Current efforts to develop retinal stem cell therapies are hindered by extremely poor graft integration. This study demonstrates that transient suppression of reactive gliosis in the retina dramatically improves engraftment of intraocular stem cell transplants.
Abstract
Purpose.
Intraocular stem cell transplantation may be therapeutic for retinal neurodegenerative diseases such as glaucoma via neuronal replacement and/or neuroprotection. However, efficacy is hindered by extremely poor retinal graft integration. The purpose was to identify the major barrier to retinal integration of intravitreally transplanted stem cells, which was hypothesized to include the cellular and/or extracellular matrix (ECM) components of the inner limiting membrane (ILM).
Methods.
Mesenchymal stem cells (MSCs) were cocultured on the vitreal surface of retinal explants. Retinal MSC migration was compared between control explants and explants in which portions of the ILM were removed by mechanical peeling; the inner basal lamina was digested with collagenase; and glial cell reactivity was selectively modulated with α-aminoadipic acid (AAA). In vivo, the MSCs were transplanted after intravitreal AAA or saline injection into glaucomatous rat eyes.
Results.
Retinal MSC migration correlated positively with the amount of peeled ILM, whereas enzymatic digestion of the basal lamina was robust but did not enhance MSC entry. In contrast, AAA treatment suppressed glial cell reactivity and facilitated a >50-fold increase in MSC migration into retinal explants. In vivo analysis showed that AAA treatment led to a more than fourfold increase in retinal engraftment.
Conclusions.
The results demonstrated that the ECM of the inner basal lamina is neither necessary nor sufficient to prevent migration of transplanted cells into the neural retina. In contrast, glial reactivity was associated with poor graft migration. Targeted disruption of glial reactivity dramatically improved the structural integration of intravitreally transplanted cells.
The potential use of stem or progenitor cell transplantation to treat neurodegenerative diseases is a subject of intensive research. The eye is a good candidate for therapeutic cell transplantation, which could benefit the retina by neuronal replacement or neuroprotection. As examples of neuronal replacement, both neonatal photoreceptor precursors1 and human embryonic stem cell–derived retinal progenitors2 can functionally replace photoreceptors when transplanted into animals with retinal dystrophy. However, so far only a very small proportion of grafted cells have been shown to integrate into the host retina. Replacement of retinal ganglion cells (RGCs) presents an even greater challenge. RGCs are selectively and progressively lost in glaucoma, which is the leading cause of irreversible blindness worldwide.3 In contrast to photoreceptors, RGCs have complex afferent retinal connections and long axons that project to precise brain targets. Thus, functional replacement of RGCs necessitates overcoming numerous fundamental barriers.
Neuroprotective strategies, in which transplanted cells protect endogenous neural tissue, have also shown promising results in animal models of retinal disease. Cell transplantation slows the loss of neurons and/or preserves vision in models of inherited photoreceptor degeneration4,5 and retinal ischemia,6 and has also been investigated in models of glaucoma.7–9 The neuroprotective mechanism of action appears to include trophic factor secretion and/or modulation of inflammatory processes.8 In addition, cell transplantation may activate endogenous repair mechanisms by modulating inhibitory signals to promote axonal regrowth and neuritic sprouting.10,11
Although retinal stem cell therapy seems promising, multiple fundamental problems must be resolved before it can be applied clinically. Arguably, the most basic of these obstacles is the inhibitory barrier that prevents migration of grafted cells from the transplantation site into the retina. Overcoming this barrier is a necessary prerequisite to the long-term efficacy of retinal stem cell therapy, regardless of whether the ultimate goal is regenerative or protective in nature. Much like the rest of the mature central nervous system (CNS), the retina is implastic and relatively inhibitory to cellular migration. We and others have reported that only ∼1% of intraocularly transplanted cells commonly migrate into the retina, whereas most remain as a bolus outside of the neural tissue.1,7,12–17 This limitation appears to be common to a variety of stem cell types, including those of neural and mesenchymal lineage. Intravitreally grafted cells line the surface of the inner limiting membrane (ILM) without penetrating the host retina.15 Endogenous extracellular matrix (ECM) molecules contribute partially to the blockade of subretinal transplants, and the targeted disruption of these molecules can modestly enhance retinal integration.16–18 The contribution of basement membrane-associated proteins to the inhibitory environment has not been investigated. Moreover, the activity of nonneuronal retinal cell types may also inhibit graft migration.13,17,19 However, the relative importance of each component is largely unknown.
In this study, we systematically assessed the contributions of the inner basal lamina ECM and the retinal glial cell population to the blockade of graft migration after intravitreal transplantation. We demonstrated in vitro that, although mechanical ILM peeling facilitated graft migration, enzymatic degradation of the inner basal lamina alone, without concurrent Müller cell trauma, did not recapitulate the effect. However, treatment with a glia-specific toxin dramatically improved cell integration even when the ILM was fully intact. Furthermore, we confirmed that this latter effect was preserved in vivo, suggesting that suppression of glial reactivity may be a necessary component of future retinal stem cell transplantation therapies.
