H). Modest upregulation for IFN-γ and IL-12 (P < 0.01 for each) also was seen. Data are the mean ± SEM and represent the results of two experiments, each with 5 mice/group/time (only G and H are data from a single experiment).
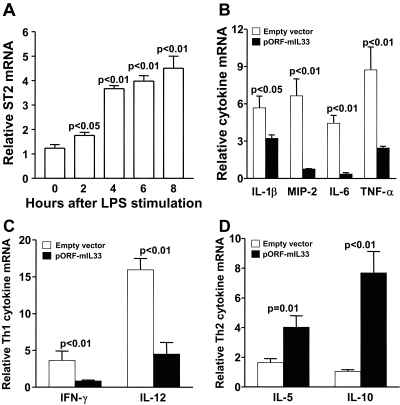
Overexpression of IL-33 Modulates Inflammation
mRNA expression of ST2 in RAW cells, determined by real-time RT-PCR, showed constitutive expression before and upregulation with time after LPS stimulation (Fig. 5A; P < 0.05, <0.01, <0.01, and <0.01 at 2, 4, 6, and 8 hours). RAW cells were transiently transfected with an IL-33 expression vector and stimulated with LPS (1 μg for 6 hours). Overexpression of IL-33 markedly decreased pro-inflammatory cytokines (Fig. 5B; P < 0.05, <0.01, <0.01, and <0.01 for IL-1β, MIP-2, IL-6, and TNF-α, respectively) and Th1-type cytokines (Fig. 5C; P < 0.01 for both IFN-γ and IL-12) while significantly increasing Th2-type cytokines (Fig. 5D; P = 0.01 and P < 0.01 for IL-5 and IL-10) compared with the empty vector control.
Figure 5.
Modulation of IL-33 in vitro. Real-time RT-PCR showed ST2 expression on RAW cells was significantly upregulated over constitutive levels after stimulation with LPS after 2 (P < 0.05), 4, 6, and 8 hours (each at P < 0.01) (A). After transfection with a vector overexpressing IL-33, RAW cells significantly downregulated mRNA for pro-inflammatory mediators (B) IL-1β (P < 0.05), MIP-2 (P < 0.01), IL-6 (P < 0.01), and TNF-α (P < 0.01). In addition, Th1-type cytokines such as IFN-γ (P < 0.01) and IL-12 (P < 0.01) (C) and Th2-type cytokines such as IL-5 (P = 0.01) and IL-10 (P < 0.01) (D) were upregulated. Data are the mean ± SEM of two similar experiments (5 samples/group/time).
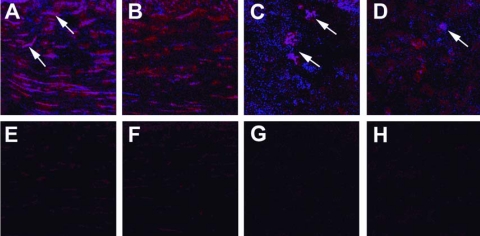
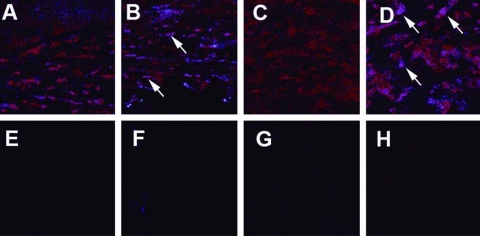
NOS2 and Arg1 Immunostaining
Immunostaining determined whether M1 or M2 polarization of F4/80 positively stained Mφ in the cornea occurred after rmIL-33 compared with PBS treatment by localizing NOS2 (M1; Figs. 6A–H) or Arg1 (M2; Figs. 7A–H). Staining for NOS2 appeared similar in PBS- compared with rmIL-33–treated corneas at 1 dpi (data not shown). At 3 dpi, more NOS2 positively stained Mφ were seen in the cornea after PBS (Fig. 6A, merged NOS2 and F4/80 staining) compared with rmIL-33 treatment (Fig. 6B, merged). By 5 dpi, less overall NOS2 staining was seen in either treatment group, but the PBS group continued to exhibit more staining than the rmIL-33 group (compare Figs. 6C, 6D; both merged). Negative controls, in which IgG was substituted for the primary antibody, are shown for both groups at 3 and 5 dpi (Figs. 6E, 6F for 3 dpi; 6G, H for 5 dpi; PBS and rmIL-33 treated, respectively). In contrast, qualitatively less intense staining for Arg1 in F4/80-positive Mφ was seen in the cornea after PBS treatment compared with rmIL-33 treatment (compare Figs. 7A, 7B; both merged) at 1 dpi. The pattern and intensity of Arg1 staining at 3 (data not shown) and 5 dpi (Figs. 7C, 7D) were similar to those at 1 dpi, with the PBS compared with the rmIL-33 treatment group exhibiting less staining for Arg1. Controls (IgG substituted for the primary antibody) are shown for both groups at 1 and 5 dpi (Figs. 7E, 7F for 1 dpi; 7G, 7H for 5 dpi; PBS and rmIL-33 treated, respectively) with no detectable background staining.
