The role of cytosolic phospholipase A2 in retinal angiogenesis is identified and characterized using relevant cell-based assays and a rodent model of retinopathy of prematurity to further elucidate the contribution of the cyclooxygenase pathway to retinal neovascularization.
Abstract
Purpose.
To identify and characterize the role of cytosolic phospholipase A2 (cPLA2) in retinal angiogenesis using relevant cell-based assays and a rodent model of retinopathy of prematurity.
Methods.
The phosphorylation states of cPLA2 and p38 MAP kinase and the expression of COX-2 were assessed by Western blot analysis in rat Müller cells. The activities of PLA2 enzymes in rat retinal lysates were assessed using a commercially available assay. Prostaglandin E2 (PGE2) and VEGF levels in Müller cell-conditioned medium and in retinal tissue samples were measured by ELISA. Rat retinal microvascular endothelial cell proliferation was measured using a BrdU assay. Efficacy of the cPLA2 inhibitor CAY10502 was tested using the rat model of oxygen-induced retinopathy (OIR) in which neovascularization (NV) was assessed by computer-assisted image analysis.
Results.
In Müller cells, hypoxia increased the phosphorylation of cPLA2 and p38 MAP kinase by 4-fold and 3-fold respectively. The cPLA2 inhibitor CAY10502 decreased hypoxia-induced PGE2 and VEGF levels in Müller cell-conditioned medium by 68.6% (P < 0.001) and 46.6% (P < 0.001), respectively. Retinal cPLA2 activity peaked 1 day after oxygen exposure in OIR rats. CAY10502 (250 nM) decreased OIR-induced retinal PGE2 and VEGF levels by 69% (P < 0.001) and 40.2% (P < 0.01), respectively. Intravitreal injection of 100 nM CAY10502 decreased retinal NV by 53.1% (P < 0.0001).
Conclusions.
cPLA2 liberates arachidonic acid, the substrate for prostaglandin (PG) production by the cyclooxygenase enzymes. PGs can exert a proangiogenic influence by inducing VEGF production and by stimulating angiogenic behaviors in vascular endothelial cells. Inhibition of cPLA2 inhibits the production of proangiogenic PGs. Thus, cPLA2 inhibition has a significant influence on pathologic retinal angiogenesis.
Angiogenesis, the formation of new capillaries from existing blood vessels, occurs during physiological processes such as reproduction, growth and development, and wound healing.1–6 Conversely, diseases such as arthritis, tumor growth, and retinopathies are characterized by pathologic, persistent angiogenesis.6–8 In the context of the retina, pathologic, persistent angiogenesis is often referred to as retinal neovascularization (NV). Age-related macular degeneration, diabetic retinopathy, and retinopathy of prematurity are potentially blinding conditions characterized by choroidal or retinal NV.
Retinal NV is often caused by tissue hypoxia.9–11 Hypoxia stimulates the activation of various intracellular signaling pathways, which lead to the production of growth factors and cytokines that stimulate quiescent endothelial cells to develop a neovascular phenotype.12–17 Of the vasoactive factors identified to date, there is considerable evidence that vascular endothelial growth factor (VEGF) is most consistently and dramatically upregulated by retinal hypoxia.18 Hypoxia induces VEGF synthesis in a number of retinal cell types, including endothelial cells, astrocytes, retinal pigment epithelial cells, Müller cells, and ganglion cells.19–23 Müller cells have been shown to be the principal source of VEGF in animal models of retinal NV.21–23
Previous studies suggest that cyclooxygenase (COX)/prostaglandin (PG)-dependent signaling mechanisms contribute to retinal VEGF production and neovascular disease.24–27 The initial step in PG biosynthesis is the liberation of arachidonic acid (AA) from membrane phospholipids by phospholipase A2 (PLA2) enzymes. There are at least 19 groups of PLA2s that are generally classified as cytosolic (cPLA2), secretory (sPLA2), or calcium-independent (iPLA2). PLA2 is activated in response to a number of stimuli including ischemia, oxidative stress, and cell signaling molecules.28 cPLA2 is activated when serines 505 and 727 are phosphorylated by p38 and p42/44 MAP kinases.29 Active cPLA2 then catalyzes the hydrolysis of membrane phospholipids at the sn-2 position, releasing AA directly into the cytoplasm.30 Free AA either diffuses out of the cell, is reincorporated into phospholipids, or is metabolized by the COX, lipoxygenase, or cytochrome P450 enzymes.30–32 There are two well-characterized COX enzymes. COX-1, a constitutive isoform, and COX-2, which is responsive to growth factors, cytokines, and environmental stimuli, catalyze the reaction between two molecules of oxygen (O2) and AA to produce prostaglandin H2 (PGH2). Cell-specific synthases catalyze isomerization, oxidation, and reduction of PGH2 to yield the prostaglandins E (PGE), F (PGF), and D (PGD).33–35
PGs may exert a proangiogenic influence by inducing the upregulation of VEGF.36–39 The following lines of evidence suggest a COX/PG-dependent component to retinal VEGF induction and subsequent NV: (1) hypoxia stimulates the upregulation of COX-2 (as well as VEGF) in Müller cells40; (2) hypoxia stimulates an approximate 3-fold increase in Müller cell PGE2 synthase (McCollum GW, et al. IOVS 2005;46:ARVO E-Abstract 2974); (3) PGE2 induces the upregulation of VEGF and basic fibroblast growth factor (bFGF; a potent angiogenesis inducer) in Müller cells39; (4) in vitro data show that amfenac, a nonsteroidal anti-inflammatory drug (NSAID), dose dependently inhibits hypoxia-induced VEGF production in Müller cells41; (5) cPLA2, COX, and VEGF are coordinately upregulated during the post-oxygen treatment phase (retinal hypoxia) in the rat model of oxygen-induced retinopathy (OIR) (Lukiw JW, et al. IOVS 2002;46:ARVO E-Abstract 2974) and in retinal endothelial cells exposed to hypoxia42; and (6) NSAIDs that inhibit COX and, consequently, PG synthesis, reduce the NV response in rodent models of OIR.24–27
In these studies, cPLA2-dependent mechanisms of retinal angiogenesis were investigated. In vitro experiments used Müller and endothelial cells as models of the primary VEGF-producing cell type and the proliferating cell type of neovascular lesions, respectively. Consequently, cPLA2 activity, VEGF levels, and PGE2 levels were measured in the Müller cells, and proliferation was measured in endothelial cells in response to inhibiting cPLA2. In vivo experiments using the rat OIR model were structured to complement and build on the in vitro studies; to that end, we measured the relative contribution of PLA2 isoforms, cPLA2 activity, VEGF levels, PGE2 levels, and neovascular areas with cPLA2 inhibition.
