TNF-α, a proinflammatory cytokine, is elevated in the cornea and aqueous humor during corneal allograft rejection. In this study, the authors investigated the molecular mechanism by which the cytokine induces barrier dysfunction in the corneal endothelium.
Abstract
Purpose.
TNF-α is elevated in the cornea and aqueous humor during allograft rejection and anterior uveitis. The authors investigated the involvement of p38 MAP kinase in the TNF-α–induced loss of barrier integrity in monolayers of cultured bovine corneal endothelial cells.
Methods.
Transendothelial electrical resistance (TER), a measure of barrier integrity, was determined by electrical cell-substrate impedance sensing. Barrier integrity was further assessed in terms of permeability to FITC dextran. Reorganization of the apical junctional complex (AJC) in response to TNF-α was visualized by immunofluorescence. The expression of TNF-α receptors was confirmed by RT-PCR. Activation of p38 MAP kinase in response to TNF-α was determined by Western blot analysis.
Results.
Exposure to TNF-α induced a continuous decline in TER that persisted for more than 20 hours. It also led to a significant increase in permeability to FITC dextran. At the AJC, the cytokine caused disassembly of microtubules, disruption of perijunctional actomyosin ring (PAMR), and dislocation of ZO-1 and cadherins. Western blot analysis showed that TNF-α also led to the activation of p38 MAP kinase. All these responses to the cytokine were opposed by treatment with SB-203580, a selective p38 MAP kinase inhibitor. TNFR1, but not TNFR2, was expressed in untreated cells with no change in the expression pattern on treatment with the cytokine.
Conclusions.
TNF-α breaks down the barrier integrity of corneal endothelium, concomitant with the disruption of PAMR, remodeling of AJC, and disassembly of microtubules. These effects are mediated by transient activation of p38 MAP kinase. Thus, the TNF-α–induced barrier dysfunction in the corneal endothelium can be suppressed by inhibitors of p38 MAP kinase and agents downstream of the kinase that affect the cytoskeleton.
The corneal endothelium maintains stromal deturgescence, which is required for corneal transparency. The glycosaminoglycans of the stroma pose a threat to stromal deturgescence because of their tendency to imbibe water across the endothelium. This fluid leak into the stroma is restricted by the putative barrier function associated with the corneal endothelium. The barrier integrity of the endothelium, in conjunction with its active fluid pump mechanism,1,2 is responsible for the stromal deturgescence. Furthermore, tight junctions (TJs) of the endothelium, in addition to their innate role in the maintenance of barrier integrity, are also intrinsically coupled to the fluid pump activity. This is because the TJs also maintain polarity of the transmembrane proteins, which are involved in active fluid transport. Barrier integrity is also critical to sustain local osmotic gradients, which elicit active fluid movement.3,4 Hence, barrier integrity of the endothelium is indispensable for the maintenance of stromal hydration control; therefore, it is crucial for corneal transparency.
Despite a continuous age-related loss of endothelial cells (∼0.5% per year), stromal hydration is maintained as long as the cell density is greater than 700 cells/mm2.1,5 When the corneal endothelium is subjected to inflammation, disease, or surgical trauma, a loss of stromal hydration control is induced concomitant with a rapid decline in cell density.1,6 Loss of corneal transparency because of decompensated endothelium is a major indication for corneal transplantation, of which there are approximately 40,000 annually in the United States.7 Even after transplantation, survival of the endothelium is a major concern6 because the proinflammatory mediators, which are released secondary to immune response, are known to affect gene expression8 and barrier integrity in other cell types. A basic understanding of the mechanisms involved in barrier dysfunction is, therefore, essential to develop therapeutic strategies that could be used to rescue transplanted corneas from endothelial failure.
TNF-α, a 17-kDa proinflammatory cytokine, is thought to play a major role in corneal endothelial dysfunction during allograft rejection9,10 and anterior uveitis.11 Its levels are elevated in the aqueous humor of rabbits undergoing allograft rejection.10,12 Prolongation of endothelial graft survival was observed by interfering with the activities of TNF-α through the administration of TNFR-Ig, a recombinant TNF receptor.10 In general, TNF-α is known to have pathophysiological influence in many cell types through mechanisms leading to apoptosis,13 loss of barrier integrity,14,15 and prolongation of the immune response through enhanced expression of cellular adhesion molecules.16
In a study involving rabbit corneal endothelium, Watsky et al.17 showed that TNF-α increases the permeability to carboxyfluorescein, concomitant with disruption of the actin cytoskeleton. Although they found that 8 Br-cAMP (a membrane-permeable analog of cAMP) opposed the response to TNF-α, the molecular mechanisms underlying the barrier dysfunction and mechanism of rescue by elevated cAMP remain unknown. In several studies involving vascular endothelium, TNF-α is known to induce barrier dysfunction concomitant with the disruption of actin cytoskeleton14,18 and microtubule disassembly15 through mechanisms involving the activation of reactive oxygen species (ROS),19 RhoA GTPase,20 MAP kinases,15 transcriptional activation of myosin light chain kinase,18,21 and heat shock protein 27 (Hsp27).22 However, a preponderance of evidence collected to date seems to indicate a major role for p38 MAP kinase because it is also implicated in the activation of ROS production through components of the NADPH complex,23 Hsp27,22 and microtubule disruption.15,24 Therefore, in the present study, for the first time, we have investigated the involvement of p38 MAP kinase in the TNF-α–induced breakdown of the barrier integrity of corneal endothelium. Our results demonstrate that the TNF-α–induced activation of p38 MAP kinase is a crucial event that results not only in the loss of barrier integrity but also in the disruption of cytoskeleton and the remodeling of apical junctional complex (AJC).