Figure 6.
Immunostaining for NOS2. Dual immunofluorescence staining for F4/80 (Mφ) and NOS2 in the corneas of rmIL-33 and PBS-treated B6 mice at 3 (A, B, E, F) and 5 (C, D, G, H) dpi. In merged images (F4/80, red; NOS2, blue), more intense NOS2 staining in cornea was seen in PBS-treated mice at 3 (A) and 5 (C) dpi compared with rmIL-33–treated corneas (B, D). Controls (primary antibody replaced with IgG) are shown for both groups at 3 and 5 dpi (E, F, for 3 dpi; G, H, for 5 dpi) with negligible backgroundstaining. Original magnification, ×400.
Figure 7.
Immunostaining for Arg1. Dual immunostaining for F4/80 (Mφ) and Arg1 in the corneas of rmIL-33– and PBS-treated B6 mice at 1 (A, B, E, F) and 5 (C, D, G, H) dpi. In merged images (F4/80 = red; Arg1 = blue), more intense Arg1 staining was seen in the cornea of rmIL-33–treated mice at 1 (B) and 5 (D) dpi than in PBS-treated mice (A, C, 1 and 5 dpi, respectively). Negligible background staining was seen in controls, in which the primary antibody was replaced with IgG (shown for both groups at 1 and 5 dpi [E, F, for 1 dpi; G, H, for 5 dpi; PBS and rmIL-33 treated, respectively]). Original magnification, ×400.
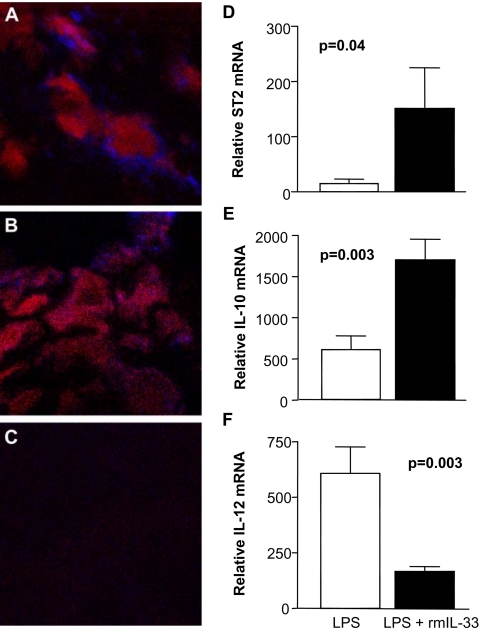
Immunostaining and RT-PCR
Dual immunolabeling with antibodies specific for Mφ and ST2 showed a qualitative upregulation of ST2 on F4/80-positive Mφ in the cornea of infected B6 mice treated with rmIL-33 (Fig. 8A) compared with PBS (Fig. 8B). No staining was seen when IgG was substituted for the primary antibody (Fig. 8C). In vitro, ST2 expression was assessed on peritoneal Mφ from B6 mice stimulated in culture with 1 μg/mL LPS with or without 0.5 μg/mL rmIL-33 (Fig. 8D). After 18 hours, Mφ with added rmIL-33 expressed significantly higher levels of ST2 transcript (P = 0.04) than Mφ with added rmIL-33. In addition, in the presence of rmIL-33, mRNA levels of the M2 cytokine IL-10 (Fig. 8E) were elevated (P = 0.003), whereas levels of the M1 cytokine IL-12 (Fig. 8F) were reduced (P = 0.003).