Materials and Methods
Rat Oxygen Treatment
All animal experiments were approved by the Vanderbilt University School of Medicine Animal Care and Use Committee, and they were conducted according to the principles expressed in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Within 4 hours of birth, litters of Sprague-Dawley rat pups and their mothers were exposed to alternating 24-hour periods at 50% and10% oxygen for 14 days. This variable oxygen treatment protocol predisposed the rat pups to OIR. Hereafter, these rats are referred to as OIR rats. Age-matched control rats were maintained in ambient (20.9% oxygen) normoxia. These rats are referred to as room air (RA) rats. After variable oxygen treatment, the OIR rats were returned to room air for up to 6 days, allowing time for retinal NV to develop. We refer to the timing of kill and assessment with two numbers, one representing the time in variable oxygen and one representing the postexposure period. Hence, rats killed immediately on removal from exposure are termed 14(0), whereas rats killed at the end of the 6-day postexposure period are referred to as 14(6).
cPLA2 Activity Assay
Rat pups were killed on 14(0), 14,(1) 14(3), and 14(6). Retinas were collected, and protein was extracted with lysis buffer solution (80 mM Hepes [pH 7.4], 150 mM NaCl, 10 mM CaCl2, 4 mM Triton X-100, 30% glycerol, and 1 mg/mL bovine serum albumin). The protein extracts were then assayed for cPLA2 activity (cPLA2 Assay Kit; Cayman Chemical, Ann Arbor, MI). Samples were either treated with 5 μM bromoenol lactone and thioether amide-PC (Cayman Chemical, Ann Arbor, MI) to block iPLA2 and sPLA2 activity, respectively, or were centrifuged using a membrane filter with a 30,000 MWt cutoff (Amicon Microcon Filter; Millipore Corporation, Bedford, MA) to separate the smaller iPLA2 and sPLA2 enzymes from cPLA2. Both methods yielded similar results. Cytosolic PLA2 was also pharmacologically inhibited in some samples with either CAY10502, a cPLA2-specific inhibitor, or methylarachidonyl fluorophosphate (MAFP), a cPLA2 and iPLA2-selective inhibitor (Cayman Chemical, Ann Arbor, MI). This experiment was conducted three times. Samples sizes of n = 4 were used for experiments examining cPLA2 activity over time and n = 5 for experiments testing the relative activity of the PLA2 isoforms.
Müller Cell Isolation and Culture
Müller cells were isolated from the retinas of 1-week-old Long-Evans rat pups, according the procedure described by Hicks and Courtois.43 Cells from passages four to six were used in the following experiments. The cells were grown in 10% serum DMEM low glucose (Mediatech, Inc., Manassas, VA) to 70% confluence and were maintained in normoxia for 24 hours. After 24 hours, cells were exposed to hypoxia for 12 hours (BBL GasPak system; Becton, Dickinson and Company, Sparks, MD) in the absence and presence of a cPLA2 inhibitor, CAY10502, at final concentrations of 5, 20, and 50 nM. Some cultures were lysed for Western blot analysis, and others were prepared for PGE2 and VEGF ELISA (Quantikine Colorimetric Sandwich ELISA; R&D Systems, Minneapolis, MN). When assaying for PGE2, each experiment included the following controls: no treatment, vehicle treatment (0.1% DMSO), and lipopolysaccharide (LPS; 1 μg/mL) treatment (positive control). These experiments were conducted four times, with n = 4 for each treatment group.
Endothelial Cell Isolation and Culture
Rat retinal microvascular endothelial cells (RRMECs) were isolated by the method developed by Matsubara et al.44 Passages four to seven were used in cell proliferation assays. RRMECs were seeded in 10% serum EBM at 3 × 103 cells/well in a 96-well plate. RRMECs were serum starved for 12 hours and then treated with 1% serum medium in the absence or presence of 25 ng/mL VEGF. Cells treated with VEGF received one of several concentrations (0.1–100 nM) of CAY10502 for 24 hours Cells were then labeled with BrdU for 12 hours, and BrdU incorporation was quantified with a colorimetric ELISA (Roche, Indianapolis, IN). For all treatment groups, n = 5.