Materials and Methods
TNF-α (biological activity of 2 × 107 U/mg; endotoxin-free), α-tubulin antibody, cadherin antibody, and FITC dextran (10 kDa) were purchased from Sigma-Aldrich (St. Louis, MO). SB-203580 and ZVAD were obtained from EMD Biosciences (La Jolla, CA), and reagent was obtained from Invitrogen (Trizol; Carlsbad, CA). Anti-phospho-p38 MAP kinase and anti-p38 MAP kinase were from Cell Signaling Technology (Beverly, MA), and ZO-1 antibody was from Zymed Laboratories (Grand Island, NY). Texas Red–conjugated phalloidin, goat anti-mouse Alexa-488, and antifade agent were purchased from Molecular Probes (Eugene, OR). Enhanced chemiluminescence kit was obtained from Thermo Scientific (Rockford, IL). Electrodes (8W10E+) for TER measurements were procured from Applied Biophysics Inc. (Troy, NY).
Cell Culture
Primary cultures of bovine corneal endothelial cells (BCECs) from freshly isolated eyes were established as previously described.25 The growth medium contained Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum and an antibiotic-antimycotic mixture (penicillin 100 U/mL, streptomycin 100 μg/mL, and amphotericin-B 0.25 μg/mL). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The medium was replaced every 2 to 3 days. First-passage cells were used in all experiments. In all experiments, TNF-α was used at 20 ng/mL with and without pretreatment of SB-203580 (20 μM) 1 hour before the addition of TNF-α.
RT-PCR Assay
Total RNA was isolated from cells using reagent (Trizol; Invitrogen) according to the manufacturer's instructions and was quantified by absorption at 260 nm. Genomic DNA contamination was removed from RNA samples by DNA digestion. Reverse transcription and polymerase chain reaction were performed simultaneously using a single-step PCR kit (Superscript III; Invitrogen). Each reaction contained 2 μL template RNA, 5 μM each primer, Taq DNA polymerase, and 1:2 dilution of 2× reaction mixture in a final volume of 50 μL. The PCR program for cDNA synthesis and subsequent PCR reaction for amplification consisted of the following cycles: step 1, 56°C for 30 minutes; step 2, 94°C for 2 minutes; step 3, 28 cycles of 94°C for 30 seconds, 58°C for 25 seconds, and 72°C for 25 seconds; step 4, 72°C for 5 minutes for a final elongation phase. 10 μL from each reaction were run along with 100-bp ladder marker (Fermentas Inc., Glen Burnie, MD) on a 1.5% (wt/vol) agarose gel containing gel red (Biotium Inc., Hayward, CA) diluted to a concentration of 1×. The resultant bands were visualized and photographed (Genesnap; Syngene, Frederick, MD). The sequence of oligonucleotide primers and the size of amplified DNA fragments were as follows: TNF-α receptor (TNFR) 1, 5′-ACG AAA TGT TCC AGG TGG AG-3′ (sense) and 5′-GTC TTT ACC AGT TGA AGG TCG G-3′ (antisense), 290-bp fragment; TNFR2, 5′-AGC AGC ACG GAC AAG AGG-3′ (sense) and 5′-GCA AAC ATT GAC AAT GCA GG-3′ (antisense), 162-bp fragment. The primers 5′-ATC AAG GAG AAG CTC TGC TAC G-3′ (sense) and 5′-TTG AAG GTA GTT TCG TGA ATG C-3′ (antisense) (270-bp fragment) were used as controls for the amplification of β-actin.
Immunocytochemistry
Cells grown on coverslips were washed with PBS after the desired drug treatments, fixed with 3.7% paraformaldehyde, and permeabilized using 0.2% Triton X-100 for 5 minutes. This was followed by staining for F-actin by phalloidin conjugated to Texas Red (1:1000) for 45 to 60 minutes at room temperature. Cells were fixed and permeabilized with 0.01% saponin in PBS. Then the cells were exposed to blocking buffer for 45 minutes, followed by incubation with the desired antibody. The antibodies—α-tubulin (1:1000), ZO-1 (1:25), and cadherin (1:1000)—were diluted overnight at 4°C in a mixture consisting of equal volumes of 0.01% saponin in PBS and goat serum. This was followed by washing and incubation with secondary antibody (goat anti-mouse IgG Alexa Fluor 488 at 1:1000). Cells without exposure to the respective primary antibody were used as controls for immunostaining. Stained cells were mounted using antifade agent with DAPI and were visualized using an epifluorescence microscope equipped with a 60× oil immersion objective and 1.2 NA (Nikon, Tokyo, Japan).
Transendothelial Electrical Resistance
TER was measured by electric cell-substrate impedance sensing (ECIS) device (ECIS 1600R; Applied Biophysics, Troy, NY), as described previously.26 Cells were seeded at a density of 5 × 105 cells/mL on gold electrodes (8W10E+; Applied Biophysics, Inc.) and grown to confluence, which was confirmed by the steady state of TER. The wells were then washed twice with serum-free media and allowed to equilibrate for 1 hour before drug treatment. The resistive portion of impedance (TER) normalized to its initial value at time 0 was monitored continuously and taken as a measure of barrier integrity.
Measurement of Permeability to FITC Dextran
Cells were grown on 0.2-μm pore-size collagen IV (1 mg/mL)-coated tissue culture inserts (Nunc; Fisher Scientific, Pittsburgh, PA) to confluence for approximately 7 days. The monolayers were then serum-starved for 1 hour and either left untreated or exposed in triplicate with desired agents. After the treatments, FITC-dextran (10 kDa), dissolved in the Ringer's solution, was introduced into the apical compartment at a concentration of 0.4 μg/mL. After 2 hours of incubation at 37°C, samples were taken from the basolateral chamber for fluorescence measurements. Fluorescence was measured by excitation at 492 nm, and the emission was collected at 520 nm.
Western Blot Analysis
Confluent cells were serum starved overnight before treatment with desired agents. Then the cells were lysed with NP40 lysis buffer (Invitrogen) containing protease inhibitor cocktail (Sigma-Aldrich) and a 5× lane marker reducing sample buffer (Thermo Scientific, Waltham, MA). The lysates were transferred to a microcentrifuge tube kept on ice and sonicated for 10 to 15 seconds. Then the lysates were centrifuged at 14,000g for 10 minutes, followed by heating at 95°C to 100°C for 5 minutes. The samples were cooled to room temperature, centrifuged, and resolved by electrophoresis. This was followed by transfer onto nitrocellulose membrane and incubation with the primary antibody (anti-phospho p38 MAP kinase; 1:1000 dilution) overnight at 4°C. The blots were washed with wash buffer followed by incubation with peroxidase-conjugated secondary antibody (anti-rabbit IgG) for 1 hour at room temperature. The blots were visualized using an enhanced chemiluminescence kit. Then the blots were stripped and probed for total p38 MAP kinase using anti-p38 MAP kinase antibody (1:1000 dilution).