Figure 8.
Immunostaining and real-time RT-PCR. Dual immunostaining for F4/80 (Mφ) and ST2 in the cornea of rmIL-33 compared with PBS-treated B6 mice. More staining for ST2 (blue) was associated with Mφ (red) in rmIL-33–-treated mice (A) than in PBS-treated mice (B) cornea. Substitution of the primary antibodies with IgG showed no detectable staining (C). Original magnification, ×1000. Real-time RT-PCR analysis of LPS-stimulated peritoneal-elicited Mφ, with or without rmIL-33, showed a significant upregulation of ST2 on Mφ after LPS stimulation in the presence of rmIL-33 (P = 0.04) (D). Upregulation of IL-10 (E; P = 0.003) and downregulation of IL-12 (F; P = 0.003) were observed after LPS, together with rmIL-33 treatment. Data for RT-PCR are the mean ± SEM of two similar experiments (5 samples/group/time).
Discussion
The TLRs constitute a family of proteins involved in the initial phase of host defense against invading pathogens32,37 and act as primary sensors of microbial products to activate signaling pathways leading to the induction of innate immune and inflammatory responses.35 TLRs belong to a larger family of proteins that include receptors for cytokines such as IL-1 and IL-18, which are pro-inflammatory. Overall, TIR domain-containing superfamily members can be divided into three subgroups, all of which induce an inflammatory response except SIGIRR (single immunoglobulin domain IL-1R related) and ST2. The latter was originally identified in murine fibroblasts as a late response gene induced by serum. Thus, ST2 serves an important selective negative regulatory function of TIR domain-containing receptors, providing an explanation for its involvement in Th2-cell responses.15,34 ST2 was the first identified member of the TIR family unable to activate NF-κB but still able to activate MAP kinases. In the cornea, previous work from this laboratory has shown that ST2 is critical in resistance to P. aeruginosa keratitis, functioning to reduce corneal infection (bacterial load) and inflammation by negatively regulating pro-inflammatory cytokines, inhibiting type-1 immunity, and upregulating type-2 cytokine production, particularly IL-10.36 Nonetheless, involvement of its ligand, IL-33, and its cellular distribution remained unknown until the present study. In this regard, in addition to being a stable cell marker on Th2 effector cells, IL-33 is a member of the IL-1 family, which includes IL-1β and IL-18. Like those cytokines, IL-33 also was found to have strong immunomodulatory functions.22 However, in contrast to IL-1β and IL-18, which promote Th1-associated responses, IL-33, the ligand for the ST2 receptor, predominantly induces the production of Th2 cytokines such as IL-5 and IL-13. In this regard, the ST2 gene encodes two isoforms of ST2 protein: ST2L, a transmembrane form, and soluble ST2, a secreted form that is able to serve as a decoy receptor for IL-33. ST2L is preferentially expressed on Th2 cells but not on Th1 cells37 and can have profound suppressive effects on innate and adaptive immune responses.22 The soluble variant of ST2 can suppress pro-inflammatory cytokine production by Mφ activated with LPS. IL-33 has been detected in epithelial cells from the bronchus and small airways and from fibroblasts and smooth muscle cells,24,25 but no information has been available about IL-33 in diseases such as bacterial keratitis. The study reported herein is the first to test the expression and functional role of IL-33 in this disease. We have shown that ocular infection with P. aeruginosa upregulated IL-33. In fact, resistant (Th2 responsive)3 