Assessment of COX-2, p38, and cPLA2 Levels in Müller Cells
For Western blot analysis, 3 × 106 Müller cells were pooled in 300 μL cold lysis buffer (150 mM NaCl, 1.0% TritonX-100, 0.1% SDS, 50 mM Tris-HCl, 100 μg/mL phenylmethylsulfonyl fluoride, 1 mM orthovanadate, 0.3 μg/mL EDTA, 0.5% deoxycholate acid, 50 μM NaF, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, and 1.0 mg/mL aprotinin) and were homogenized by sonication at 4°C. The samples were then centrifuged at 5000 rpm for 15 minutes at 4°C. Protein concentrations were determined using a BCA kit (Pierce Biotechnology, Rockford, IL). The volume of each sample was adjusted to a protein concentration of 2.5 μg/μL with cold lysis buffer containing protease inhibitors. Samples were resolved by SDS-PAGE and transferred to 0.2 μm nitrocellulose membranes (Bio-Rad). Nitrocellulose membranes were blocked with TBST-1% bovine serum albumin (Sigma) and probed with primary antibodies. Either goat anti-mouse IgG HRP (Chemicon, Temecula, CA) or goat anti-rabbit IgG-HRP (Chemicon) secondary antibodies were applied to the membranes, which were then developed with enhanced chemiluminescence (Amersham, Piscataway, NJ). The following primary antibodies were used in this experiment: anti-cPLA2, phosphoSer505-cPLA2, -p38, and -phosphoThr180/Tyr182-p38 (Cell Signaling Technology), and anti-COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA). Each Western blot was repeated three times.
Intravitreal Injections
Rats were anesthetized by isoflurane (Terrell, Meridian, ID) inhalation, and a single drop of 0.5% proparacaine (Allergan; Hormigueros, PR) was topically applied to the cornea before intravitreal injection. For all intravitreal injections, the globe was penetrated posterior to the ora ciliaris retinal using a 30-gauge needle with a 19° bevel and a 10-μL syringe (Hamilton Co., Reno, NV). The needle was advanced to the posterior vitreous while a steep angle was maintained to avoid contact with the lens. The injection bolus (5 μL) was delivered near the trunk of the hyaloid artery, proximal to the posterior pole of the retina. After injection, a topical antibiotic suspension (neomycin and polymyxin B sulfates and gramicidin; Monarch Pharmaceuticals, Bristol, TN) was applied. Noninjected eyes were also treated with topical proparacaine and antibiotic to control for the potential of these agents to influence retinal vessel growth.
Drug Treatment
At 14(0), a time of high retinal VEGF expression in this model, eyes from OIR and RA rats remained uninjected, or were injected with 5 μL vehicle (0.1% DMSO), MAFP, or CAY10502 at doses ranging from 0.5 to 100 μM or 2.5 to 100 nM, respectively. These doses were initially chosen based on published IC50 data45–47 and were confirmed empirically.
Quantification of Retinopathy
OIR rats were euthanatized by decapitation on 14(6). Rat eyes were enucleated, and the neural retinas were dissected and placed in cmf-PBS with 10% formaldehyde solution (37% formaldehyde solution; Fisher Scientific, Fair Lawn, NJ) overnight at 4°C. The retinal vasculature was stained for ADPase activity, according to a previously described method48 adapted for use herein.49,50 Images of ADPase-stained retinas were digitized, captured, and displayed at 20× magnification. The total retinal area and the retinal area containing blood vessels were traced on the monitor face with an interactive stylus pen (FTG Data Systems, Stanton, CA).51 The number of pixels within these areas was converted to square millimeters. Measurements of this parameter were recorded.
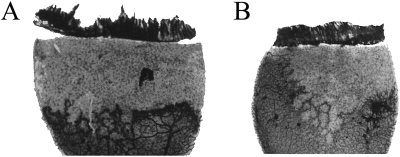
To determine the effect of the various treatments on pathologic angiogenesis, the extent of retinal NV was assessed in flat-mounted retinas stained for ADPase activity. Representative retinal flatmounts of vehicle- and CAY10502-treated rats are shown (see Fig. 7). Abnormal preretinal NV was assessed by digitally measuring NV area. Digitized images of the retinas were captured and displayed at 65× magnification. Preretinal vessels were then traced on a computer monitor with an interactive stylus pen (FTG Data Systems). The pixels contained in the areas of NV were totaled for each retina and converted to square millimeter (Photoshop CS; Adobe Systems Inc., San Jose, CA). The operator was masked with respect to the treatment. Where there was a question of the preretinal nature of a vessel tuft, the tissue was evaluated with a light microscope at 200× magnification using the plane of focus. This method of estimation correlates well (r2 = 0.947) with the clock hour method of estimation52 and yields normally distributed data that allow statistically significant differences between treatment groups to be determined by analysis of variance. The retinas of age-matched RA rats showed no abnormalities and were not included in statistical analyses.
Figure 7.
Comparison of two representative retinal quadrants from eyes treated with 0.01% DMSO vehicle (A) or the cPLA2 inhibitor CAY10502 at 100 nM (B).
Statistical Analysis
Statistically significant differences in average cPLA2 activities, average PGE2 and VEGF levels, and average NV areas between treatment and control groups were determined by analysis of variance with a Bonferroni/Dunn post hoc procedure. P ≤ 0.05 was considered significant. The experiment was repeated three times. For each experiment, n = 8 to 12 eyes for each treatment group.
Results
Effect of OIR on Rat Retinal cPLA2 Activity
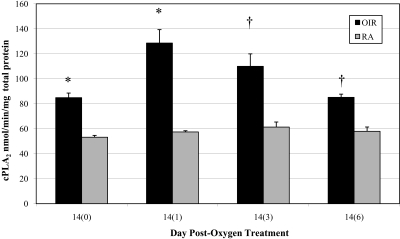
Retinal tissue lysates from OIR and age-matched RA rats (Fig. 1) were assayed for cPLA2 activity. At every time point, the cPLA2 activity in OIR retinas was significantly higher than the activity in RA retinas, which was unchanged. The greatest difference was at 14(1), when the cPLA2 activities in OIR and RA retinas were 128.58 ± 36.04 and 57.40 ± 2.78 nmol/min/mg total protein (P = 0.0076), respectively.