Results
Effect of TNF-α on Transendothelial Electrical Resistance
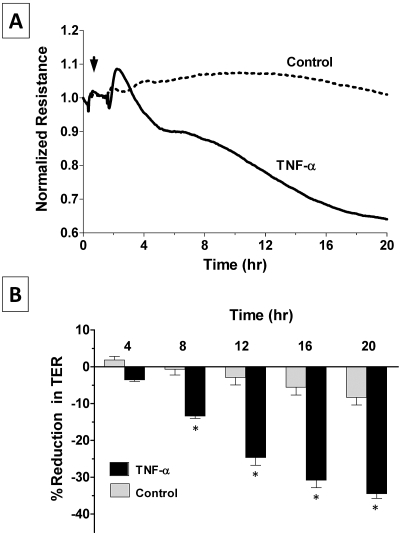
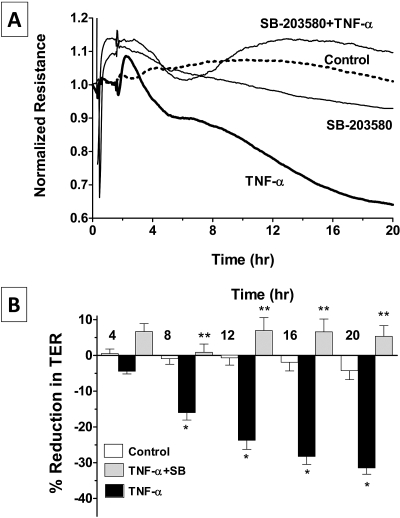
We followed the dynamics of TER, which is a measure of barrier integrity, in real time using the well-established ECIS approach,24,27–29 as also shown in our previous study.30 Exposure to TNF-α (20 ng/mL) induced a biphasic decline in TER after a delay of approximately 2 hours. The first phase of decline was usually rapid compared with the second phase, which lasted for more than 20 hours (Fig. 1A). The decline in TER every 4 hours after exposure to the cytokine, from experiments similar to those shown in Figure 1A, is summarized in Figure 1B. It may be noted that the TNF-α–induced decline is statistically significant after 8 hours of exposure.
Figure 1.
Effect of TNF-α on the dynamics of TER. Changes in TER in terms of normalized resistance were monitored with or without the presence of 20 ng/mL TNF-α by ECIS. (A) In untreated cells, the TER remains steady without any major reduction, whereas TNF-α induces a decrease in TER after a delay of approximately 2 to 3 hours and continues beyond 20 hours. (B) Bar graph of experiments similar to that shown in (A). The mean percentage reduction in TER induced by TNF-α is significantly greater than control after 8 hours of exposure. Error bars represent the SEM (n = 6). *P < 0.001; significantly different from control.
Role of Apoptosis in the TNF-α Response
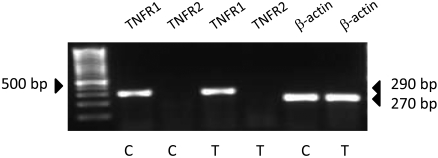
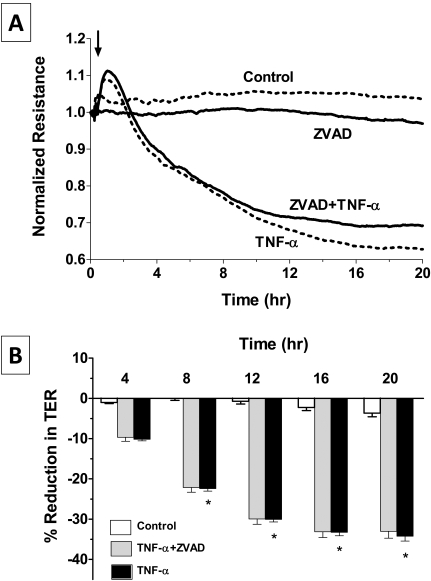
As a pleiotropic cytokine, TNF-α–induced dysfunction is known to involve apoptosis20 and to break down the barrier integrity.14,15,17 To further investigate whether the TER response is secondary to TNF-α-mediated apoptosis via TNFR1 signaling,31 we first tested the expression of TNFRs, namely TNFR1 and TNFR2, at the mRNA level. RT-PCR results (Fig. 2) show a positive band at the expected size of 290 bp for TNFR1 in untreated and TNF-α)-treated cells. However, the transcript for TNFR2 (expected size, 162 bp) is absent. Therefore, the TER response in Figure 1 could be attributed to signaling downstream of TNFR1. We then measured the TER response in the presence of ZVAD [Z-Val-Ala-Asp (ZVAD)-fluoromethylketone (fmk)], a pan-caspase inhibitor to rule out the involvement of apoptosis.32 Pretreatment with 100 μM ZVAD for 1 hour failed to oppose the TNF-α–induced decline in TER (Fig. 3), suggesting that the response of the cytokine is independent of apoptosis.
Figure 2.
Expression of TNF-α receptors. Cells were either untreated (lanes 2, 3, 6) or were treated with 20 ng/mL TNF-α for 20 hours (lanes 4, 5, 7). mRNA analysis demonstrated expression of the transcript for TNFR1 receptor (290 bp) but not for TNFR2 receptor (162 bp). As a control, β-actin is shown (270 bp).
Figure 3.
Influence of apoptosis in the TNF-α response. (A) Cells were pretreated with 100 μM ZVAD, a pan-caspase inhibitor, for 1 hour with or without 20 ng/mL TNF-α, and the changes in TER were monitored. ZVAD does not attenuate the decline in TER induced by TNF-α. Bar graph of experiments similar to that shown in (A). The mean percentage reduction in TER induced by TNF-α is significantly greater than control after 8 hours of exposure. Error bars represent the SEM (n = 6). *P < 0.001; significantly different from control. The mean percentage reductions in TER in TNF-α–treated cells and cells pretreated with ZVAD are not statistically different (P > 0.05).