mice exhibited higher mRNA and protein expression levels constitutively and after infection than did susceptible (Th1-responsive)3 mice whose corneas were perforated. Thus, it is tempting to hypothesize that in BALB/c mice, constitutively elevated expression of IL-33 may exert a significant posttranslational regulative effect that could contribute to or prime the resistance response. In fact, injection of susceptible B6 mice with rmIL-33 protein promoted the resistance response of these mice by decreasing corneal disease and the PMN infiltrate and bacterial count in the cornea. In addition, treatment shifted Mφ polarization in the cornea from M1 to an M2 profile, increased ST2 expression, and shifted mRNA expression levels from IL-12 to IL-10 production. In the absence of the Mφ, rmIL-33 treatment also upregulated Th2-type T-cell cytokines, including IL-4, -5, and -10, but Th1-type cytokines also were modestly elevated. These data suggest, and are consistent with other data showing,38 that CD4+ Th0-type T cells are responsive to rmIL-33 treatment. The data also illustrate the importance of Mφ modulation of the host response in this disease because in the absence of this cell, Th1-type cytokines also were slightly upregulated, an unexpected finding. In addition, these data are consistent with past studies showing that Mφ from ST2-deficient mice produced significantly more IL-12 than cells from wild-type mice when stimulated with the TLR4 ligand LPS and that ST2 negatively regulated IL-1R, TLR2, TLR4, and TLR9 signaling.37 IL-1 itself upregulates the expression of many genes important in the initiation and development of the inflammatory state and other immune processes, including bacterial keratitis.37,39 Thus, IL-1 is critical for maintaining the health of the organism and, in the cornea, has been dubbed its “alarm bell.” Imbalance of this potent cytokine can lead to the development of disease and can contribute to perforation39 or destruction of the cornea. TLR signaling leads to the induction and activation of many genes similar to IL-1; thus, the regulation of TLRs is required for immune system homeostasis. Although SIGIRR is also an inhibitory member of the IL-1R1-TLR superfamily, its expression pattern differs. SIGIRR is restricted primarily to epithelial cells, whereas ST2 is expressed on macrophages and Th2 cells, and its ability to modulate TLR signaling may be even more important than SIGIRR in terms of immune responsiveness.15 Nonetheless, SIGIRR is important in resistance to P. aeruginosa corneal infection and functions to downregulate type 1 immunity and to negatively regulate IL-1 and TLR4 signaling.40
In summary, the data presented herein indicate that IL-33 is constitutively expressed disparately in the corneal epithelium of B6 (less) and BALB/c (more) mice before and after P. aeruginosa infection. In addition, we provide substantial evidence that IL-33 is critical for host resistance and functions in vivo by binding to Mφ, favors an M2 (Arg1) rather than an M1 (NOS2) phenotype, promotes type 2 cytokine (e.g., IL-10) production, and negatively regulates type 1 cytokine production and reduces pro-inflammatory cytokine expression. In the absence of Mφ, Th2 cytokines are upregulated, but Th1 cytokines are also increased. These data suggest that IL-33 plays a protective role in corneal defense against bacterial infection and that modulation of IL-33 signaling may be used as a novel therapeutic reagent for the treatment of P. aeruginosa keratitis.
Footnotes
Supported by National Institutes of Health Grants R01 EY016058 and P30EY004068.