Figure 1.
Retinal cPLA2 activity ± SEM in OIR and RA rats between 14(0) and 14(6). *P < 0.01, and †P < 0.02; OIR versus RA.
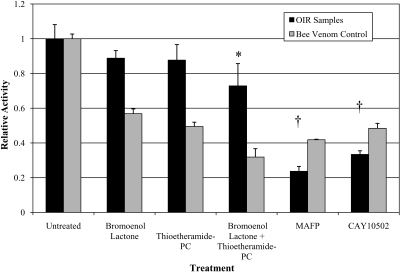
At least three isoforms—cPLA2, iPLA2, and sPLA2—significantly contribute to total retinal PLA2 activity. To estimate the relative contribution of cPLA2, we treated retinal tissue lysates from 14(1) OIR rats with isoform-selective inhibitors (Fig. 2). The addition of bromoenol lactone (iPLA2-selective inhibitor) or thioetheramide-PC (sPLA2-selective inhibitor) to retinal lysates did not significantly decrease PLA2 activity compared with control. When these inhibitors were combined, only a 27.1% ± 11.3% decrease was observed (P = 0.036). The addition of MAFP (cPLA2 and iPLA2 inhibitor) resulted in a 76.3% ± 3.5% decrease in activity compared with control (P < 0.001), and the more specific cPLA2 inhibitor, CAY10502, showed a 66.6% ± 2.6% decrease in activity (P < 0.001). These data suggest that as much as two-thirds of the retinal PLA2 activity is due to cPLA2. Therefore, inhibiting cPLA2 may be the optimal means by which to inhibit the release of AA in the retina. In Figure 2, bee venom refers to the effect of the individual PLA2 inhibitors on a sample composed primarily of PLA enzymes with a similar contribution from each of the tested isoforms.
Figure 2.
Relative PLA2 activity in OIR retinas at 14(1). This graph demonstrates the contribution of cPLA2 to total PLA2 activity in OIR retinas. Most of the total PLA2 activity in the retinal samples is derived from the cytosolic phospholipase family. Bromoenol lactone, iPLA2 inhibitor; thioetheramide-PC, sPLA2 inhibitor; MAFP (methylarachidonyl-fluorophosphonate), cPLA2 inhibitor with some iPLA2 inhibition; CAY10502, cPLA2α-specific inhibitor. *P < 0.05, and †P < 0.001; inhibitor versus untreated.
Effect of Hypoxia on the Phosphorylation of cPLA2 and the Expression of Associated Enzymes and Signaling Intermediates
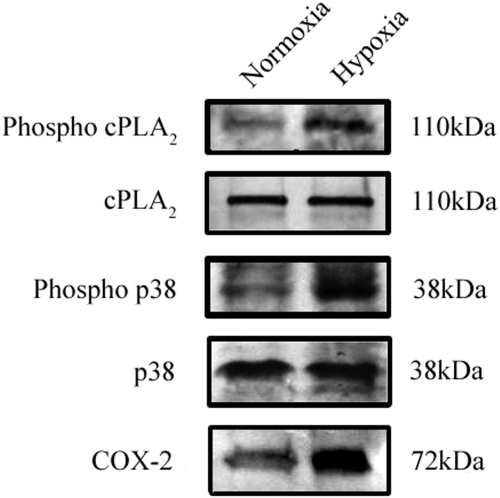
In Müller cells, the phosphorylation of p38 MAP kinase demonstrated a 4-fold increase in response to hypoxia. MAP kinase p38 is an upstream activator of cPLA2.53 Hypoxia also caused a 3-fold increase in the phosphorylation of Ser505 on cPLA2 and concomitantly induced an approximate 2-fold increase in the level of the COX-2 protein (Fig. 3).
Figure 3.
The effect of hypoxia on cPLA2 activation and related proteins in rat Müller cells. Hypoxia increases the phosphorylation of cPLA2 and p38 and the total protein levels of COX-2.
Effect of cPLA2 Inhibition on Hypoxia-Induced PGE2 and VEGF Expression in Rat Müller Cells
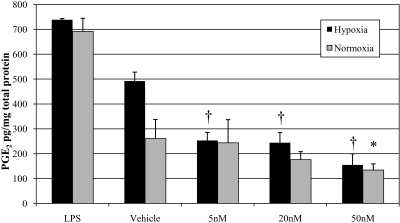
Conditioned media from normoxic and hypoxic rat Müller cells treated with LPS (positive control), vehicle, and 5 to 50 nM CAY10502 was assayed for PGE2 by ELISA (Fig. 4). In both normoxia and hypoxia, LPS treatment increased PGE2 levels compared with vehicle. A 1.9-fold increase in PGE2 was observed in hypoxic cells treated with vehicle (492.04 ± 32.5 vs. 260.58 ± 93.15 pg/mg total protein in normoxia; P = 0.0034). Notably, CAY10502 inhibited hypoxia-induced PGE2 production in a dose-dependent manner: 5 nM, 253.24 ± 36.03 (P < 0.001); 20 nM, 244.16 ± 40.67 (P < 0.001); and 50 nM, 154.50 ± 44.77 pg/mg (P < 0.001). There was also a dose-dependent decrease in PGE2 production by normoxic cultures treated with CAY10502 compared with vehicle; however, only the 50-nM concentration (134.06 ± 25.47 pg/mg [P = 0.040]) yielded statistical significance.