Role of p38 MAP Kinase in the TNF-α Response
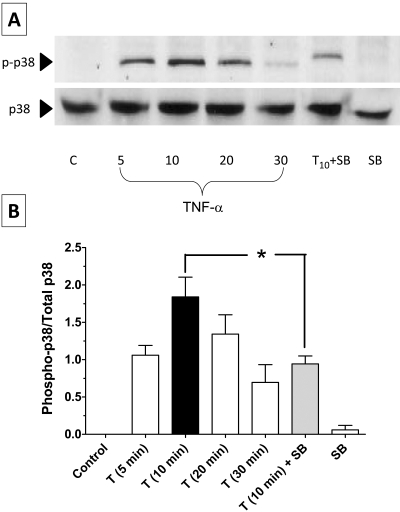
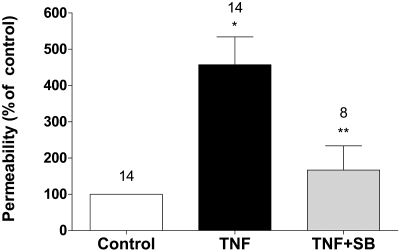
p38 MAP kinase is a stress kinase activated by a variety of cellular stresses, including inflammatory cytokines. Specifically, p38 MAP kinase is thought to play a major role in barrier dysfunction in response to TNF-α.15,33 Among the four known isoforms of the kinase (α, β, γ, δ), p38α (also known as SAPK2a and MAPK14) isoform is ubiquitously expressed and susceptible to dual phosphorylation by MKK3 and MKK6 at its Thr180 and Tyr182 residues. This is known to result in an activation of p38 MAP kinase, which eventually leads to inflammatory responses attributed to the cytokine.34 Activated p38 MAP kinase has been shown to phosphorylate and activate MAPKAP kinase 2 and to phosphorylate the transcription factor ATF-2. Recent studies demonstrated that SB203580, a pyridinyl imidazole inhibitor, acts via the ATP competitive mechanism and inhibits only the activity of the p38 MAP kinase but does not inhibit p38 MAP kinase expression in vitro or in vivo.35,36 As shown in Figure 4A, exposure to the cytokine led to activation of the kinase within 5 minutes, peaked at 10 minutes, and decreased by 30 minutes. Pretreatment with 20 μM SB-203580 for 1 hour attenuated the phosphorylation at 10 minutes (Fig. 4A). The ratio of phosphorylated to total p38 MAP kinase from densitometric analysis is summarized in Figure 4B. We further tested for the contribution of p38 MAP kinase in the TER response using SB-203580. As shown in Figure 5A, pretreatment with SB-203580 completely inhibited the decrease in TER induced by TNF-α (Fig. 5B). The efficacy of SB-203580 and the TER response as a measure of barrier integrity was further confirmed by assessing permeability to FITC dextran (10 kDa) across BCEC monolayers on porous supports. As shown in Figure 6, treatment with TNF-α significantly increases permeability compared with untreated cells. Further, the increase in permeability is attenuated on pretreatment with SB-203580. In summary, measurements of both TER and permeability to FITC dextran indicate that the TNF-α–induced breakdown of barrier integrity in corneal endothelium involves p38 MAP kinase.
Figure 4.
Influence of TNF-α on p38 MAP kinase activation. p38 MAP kinase activation after treatment with 20 ng/mL TNF-α at different time points, with or without pretreatment with 20 μM SB-203580, was confirmed by immunoblotting. (A) TNF-α–induced phosphorylation of p38 MAP kinase peaks at 10 minutes. However, pretreatment with SB-203580 suppressed p38 MAP kinase phosphorylation induced by TNF-α at 10 minutes. (B) Bar graph of the densitometric analysis of experiments, similar to that shown in (A). The ratio of phosphorylated to total p38 MAP kinase is maximum at 10 minutes stimulation with TNF-α. Pretreatment with SB-203580 followed by stimulation with TNF-α for 10 minutes significantly decreases the ratio. Error bars represent the SEM (n = 3). *P < 0.001; significantly different from TNF-α.
Figure 5.
p38 MAP kinase-dependent TNF-α response on TER. (A) Cells were pretreated with 20 μM SB-203580 for 1 hour with or without 20 ng/mL TNF-α. SB-203580 opposes the decrease in TER induced by TNF-α. (B) Bar graph of experiments similar to that shown in (A). The mean percentage reduction in TER induced by TNF-α is significantly greater than control after 8 hours of exposure. Error bars represent the SEM (n = 6). *P < 0.001; significantly different from control. SB-203580 significantly opposes the TNF-α–induced reduction in TER after 8 hours of exposure. **P < 0.001; significantly different from TNF-α.
Figure 6.
p38 MAP kinase-dependent TNF-α response on endothelial permeability. Changes in permeability in response to TNF-α, with or without pretreatment with SB-203580, were ascertained by quantifying the flux of FITC dextran (10 kDa) across cells grown on porous culture inserts. Treatment with 20 ng/mL TNF-α for 20 hours significantly increases the flux of FITC dextran in comparison with untreated cells (control). Error bars represent the SEM (n = 8). *P < 0.001; significantly different from control. Pretreatment with 20 μM SB-203580 for 1 hour significantly attenuates the TNF-α–induced increase in permeability. **P < 0.01; significantly different from TNF-α.