Disclosure: L.D. Hazlett, None; S.A. McClellan, None; R.P. Barrett, None; X. Huang, None; Y. Zhang, None; M. Wu, None; N. van Rooijen, None; E. Szliter, None
References
- 1.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res 2004;23:1–30 [DOI] [PubMed] [Google Scholar]
- 2.Szliter EA, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol 2007;178:1105–1114 [DOI] [PubMed] [Google Scholar]
- 3.Hazlett LD, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 compared with Th2 responsive. Invest Ophthalmol Vis Sci 2000;41:805–810 [PubMed] [Google Scholar]
- 4.Huang X, Hazlett LD. Analysis of Pseudomonas aeruginosa corneal infection using an oligonucleotide microarray. Invest Ophthalmol Vis Sci 2003;44:3409–3416 [DOI] [PubMed] [Google Scholar]
- 5.Soehnlein O, Kenne E, Rotzius P, Eriksson EE, Lindbom L. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin Exp Immunol 2007;151:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science 2003;300:1524–1525 [DOI] [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999;285:732–736 [DOI] [PubMed] [Google Scholar]
- 8.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 1999;274:10689–10692 [DOI] [PubMed] [Google Scholar]
- 9.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol 2008;181:3733–3739 [DOI] [PubMed] [Google Scholar]
- 10.Mills CD, Kincaid K, Alt J, Heilman M, Hill A. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–6173 [DOI] [PubMed] [Google Scholar]
- 11.Joshi AD, Raymond T, Coelho AL, Kunkel SL, Hogaboam CM. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J Leuk Biol 2008;83:314–324 [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Massimo L, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–555 [DOI] [PubMed] [Google Scholar]
- 13.O'Neill LA. SIGIRR puts the brakes on Toll-like receptors. Nat Immunol 2003;4:823–824 [DOI] [PubMed] [Google Scholar]
- 14.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 2005;5:446–458 [DOI] [PubMed] [Google Scholar]
- 15.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol 2004;5:373–379 [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun 1997;235:474–478 [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J 1994;13:1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol 1998;161:4866–4874 [PubMed] [Google Scholar]
- 19.Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med 1999;190:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 2000;191:1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–490 [DOI] [PubMed] [Google Scholar]
- 22.Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med 2008;205:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 2007;19:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol 2007;142:265–273 [DOI] [PubMed] [Google Scholar]
- 25.Huang X, McClellan SA, Barrett RP, Hazlett LD. IL-18 contributes to host resistance against infection with Pseudomonas aeruginosa through induction of IFN-gamma production. J Immunol 2002;168:5756–5763 [DOI] [PubMed] [Google Scholar]
- 26.Kwon B, Hazlett LD. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J Immunol 1997;159:6283–6290 [PubMed] [Google Scholar]
- 27.Hazlett LD, Moon MM, Strejc M, Berk RS. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci 1987;28:1978–1985 [PubMed] [Google Scholar]
- 28.Williams RN, Paterson CA, Eakins KE, Bhattacherjee P. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr Eye Res 1982;2:465–470 [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanisms of action, preparation of liposomes and applications. J Immunol Meth 1994;174:83–93 [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Tumpey TM, Staats HF, van Rooijen N, Oakes JE, Lausch RN. Role of macrophages in restricting Herpes simplex virus type 1 growth after ocular infection. Invest Ophthalmol Vis Sci 2000;41:1402–1409 [PubMed] [Google Scholar]
- 31.McClellan SA, Huang X, Barrett RP, van Rooijen N, Hazlett LD. Macrophages restrict P. aeruginosa growth, regulate PMN influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J Immunol 2003;170:5219–5227 [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 2005;46:4209–4216 [DOI] [PubMed] [Google Scholar]
- 33.Fortier AH. Isolation of murine macrophages. Curr Protoc Immunol 2001;14:14.1 [DOI] [PubMed] [Google Scholar]
- 34.Liew FY, Liu H, Xu D. A novel negative regulator for IL-1 receptor and Toll-like receptor 4. Immunol Lett 2005;96:27–31 [DOI] [PubMed] [Google Scholar]
- 35.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2000:RE1:1–11 [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Du W, Barrett RP, Hazlett LD. ST2 is essential for Th2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 2007;48:4626–4633 [DOI] [PubMed] [Google Scholar]
- 37.Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper cells. J Exp Med 1998;187:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakkar R, Lee RT. The Il-33/ST2 pathway therapeutic target and novel biomarker. Nat Rev 2008;7:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudner X, Kernacki KA, Barrett RP, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol 2000;164:6576–6582 [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by downregulating type-1 immunity and IL-1R1 and TLR-4 signaling. J Immunol 2006;177:548–556 [DOI] [PubMed] [Google Scholar]