Figure 4.
The effect of cPLA2 inhibition on PGE2 in normoxic and hypoxic rat Müller cells. Cells were pretreated in normoxia for 24 hours and then were treated with normoxia or hypoxia plus drug for 12 hours. The cPLA2 inhibitor CAY10502 led to a dose-dependent reduction in PGE2 production. *P < 0.05, and †P < 0.001 relative to vehicle (0.1% DMSO).
Conditioned media from normoxic and hypoxic Müller cells treated with vehicle or 5 to 50 nM CAY10503 was assayed for VEGF by ELISA. Predictably, hypoxia caused a statistically significant increase in VEGF production by untreated and vehicle-treated cultures. VEGF levels were 41.39 ± 4.07 and 77.88 ± 6.62 pg/mg total protein for vehicle-treated normoxic and hypoxic cultures, respectively. CAY10502 treatment caused a dose-dependent decrease in hypoxia-induced VEGF production. The VEGF levels for 5, 20, and 50 nM CAY10502-treated hypoxic cultures were 60.90 ± 7.86 (P = 0.016), 57.99 ± 10.63 (P = 0.019), and 41.60 ± 5.11 pg/mg (P = 0.00013), respectively. However, in normoxic cultures treated with 5 to 50 nM CAY10502, VEGF levels were significantly different from vehicle-treated cultures (41.39 ± 4.07) only at the 50-nM concentration (33.69 ± 2.64 pg/mg; P = 0.019). A similar dose-dependent decrease in VEGF production was observed with MAFP treatment (data not shown).
Effect of cPLA2 Inhibition on VEGF-Induced RRMEC Proliferation
RRMECs stimulated with VEGF in serum-free growth medium demonstrated a 69.2% increase in proliferation compared with cells maintained in serum-free medium alone. RRMECs treated with 35 or 50 nM CAY10502, demonstrated significant reductions in VEGF-induced proliferation (64.3% [P = 0.010] and 84.1% [P = 0.012]), respectively) compared with cultures treated with VEGF alone. This experiment was also conducted using human retinal microvascular endothelial cells (HRMECs), yielding identical results (data not shown).
Effect of cPLA2 Inhibition on PGE2 and VEGF Induction in Rat OIR
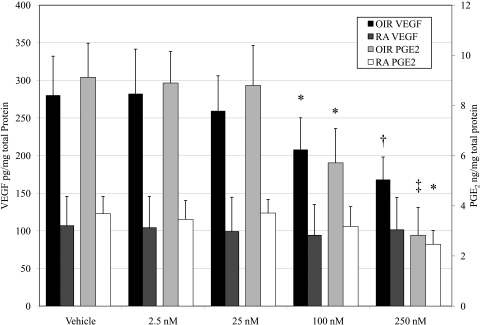
At 14(0), OIR and age-matched RA rats received intravitreal injections of vehicle or 2.5, 25, 100, or 250 nM CAY10502. Retinal tissues were collected at 14(1), and PGE2 levels were determined by ELISA. As expected, OIR induced retinal PGE2. In vehicle-injected eyes, OIR rats exhibited retinal PGE2 levels of 9.125 ± 1.36 compared with 3.682 ± 0.69 ng/mg total protein in the RA rats (Fig. 5). In the OIR rats, 2.5 or 25 nM CAY10502 did not significantly affect retinal PGE2 production, whereas 100 or 250 nM decreased the OIR-induced PGE2 levels to 5.71 ± 1.37 (P = 0.012) and 2.83 ± 1.11 ng/mg (P = 0.00036), respectively. Notably, the OIR-induced retinal PGE2 production was completely abolished at the highest concentration of PLA2 inhibitor. There was no statistically significant effect of CAY10502 on PGE2 production in RA rats except at the 250-nM (highest) concentration, which reduced PGE2 to 2.45 ± 0.55 ng/mg (P = 0.031 vs. RA vehicle treatment).
Figure 5.
The effect of cPLA2 inhibition on retinal VEGF and PGE2 in OIR rats 24 hours after removal from oxygen treatment. cPLA2 inhibition by CAY10502 demonstrates a dose-responsive reduction on both retinal VEGF (dark bars with axis on the left) and PGE2 (light bars with axis on the right). *P < 0.05, †P < 0.01, and ‡P< 0.001 relative to vehicle.
In vehicle-treated eyes, OIR rats exhibited retinal VEGF levels of 279.87 ± 52.24 compared with 100.19 ± 4.88 pg/mg total protein for RA rats (Fig. 5). None of the concentrations of CAY10502 significantly affected retinal VEGF levels in RA rats. In contrast, CAY10502 treatment caused a dose-dependent decrease in retinal VEGF in OIR rats. The retinal VEGF levels of the 25, 100, and 250 nM-treated eyes were 259.07 ± 47.04 (P = NS), 207.64 ± 42.62 (P = 0.043), and 167.69 ± 34.58 pg/mg (P = 0.0039), respectively. MAFP treatment caused a similar dose-dependent decrease in retinal VEGF in OIR rats (data not shown).