Effect of TNF-α on Integrity of the Apical Junctional Complex
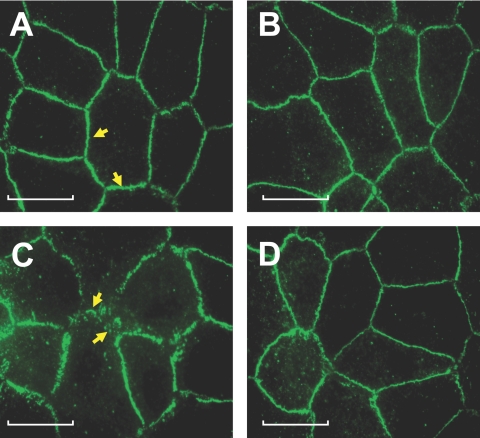
We next confirmed these findings on loss of barrier integrity by examining the organization of the AJC. In untreated cells, ZO-1 is localized contiguously at the cell-cell boundaries and accordingly stained uniformly at the cell borders (Fig. 7A). Exposure to TNF-α induced a dispersion of ZO-1 from its normal locus, indicating a breakdown of the barrier integrity (Fig. 7C). Pretreatment with SB-203580 opposed the influence of TNF-α on ZO-1 redistribution (Fig. 7D). This confirmed that the loss in TER response in Figure 1 involved the dislocation of ZO-1 by the p38 MAP kinase-dependent pathway.
Figure 7.
p38 MAP kinase-dependent TNF-α response on the distribution of ZO-1. Cells were pretreated with 20 μM SB-203580 for 1 hour with or without 20 ng/mL TNF-α for 6 hours and were assessed for distribution of ZO-1. (A–D) Representative images. (A) In untreated cells, ZO-1 distribution is contiguous at the cell-cell border (arrows). (B) On treatment with SB-203580, the pattern of ZO-1 distribution is similar to that of untreated cells. (C) TNF-α induces the dispersion of ZO-1, as evidenced by discontinuities in ZO-1 distribution (arrows). (D) SB-203580 opposes the dispersion of ZO-1 by TNF-α. Scale bars, 20 μM.
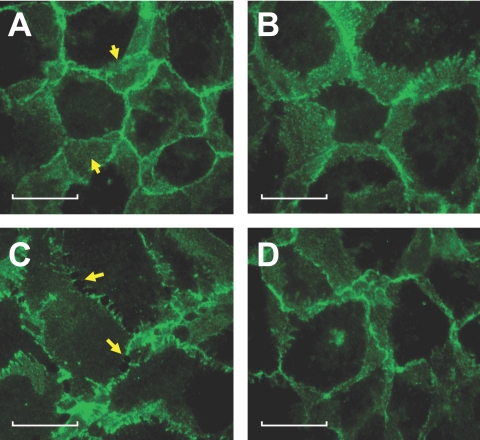
In consonance with the findings on ZO-1, we also observed disorganization of cadherins, which are associated with adherens junctions (AJs). In untreated cells, cadherins distribution is contiguous at the cell-cell boundary (Fig. 8A). Exposure to TNF-α for 6 hours disrupted the organization of cadherins, indicating a breakdown of AJs (Fig. 8C). As with ZO-1 staining, pretreatment with SB-203580 suppressed the influence of TNF-α on the redistribution of cadherins (Fig. 8D). These results show that TNF-α induces not only a breakdown of TJs, as indicated by a decrease in TER and an increase in FITC dextran permeability, but also causes disruption of the AJs. Furthermore, our results suggest that the breakdown of TJs and AJs are mediated by a p38 MAP kinase-dependent pathway.
Figure 8.
p38 MAP kinase-dependent TNF-α response on the distribution of cadherins. Cells were pretreated with 20 μM SB-203580 for 1 hour with or without 20 ng/mL TNF-α for 6 hours and were assessed for the distribution of cadherins. (A–D) Representative images. (A) In untreated cells, prominent distribution of cadherins at the region of cell-cell contacts is evident (arrows). (B) On treatment with SB-203580, the distribution of cadherins is unaltered. (C) Treatment with 20 ng/mL TNF-α for 6 hours induces compromise in cell-cell contacts through the disengagement of cadherins at the intercellular regions (arrows). (D) Pretreatment with SB-203580 opposes the influence of TNF-α on localization of cadherins. Scale bars, 20 μM.
Changes in the Cytoskeleton in Response to TNF-α
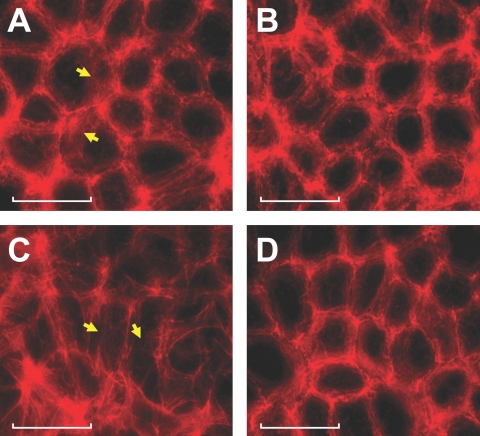
The actin cytoskeleton at the AJC, which forms a dense assembly commonly referred to as the perijunctional actomyosin ring (PAMR), is structurally and functionally coupled to cytoplasmic domains of TJs and AJs. Therefore, PAMR disruption is implicated in the regulation of barrier integrity.25,37,38 Because of this, we investigated remodeling of the PAMR in response to TNF-α. The characteristic assembly of the PAMR in untreated cells (shown in Fig. 9A) is disrupted significantly by exposure to the cytokine (Fig. 9C). However, the cytokine-induced disruption of the PAMR is suppressed by pretreatment with SB-203580 (Fig. 9D).
Figure 9.
p38 MAP kinase-dependent TNF-α response on organization of the PAMR. The influence of TNF-α on the organization of PAMR was ascertained by staining for F-actin using Texas Red conjugated to phalloidin. Cells were pretreated with 20 μM SB-203580 for 1 hour with or without 20 ng/mL TNF-α for 6 hours. (A–D) Representative images showing organization of cortical actin at the plane of AJC. (A) In untreated cells, the characteristic organization of cortical actin with intact PAMR is observed (arrows). (B) On treatment with SB-203580, the organization of PAMR is not disturbed to a large extent. (C) TNF-α induces the disruption of PAMR (arrows), leading to a change in cell morphology. (D) Pretreatment with SB-203580 opposes the TNF-α response on PAMR. Scale bars, 40 μM.