Effect of cPLA2 Inhibition on OIR Severity
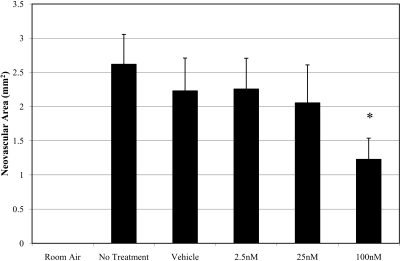
CAY10502-injected eyes demonstrated a dose-dependent inhibition of retinal NV (Fig. 6). Injection of 100 nM CAY10502 resulted in a 53.1% reduction in NV compared with vehicle treatment (P < 0.0001). Figure 7 contains representative flat-mounted retinas from vehicle-treated eyes (Fig. 7A) and 100 nM CAY10502-treated eyes (Fig. 7B). Compared with vehicle, the CAY10502-treated retina has fewer and less severe neovascular lesions.
Figure 6.
The effect of cPLA2 inhibition on NV area in rat OIR. The cPLA2 inhibitor CAY10502 led to a dose-dependent inhibition in NV area. *P < 0.0001 relative to vehicle.
A similar demonstration of efficacy was conducted using the less specific cPLA2 and iPLA2 inhibitor, MAFP. These experiments were conducted with concentrations ranging from 0.5 to 100 mM and resulted in a similar pathology-response profile (data not shown).
Discussion
In tumor angiogenesis, studies suggest PGE2 is a proangiogenic inducer of VEGF.54,55 Cheng et al.39 showed that treating rat Müller cells with PGE2 increased VEGF and bFGF secretion. Because retinal Müller cells are a primary source of VEGF in neovascular retinopathies, this study and others54,55 suggest that the COX/PG pathway plays a role in the pathologic condition. More knowledge of this pathway will improve our understanding of retinal NV. With a goal to further this knowledge, this study investigated cPLA2, a molecule upstream of prostaglandin synthesis.
Our central premise regarding the pathogenesis of retinal NV is not novel: retinal ischemia causes retinal hypoxia, leading to the induction of vasoactive factors, which activate vascular endothelial cells. To investigate the role of cPLA2 in this, we have used assays of hypoxia-induced VEGF production in Müller cells and VEGF-induced proliferation in endothelial cells. Although we have found cPLA2 to be ubiquitously produced throughout the retinal tissue (data not shown), Müller cells were specifically used in these studies because these cells have been shown to be associated with the largest induction of VEGF in the retina in response to hypoxia.21,54,55 In Müller cells, cPLA2 is expressed; p38 MAP kinase (an enzyme that activates cPLA2) and cPLA2 are phosphorylated/activated. The molecular weight of cPLA2 is 85 kDa. However, it has been reported to have an molecular weight of 110 kDa by SDS-PAGE, and our findings are consistent with this observation.56,57 These findings are also consistent with a role for cPLA2 in retinal NV. Moreover, in agreement with our previous findings and those of other studies,40 we observed increased COX-2 levels in OIR retinas and in hypoxic Müller cells (Barnett JM, et al. IOVS 2005;46:ARVO E-Abstract 4188). These coordinated events are expected to lead to increased levels of proangiogenic PGs because activation of cPLA2 liberates AA from membrane phospholipids, and AA is a necessary substrate for PG production by COX enzymes. We also observed that retinal cPLA2 activity is increased in OIR rats relative to RA rats. Accordingly, we found elevated levels of PGE2 in OIR retinas and hypoxic Müller cells and, as expected, elevated levels of VEGF.
Inhibition of PLA2 using either a cPLA2-specific compound or a cPLA2 and iPLA2-selective compound reduced or eliminated the elevation of PGE2 and VEGF in hypoxic Müller cells. Furthermore, the same inhibitors decreased VEGF-induced RRMEC and HRMEC proliferation. Several studies have shown AA release and both the cyclooxygenase and lipoxygenase pathways to be influential in endothelial cell proliferation induced by basic fibroblast growth factor, platelet-derived growth factor, and serum-containing VEGF.55,58,59 Similarly, this study's decreased endothelial proliferation, shown by cPLA2 inhibition, was likely the result of consequentially blocked VEGF-induced AA release. The effects of inhibiting PLA2 in vitro predicate our working hypothesis, modeling events of central importance to the onset of retinal angiogenesis in the in vivo setting, and our results suggest the importance of cPLA2 as a positive regulator of that process.
We further investigated the potential role of cPLA2 in vivo using rat OIR. In this biphasic animal model, phase 1 is OIR induced and leads to the attenuation of normal retinal vascular development, producing a substantial peripheral avascular zone. Phase 2 occurs when OIR rats are removed from exposure to RA. In phase 1, retinal avascularity leads to OIR-induced production of vasoactive factors and activation of vascular endothelium. In this model, the most important of these factors is the VEGF that is largely produced by the hypoxic Müller cells in the retinal avascular zone.21,54,55
Collectively, the in vivo data suggest a mechanistic link between OIR-induced increases in retinal cPLA2 activity, PGE2 and VEGF and imply that cPLA2 activity is important in the pathogenesis of rat OIR. CAY10502 reduced the retinal PLA2 activity at its maximal activity on 14(1) by approximately 66%, indicating that a majority of this activity can be attributed to the cytosolic PLA2 isoform. Retinal PGE2 and VEGF were increased in OIR rats, and intravitreal administration of CAY10502 significantly inhibited retinal levels of both. The peak in OIR-induced retinal cPLA2 activity, and increased retinal PGE2 and VEGF levels, all preceded the appearance of preretinal NV, which typically occurred at approximately 14(3) in this model.
The angiostatic capacity of the cPLA2-specific CAY10502 was tested in the rat OIR model, and the compound proved significantly efficacious at 100 nM. A similar efficacy trial was performed using the less specific inhibitor MAFP, which targets both cPLA2 and iPLA2. The two inhibitors showed similar potency in vivo, suggesting that the proangiogenic effect of retinal cPLA2 outweighs the contribution of iPLA2.
In light of our findings, cPLA2 is implicated in the pathogenesis of OIR and, by extension, human neovascular retinopathies. Notably, it appears to exert its influence both upstream and downstream of VEGF receptor activation. Although this is an important and potentially attractive feature of cPLA2, its value as a chemotherapeutic target for angiogenic conditions is limited. PLA2 enzymatic activity yields free AA, which in turn serves as a substrate for COX enzymes and PG production. PGs confer a wide range of bioactivities, many of which are completely unrelated to angiogenesis. Thus, inhibition of either PLA2 or COX represents a nonselective therapeutic strategy with the potential for a variety unintended side effects, a complication that has been clearly demonstrated.60 Our current efforts are focused on identifying the specific PGs and PG receptors responsible for the induction of VEGF and the promotion of angiogenic endothelial cell activities. We believe that inhibition of these targets holds significant therapeutic promise.
Footnotes
Supported by National Institutes of Health Grants EY07533 and EY01826; an unrestricted grant from Research to Prevent Blindness, Inc.; and a Research to Prevent Blindness Senior Scientific Investigator Award (JSP).
Disclosure: J.M. Barnett, None; G.W. McCollum, None; J.S. Penn, None
References
- 1.Klagsbrun M. Regulators of angiogenesis: stimulators, inhibitors, and extracellular matrix. J Cell Biochem 1991; 47(3): 199–200 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267(16): 10931–10934 [PubMed] [Google Scholar]
- 3.Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996; 87(7): 1153–1155 [DOI] [PubMed] [Google Scholar]
- 4.Risau W. Mechanisms of angiogenesis. Nature 1997; 386(6626): 671–674 [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003; 60(1): 107–114 [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost 2001; 86(1): 23–33 [PubMed] [Google Scholar]
- 7.Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9(6): 653–660 [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1(1): 27–31 [DOI] [PubMed] [Google Scholar]
- 9.Steinkuller PG, Du L, Gilbert C, et al. Childhood blindness. J AAPOS 1999; 3(1): 26–32 [DOI] [PubMed] [Google Scholar]
- 10.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population: the Baltimore Eye Survey. Ophthalmology 1996; 103(11): 1721–1726 [DOI] [PubMed] [Google Scholar]
- 11.Bressler NM, Bressler SB. Preventative ophthalmology: age-related macular degeneration. Ophthalmology 1995; 102(8): 1206–1211 [DOI] [PubMed] [Google Scholar]
- 12.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995; 92(12): 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17(11): 3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1α-deficient colon cancer cells. Nat Med 2005; 11(9): 992–997 [DOI] [PubMed] [Google Scholar]
- 15.Mizukami Y, Li J, Zhang X, et al. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 2004; 64(5): 1765–1772 [DOI] [PubMed] [Google Scholar]
- 16.Mizukami Y, Fujiki K, Duerr EM, et al. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem 2006; 281(20): 13957–13963 [DOI] [PubMed] [Google Scholar]
- 17.Jin HG, Yamashita H, Nagano Y, et al. Hypoxia-induced upregulation of endothelial small G protein RhoA and Rho-kinase/ROCK2 inhibits eNOS expression. Neurosci Lett 2006; 408(1): 62–67 [DOI] [PubMed] [Google Scholar]
- 18.Aiello LP. Vascular endothelial growth factor: 20th-century mechanisms, 21st-century therapies. Invest Ophthalmol Vis Sci 1997; 38(9): 1647–1652 [PubMed] [Google Scholar]
- 19.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 1995; 113(12): 1538–1544 [DOI] [PubMed] [Google Scholar]
- 20.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A 1995; 92(23): 10457–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 1995; 92(3): 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors 1997; 14(4): 229–241 [DOI] [PubMed] [Google Scholar]
- 23.Robbins SG, Rajaratnam VS, Penn JS. Evidence for upregulation and redistribution of vascular endothelial growth factor (VEGF) receptors flt-1 and flk-1 in the oxygen-injured rat retina. Growth Factors 1998; 16(1): 1–9 [DOI] [PubMed] [Google Scholar]
- 24.Nandgaonkar BN, Rotschild T, Yu K, Higgins RD. Indomethacin improves oxygen-induced retinopathy in the mouse. Pediatr Res 1999; 46(2): 184–188 [DOI] [PubMed] [Google Scholar]
- 25.Sennlaub F, Valamanesh F, Vazquez-Tello A, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 2003; 108(2): 198–204 [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2003; 44(3): 974–979 [DOI] [PubMed] [Google Scholar]
- 27.Castro MR, Lutz D, Edelman JL. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp Eye Res 2004; 79(2): 275–285 [DOI] [PubMed] [Google Scholar]
- 28.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005; 24(1): 87–138 [DOI] [PubMed] [Google Scholar]
- 29.Moolwaney AS, Igwe OJ. Regulation of the cyclooxygenase-2 system by interleukin-1β through mitogen-activated protein kinase signaling pathways: a comparative study of human neuroglioma and neuroblastoma cells. Brain Res Mol Brain Res 2005; 137(1–2): 202–212 [DOI] [PubMed] [Google Scholar]
- 30.Lin LL, Wartmann M, Lin AY, et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell 1993; 72(2): 269–278 [DOI] [PubMed] [Google Scholar]