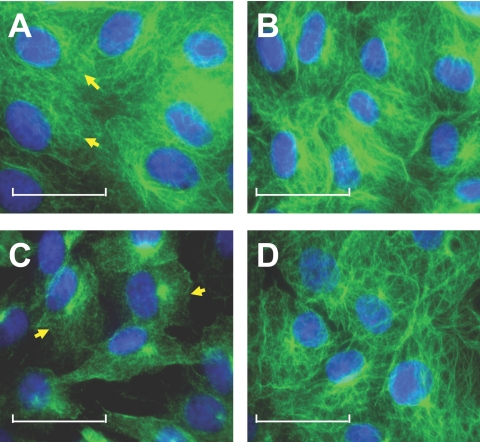
In addition to disruption of the PAMR, we have recently demonstrated that TNF-α can also induce microtubule disassembly (Shivanna M, et al. IOVS 2008;49:ARVO E-Abstract 3948). To determine whether the microtubule disassembly is also mediated by the TNF-α–induced p38 MAP kinase pathway, we immunostained for α-tubulin. The microtubule disassembly in response to TNF-α is suppressed by pretreatment with SB-203580 (Fig. 10D).
Figure 10.
p38 MAP kinase-dependent TNF-α response on microtubules. The influence of TNF-α on organization of microtubules was ascertained by immunostaining for α-tubulin. Cells were pretreated with 20 μM SB-203580 for 1 hour with or without 20 ng/mL TNF-α for 6 hours. (A–D) Representative images showing the organization of microtubules. (A) In untreated cells, microtubules exist as characteristic fibrillary extensions from around the nucleus to the cell periphery (arrows). (B) On treatment with SB-203580, the organization of microtubules is unaltered. (C) Treatment with TNF-α induces the loss of fibrillary extensions and the condensation of microtubules, an indication of microtubule disassembly (arrows). (D) Pretreatment with 20 μM SB-203580 for 1 hour opposes the TNF-α response on microtubules. Scale bars, 30 μM.
Discussion
This is the first study that suggests the involvement of p38 MAP kinase in the TNF-α–induced loss of barrier integrity in corneal endothelium. As noted, TNF-α is implicated in endothelial dysfunction during corneal allograft rejection9,10 and during anterior uveitis.11 Unlike many other cytokines (e.g., IL-2) found in the anterior chamber and cornea during these disorders,7,39,40 TNF-α is likely to have a large number of effects, including apoptosis,21,41 breakdown of the barrier integrity of the endothelium,14,15,17,18 degradation of the extracellular matrix by the induction of MMPs,42 disruption of the cytoskeleton14,15,17 and prolongation of the immune response through the induction of cell adhesion molecules.16 In this study, we focused on the loss of barrier integrity in response to the cytokine because it has been demonstrated that such a loss often is independent of apoptosis.21,43 Additional impetus for this study is based on the rationale that the barrier integrity of the endothelium complements its fluid transport function in maintaining corneal transparency.4 In several recent studies, we investigated the mechanisms by which inflammatory mediators elicit the breakdown in barrier integrity and intercellular communication of corneal endothelium through altered cytoskeleton.25,37,38,44–47 Our experimental findings, taken together, suggest that TNF-α breaks down the barrier integrity of corneal endothelium through the activation of p38 MAP kinase. This pathway, in turn, through yet unknown downstream targets, influences reorganization of the PAMR and AJC and causes disassembly of the microtubule cytoskeleton.
Changes in TER in the Response to TNF-α
Given the small electrical resistance across the corneal endothelium, we took recourse to the highly sensitive method of ECIS to quantify TER.26 Previously, we30 and others29 have used ECIS to demonstrate changes in the barrier integrity of corneal endothelial monolayers. Specifically, we have shown that exposure to cytochalasin D, an actin-severing agent, induced a dose-dependent decline in TER. This finding is in line with a number of studies that have shown that a destruction of PAMR by cytochalasin D causes a breakdown of the barrier integrity.48 Based on these observations, the decline in TER that we observed (Figs. 1, 3, 5) suggest that TNF-α induces a loss in barrier integrity. Further confirmation is given in Figure 6, in which the cytokine is shown to increase permeability of FITC dextran. These findings are also reflected in our experiments involving immunostaining of ZO-1 and cadherins (Figs. 7, 8). ZO-1, which is an intracellular adaptor molecule associated with the transmembrane proteins of the TJs, is contiguous at cell-cell borders, and its dislocation is a marked indication of the loss of barrier integrity.49 Thus, our findings in Figure 7 are consistent with the loss of TER in Figures 1, 3, and 5 and denotes a disruption of the integrity of TJs. That the cytokine also breaks down the AJs is demonstrated by dislocation of cadherins at several points along the cell-cell border (Fig. 8C, arrows). Because the AJs are critical to inducing the cell-cell tethering necessary to bring about the interaction of transmembrane proteins of the TJs, the dispersion of cadherins on exposure to TNF-α is also indicative of the breakdown of the barrier integrity. These modifications at the AJC collectively argue in favor of the loss of barrier integrity via nonapoptotic mechanisms. This is further demonstrated by our TER measurements with ZVAD, a pan-caspase inhibitor. The failure of ZVAD to oppose the TNF-α response (Fig. 3) indicates that the cytokine, at the doses used, did not cause significant apoptosis. Our additional apoptosis assay involving annexin V-propidium iodide staining showed that TNF-α did not induce significant apoptosis (% apoptotic cells; untreated cells 4.45 vs. TNF-α 5.03; n = 6 independent trials; P > 0.05) or necrosis (% necrotic cells; untreated cells 2.22 vs. TNF-α 2.81; n = 6 independent trials; P > 0.05) compared with untreated cells Thus, we conclude that the decline in TER was not through a trivial loss of cells from the monolayers but involved molecular mechanisms directed at AJC.
Activation of p38 MAP Kinase in Response to TNF-α and Its Downstream Effects
To gain further insight into the molecular mechanism involving the loss of barrier integrity, we examined the role of p38 MAP kinase because it is a major stress kinase implicated in the TNF-α–induced proinflammatory response.33,50 The cytokine-induced activation of p38 MAP kinase is demonstrated in Figure 4. As noted, phosphorylation was suppressed on pretreatment with SB-203580. The importance of the activation of p38 MAP kinase toward the loss of barrier integrity has been further evaluated. As shown in Figures 5 and 6, the TNF-α–induced decline in TER and the increase in FITC dextran permeability, respectively, are suppressed by a selective inhibitor of the kinase. It is also evident that SB-203580 significantly prevented the disruption of the cytoskeleton (Figs. 9, 10) and the dispersion of ZO-1 (Fig. 7) and cadherins (Fig. 8) at the AJC. Taken together, these results confirm that p38 MAP kinase activation plays a central role in the response to TNF-α. This claim is also consistent with our finding that TNFR1 is expressed in the endothelium because the receptor is known to induce TJ rearrangement during the loss of intestinal barrier function.51
Remodeling of AJC in Response to TNF-α
It has been shown previously that p38 MAP kinase activation by TNF-α can lead to changes in organization of the cytoskeleton and AJC.15,52 In this study, we examined the influence of TNF-α on the organization of the cytoskeleton and AJC with or without SB-203580. Results in Figures 7 to 10 suggest that p38 MAP kinase is upstream of the dislocation of ZO-1, disengagement of cadherins, and disruption of the cytoskeleton. The disruption of cytoskeleton, especially that of the actin cytoskeleton (specifically, PAMR), may be associated with the phosphorylation of Hsp27, which is activated downstream of p38 MAP kinase.53 Alternatively, disruption of the cytoskeleton could also be attributed to the loss of focal adhesion secondary to the activation of MMPs, which are implicated as downstream targets of p38 MAP kinase.54 Although our results, especially those involving TER in the presence of SB-203580 (Fig. 5), suggest that p38 MAP kinase is the major pathway in the disruption of barrier integrity, other important mechanisms, such as the activation of c-Jun N-terminal kinase JNK50 and Rho kinase18,20 and the transcriptional upregulation of MLCK18,21 demonstrated in other cell types, may also play a role.
It has been demonstrated in recent studies that p38 MAP kinase-induced loss of barrier integrity is mediated by ROS, possibly through protein tyrosine phosphorylation.55 This is in line with a previous study that suggests that ROS activation is regulated by TNF-α signaling.56 Recent studies also suggest that redox imbalance contributes to alteration in the composition or spatial distribution of endothelial junctional proteins.57 Although further studies are warranted, based on these studies, we speculate that TNF-α–induced loss of barrier integrity downstream of p38 MAP kinase signaling may also involve ROS upregulation.
In conclusion, for the first time, we demonstrate the activation of p38 MAP kinase as the key molecular mechanism in the TNF-α–induced loss of barrier integrity of corneal endothelium. Hence, inhibition of p38 MAP kinase can be a therapeutic strategy to overcome endothelial dysfunction after corneal transplantation.
Footnotes
Supported by National Institutes of Health Grant R21-EY019119 and by a faculty research grant from the Indiana University at Bloomington (SPS).
Disclosure: M. Shivanna, None; G. Rajashekhar, None; S.P. Srinivas, None
References
- 1.Edelhauser HF. The balance between corneal transparency and edema: the Proctor Lecture. Invest Ophthalmol Vis Sci 2006;47:1754–1767 [DOI] [PubMed] [Google Scholar]
- 2.Riley M. Pump and leak in regulation of fluid transport in rabbit cornea. Curr Eye Res 1985;4:371–376 [DOI] [PubMed] [Google Scholar]
- 3.Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res 2003;22:69–94 [DOI] [PubMed] [Google Scholar]
- 4.Riley MV, Winkler BS, Starnes CA, Peters MI, Dang L. Regulation of corneal endothelial barrier function by adenosine, cyclic AMP, and protein kinases. Invest Ophthalmol Vis Sci 1998;39:2076–2084 [PubMed] [Google Scholar]
- 5.Bourne WM. Biology of the corneal endothelium in health and disease. Eye 2003;17:912–918 [DOI] [PubMed] [Google Scholar]
- 6.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci 2003;44:3326–3331 [DOI] [PubMed] [Google Scholar]
- 7.George AJ, Larkin DF. Corneal transplantation: the forgotten graft. Am J Transplant 2004;4:678–685 [DOI] [PubMed] [Google Scholar]
- 8.Torres PF, De Vos AF, van der Gaag R, Martins B, Kijlstra A. Cytokine mRNA expression during experimental corneal allograft rejection. Exp Eye Res 1996;63:453–461 [DOI] [PubMed] [Google Scholar]
- 9.Niederkorn JY, Mayhew E, Mellon J, Hegde S. Role of tumor necrosis factor receptor expression in anterior chamber-associated immune deviation (ACAID) and corneal allograft survival. Invest Ophthalmol Vis Sci 2004;45:2674–2681 [DOI] [PubMed] [Google Scholar]
- 10.Rayner SA, King WJ, Comer RM, et al. Local bioactive tumour necrosis factor (TNF) in corneal allotransplantation. Clin Exp Immunol 2000;122:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstedt EW, Baarsma GS, Kuijpers RW, van Hagen PM. Anti-TNF-alpha therapy for sight threatening uveitis. Br J Ophthalmol 2005;89:533–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayner SA, Larkin DF, George AJ. TNF receptor secretion after ex vivo adenoviral gene transfer to cornea and effect on in vivo graft survival. Invest Ophthalmol Vis Sci 2001;42:1568–1573 [PubMed] [Google Scholar]
- 13.Petrache I, Birukov K, Zaiman AL, et al. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 2003;17:407–416 [DOI] [PubMed] [Google Scholar]
- 14.Goldblum SE, Ding X, Campbell-Washington J. TNF-alpha induces endothelial cell F-actin depolymerization, new actin synthesis, and barrier dysfunction. Am J Physiol 1993;264:C894–C905 [DOI] [PubMed] [Google Scholar]
- 15.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 2003;28:574–581 [DOI] [PubMed] [Google Scholar]
- 16.Okada N, Fukagawa K, Takano Y, et al. The implications of the upregulation of ICAM-1/VCAM-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci 2005;46:4512–4518 [DOI] [PubMed] [Google Scholar]