- 31.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal 1995; 12(2–3): 83–117 [DOI] [PubMed] [Google Scholar]
- 32.Kramer RM, Roberts EF, Hyslop PA, et al. Differential activation of cytosolic phospholipase A2 (cPLA2) by thrombin and thrombin receptor agonist peptide in human platelets: evidence for activation of cPLA2 independent of the mitogen-activated protein kinases ERK1/2. J Biol Chem 1995; 270(24): 14816–14823 [DOI] [PubMed] [Google Scholar]
- 33.Rouzer CA, Marnett LJ. Structural and functional differences between cyclooxygenases: fatty acid oxygenases with a critical role in cell signaling. Biochem Biophys Res Commun 2005; 338(1): 34–44 [DOI] [PubMed] [Google Scholar]
- 34.Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2: mechanisms of catalysis and inhibition. J Biol Chem 1999; 274(33): 22903–22906 [DOI] [PubMed] [Google Scholar]
- 35.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 2004; 56(3): 387–437 [DOI] [PubMed] [Google Scholar]
- 36.Chang SH, Liu CH, Wu MT, Hla T. Regulation of vascular endothelial cell growth factor expression in mouse mammary tumor cells by the EP2 subtype of the prostaglandin E2 receptor. Prostaglandins Other Lipid Mediat 2005; 76(1–4): 48–58 [DOI] [PubMed] [Google Scholar]
- 37.Hoper MM, Voelkel NF, Bates TO, et al. Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol 1997; 17(6): 748–756 [DOI] [PubMed] [Google Scholar]
- 38.Hatazawa R, Tanigami M, Izumi N, et al. Prostaglandin E2 stimulates VEGF expression in primary rat gastric fibroblasts through EP4 receptors. Inflammopharmacology 2007; 15(5): 214–217 [DOI] [PubMed] [Google Scholar]
- 39.Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci 1998; 39(3): 581–591 [PubMed] [Google Scholar]
- 40.Ju WK, Kim KY, Neufeld AH. Increased activity of cyclooxygenase-2 signals early neurodegenerative events in the rat retina following transient ischemia. Exp Eye Res 2003; 77(2): 137–145 [DOI] [PubMed] [Google Scholar]
- 41.Penn JS, Song Q, Odonkor C. Nepafenac metabolite, amfenac, inhibits VEGF-induced angiogenesis Presented at: 57th Southeast/61st Southwest Regional Meeting; November 1–4, 2005; Memphis, TN Abstract 529 [Google Scholar]
- 42.Lukiw WJ, Ottlecz A, Lambrou G, et al. Coordinate activation of HIF-1 and NF-κB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci 2003; 44(10): 4163–4170 [DOI] [PubMed] [Google Scholar]
- 43.Hicks D, Courtois Y. The growth and behaviour of rat retinal Muller cells in vitro, 1: an improved method for isolation and culture. Exp Eye Res 1990; 51(2): 119–129 [DOI] [PubMed] [Google Scholar]
- 44.Matsubara TA, Murata TA, Wu GS, Barron EA, Rao NA. Isolation and culture of rat retinal microvessel endothelial cells using magnetic beads coated with antibodies to PECAM-1. Curr Eye Res 2000; 20(1): 1–7 [PubMed] [Google Scholar]
- 45.Riendeau D, Guay J, Weech PK, et al. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J Biol Chem 1994; 269(22): 15619–15624 [PubMed] [Google Scholar]
- 46.Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta 1996; 1302(1): 55–60 [DOI] [PubMed] [Google Scholar]
- 47.Ludwig J, Bovens S, Brauch C, Elfringhoff AS, Lehr M. Design and synthesis of 1-indol-1-yl-propan-2-ones as inhibitors of human cytosolic phospholipase A2α. J Med Chem 2006; 49(8): 2611–2620 [DOI] [PubMed] [Google Scholar]
- 48.Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol 1992; 110(2): 267–276 [DOI] [PubMed] [Google Scholar]
- 49.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci 1993; 34(3): 576–585 [PubMed] [Google Scholar]
- 50.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci 1995; 36(10): 2063–2070 [PubMed] [Google Scholar]
- 51.Penn JS, Rajaratnam VS, Collier RJ, Clark AF. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2001; 42(1): 283–290 [PubMed] [Google Scholar]
- 52.Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci 2003; 44(4): 1722–1731 [DOI] [PubMed] [Google Scholar]
- 53.Kramer RM, Roberts EF, Um SL, et al. p38 Mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets: evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem 1996; 271(44): 27723–27729 [DOI] [PubMed] [Google Scholar]
- 54.Gallo O, Franchi A, Magnelli L, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer: implications for tumor angiogenesis and metastasis. Neoplasia 2001; 3(1): 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majima M, Isono M, Ikeda Y, et al. Significant roles of inducible cyclooxygenase (COX)-2 in angiogenesis in rat sponge implants. Jpn J Pharmacol 1997; 75(2): 105–114 [DOI] [PubMed] [Google Scholar]
- 56.Clark JD, Lin LL, Kriz RW, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 1991; 65(6): 1043–1051 [DOI] [PubMed] [Google Scholar]
- 57.Kramer RM, Roberts EF, Manetta J, Putnam JE. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem 1991; 266(8): 5268–5272 [PubMed] [Google Scholar]
- 58.Dethlefsen SM, Shepro D, D'Amore PA. Arachidonic acid metabolites in bFGF-, PDGF-, and serum-stimulated vascular cell growth. Exp Cell Res 1994; 212(2): 262–273 [DOI] [PubMed] [Google Scholar]
- 59.Leahy KM, Ornberg RL, Wang Y, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res 2002; 62(3): 625–631 [PubMed] [Google Scholar]
- 60.Mukherjee D. Selective cyclooxygenase-2 (COX-2) inhibitors and potential risk of cardiovascular events. Biochem Pharmacol 2002; 63(5): 817–821 [DOI] [PubMed] [Google Scholar]