- 17.Watsky MA, Guan Z, Ragsdale DN. Effect of tumor necrosis factor alpha on rabbit corneal endothelial permeability. Invest Ophthalmol Vis Sci 1996;37:1924–1929 [PubMed] [Google Scholar]
- 18.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol 2007;213:221–228 [DOI] [PubMed] [Google Scholar]
- 19.Gertzberg N, Neumann P, Rizzo V, Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 2004;286:L37–L48 [DOI] [PubMed] [Google Scholar]
- 20.Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun 2003;306:244–249 [DOI] [PubMed] [Google Scholar]
- 21.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1168–L1178 [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Yerukhimovich M, Gaarde WA, Popoff IJ, Doerschuk CM. MKK3 and -6-dependent activation of p38alpha MAP kinase is required for cytoskeletal changes in pulmonary microvascular endothelial cells induced by ICAM-1 ligation. Am J Physiol Lung Cell Mol Physiol 2005;288:L359–L369 [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Horie T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br J Pharmacol 2001;132:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 2005;289:L75–L84 [DOI] [PubMed] [Google Scholar]
- 25.Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res 2004;79:477–486 [DOI] [PubMed] [Google Scholar]
- 26.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A 1992;89:7919–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birukova AA, Birukov KG, Adyshev D, et al. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 2005;204:934–947 [DOI] [PubMed] [Google Scholar]
- 28.Birukova AA, Smurova K, Birukov KG, et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 2004;201:55–70 [DOI] [PubMed] [Google Scholar]
- 29.Yin F, Watsky MA. LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Invest Ophthalmol Vis Sci 2005;46:1927–1933 [DOI] [PubMed] [Google Scholar]
- 30.Jalimarada SS, Shivanna M, Kini V, Mehta D, Srinivas SP. Microtubule disassembly breaks down the barrier integrity of corneal endothelium. Exp Eye Res 2009;89:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Lamki RS, Wang J, Vandenabeele P, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 2005;19:1637–1645 [DOI] [PubMed] [Google Scholar]
- 32.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE 2006;2006:pe44 [DOI] [PubMed] [Google Scholar]
- 33.Nwariaku FE, Chang J, Zhu X, et al. The role of p38 MAP kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock 2002;18:82–85 [DOI] [PubMed] [Google Scholar]
- 34.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995;9:726–735 [PubMed] [Google Scholar]
- 35.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun 1999;263:825–831 [DOI] [PubMed] [Google Scholar]
- 36.Young PR, McLaughlin MM, Kumar S, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem 1997;272:12116–12121 [DOI] [PubMed] [Google Scholar]
- 37.Satpathy M, Gallagher P, Jin Y, Srinivas SP. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp Eye Res 2005;81:183–192 [DOI] [PubMed] [Google Scholar]
- 38.Srinivas SP, Satpathy M, Guo Y, Anandan V. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Invest Ophthalmol Vis Sci 2006;47:4011–4018 [DOI] [PubMed] [Google Scholar]
- 39.Niederkorn JY. Mechanisms of corneal graft rejection: the sixth annual Thygeson Lecture, presented at the Ocular Microbiology and Immunology Group meeting, October 21, 2000. Cornea 2001;20:675–679 [DOI] [PubMed] [Google Scholar]
- 40.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res 2007;32:1005–1016 [DOI] [PubMed] [Google Scholar]
- 41.Sagoo P, Chan G, Larkin DF, George AJ. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci 2004;45:3964–3973 [DOI] [PubMed] [Google Scholar]
- 42.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 2004;25:1543–1549 [DOI] [PubMed] [Google Scholar]
- 43.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003;171:6164–6172 [DOI] [PubMed] [Google Scholar]
- 44.Srinivas SP, Satpathy M, Gallagher P, Lariviere E, Van Driessche W. Adenosine induces dephosphorylation of myosin II regulatory light chain in cultured bovine corneal endothelial cells. Exp Eye Res 2004;79:543–551 [DOI] [PubMed] [Google Scholar]
- 45.D'Hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophthalmol Vis Sci 2007;48:120–133 [DOI] [PubMed] [Google Scholar]
- 46.D'Hondt C, Srinivas SP, Vereecke J, Himpens B. Adenosine opposes thrombin-induced inhibition of intercellular calcium wave in corneal endothelial cells. Invest Ophthalmol Vis Sci 2007;48:1518–1527 [DOI] [PubMed] [Google Scholar]
- 47.Ponsaerts R, D'Hondt C, Bultynck G, Srinivas SP, Vereecke J, Himpens B. The myosin II ATPase inhibitor blebbistatin prevents thrombin-induced inhibition of intercellular calcium wave propagation in corneal endothelial cells. Invest Ophthalmol Vis Sci 2008;49:4816–4827 [DOI] [PubMed] [Google Scholar]
- 48.Matthews JB, Tally KJ, Smith JA, Awtrey CS. F-actin differentially alters epithelial transport and barrier function. J Surg Res 1994;56:505–509 [DOI] [PubMed] [Google Scholar]
- 49.Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2004;45:675–684 [DOI] [PubMed] [Google Scholar]
- 50.Winston BW, Chan ED, Johnson GL, Riches DW. Activation of p38mapk, MKK3, and MKK4 by TNF-alpha in mouse bone marrow-derived macrophages. J Immunol 1997;159:4491–4497 [PubMed] [Google Scholar]
- 51.Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol 2008;294:G938–G947 [DOI] [PubMed] [Google Scholar]
- 52.Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 2004;286:G367–G376 [DOI] [PubMed] [Google Scholar]
- 53.Kiemer AK, Weber NC, Furst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res 2002;90:874–881 [DOI] [PubMed] [Google Scholar]
- 54.Kim KC, Lee CH. MAP kinase activation is required for the MMP-9 induction by TNF-stimulation. Arch Pharm Res 2005;28:1257–1262 [DOI] [PubMed] [Google Scholar]
- 55.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal 2003;5:723–730 [DOI] [PubMed] [Google Scholar]
- 56.Rajashekhar G, Grow M, Willuweit A, Patterson CE, Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics 2007;31:104–113 [DOI] [PubMed] [Google Scholar]
- 57.Zhao X, Alexander JS, Zhang S, et al. Redox regulation of endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol 2001;281:L879–L886 [DOI] [PubMed] [Google Scholar]