Materials and Methods
Animals
Adult (8–12-week-old) male Sprague-Dawley rats were housed in light- and temperature-controlled conditions. All procedures were performed in accordance with U.K. Home Office regulations and the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research.
Reagents
Collagenase (Sigma-Aldrich, St. Louis, MO) was diluted in PBS to achieve the indicated dosages (Table 1). Human plasmin (Sigma-Aldrich) was suspended in PBS (25 U/mL). α-Aminoadipic acid (AAA; Sigma-Aldrich) was suspended in PBS to achieve the indicated dosages (Table 1), and the pH was adjusted to 7.4. All substances were added to the RGC surface of retinal explants in a volume of 2 μL or injected intravitreally in a volume of 2 μL (collagenase) or 5 μL (AAA). In vivo, AAA was used at a concentration of 100 μg/μL.
Table 1.
In Vitro Engraftment Summary
| Treatment | Total MSCs |
% MSCs within Retina |
||||
|---|---|---|---|---|---|---|
| Control | Treated | P* | Control | Treated | P† | |
| 0.2 U Collagenase injected | 10,188 ± 1,329 | 5,930 ± 1,217 | 0.06 | 0.36 ± 0.14 | 0.66 ± 0.21 | 0.34 |
| 0.05 U Collagenase injected | 9,480 ± 1,306 | 8,815 ± 806 | 0.68 | 0.69 ± 0.26 | 0.98 ± 0.19 | 0.34 |
| 0.02 U Collagenase injected | 8,255 ± 1,557 | 9,275 ± 2,383 | 0.73 | 1.54 ± 0.36 | 3.67 ± 1.43 | 0.34 |
| 0.2 U Collagenase in vitro | 9,853 ± 857 | 12,075 ± 1,179 | 0.18 | 0.20 ± 0.03 | 0.32 ± 0.04 | 0.06 |
| 0.05 U Plasmin in vitro | 17,975 ± 1,275 | 18,213 ± 3,003 | 0.94 | 0.33 ± 0.10 | 0.54 ± 0.34 | 0.99 |
| 200 μg AAA in vitro | 14,895 ± 1,590 | 24,873 ± 3,669 | 0.03 | 0.75 ± 0.37 | 39.32 ± 11.39 | <0.001 |
| 20 μg AAA in vitro | 14,248 ± 3,434 | 8,795 ± 4,192 | 0.35 | 0.64 ± 0.48 | 38.35 ± 8.1 | 0.029 |
Summary of retinal engraftment by MSCs cocultured on the vitreal surface of retinal explants. Injected, substance was injected intravitreally before explantation and coculture. In vitro, substance was applied to retinal tissue after explantation but before coculture. Data are expressed as the mean ± SEM.
Unpaired t-test.
Mann-Whitney U test.
Organotypic Retinal Explant Tissue Culture
Retinal tissue obtained from adult rats was cultured as previously described.15 We found that the dissection procedure could be varied slightly to preserve or remove the ILM. For ILM preservation, the anterior segment was removed after circumferential incision of the globe 1 mm posterior to the ora serrata, in an attempt to remove the vitreous base (which is the strongest point of adhesion between the vitreous and the ILM) from the posterior eye cup. In contrast, for ILM removal, the globe was incised along the peripheral cornea in an attempt to leave the vitreous base attached to the posterior eye cup. On removal of the vitreous from the posterior eye cup, the ILM tended to be concomitantly peeled off of the surface of the retina, where the vitreous base was preserved while remaining attached to the retina, where the vitreous base had been dissected along with the anterior segment. In all experiments involving enzymatic basal lamina digestion or suppression of glial reactivity, the vitreous was not removed from the retinal tissue. Retinal tissue was cultured RGC side up on culture inserts (Millipore; Millicell Inc., Cork, Ireland) in medium composed of neuronal cell culturing medium (Neurobasal-A), B27 supplement (2%), N2 supplement (1%), l-glutamine (0.8 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) (all components from Invitrogen, Inc., Carlsbad, CA) at 35°C and 5% CO2. Half of the medium was exchanged on day 1 and every second day thereafter.
Laser-Induced Ocular Hypertension
Ocular hypertension was induced by using a modification of the method developed by Levkovitch-Verbin et al.20 Briefly, anesthetized rats were placed in front of a slit lamp equipped with a 532-nm diode laser, which delivered 0.7-W pulses for 0.6 second. Fifty to 60 laser pulses were directed to the trabecular meshwork 360° around the circumference of the left cornea only. The animals were treated twice, 1 week apart. Contralateral fellow eyes served as the untreated control. Intraocular pressure (IOP) was measured bilaterally before and 24 hours after each laser treatment, and then weekly thereafter, with a rebound tonometer (TonoLab; Tiolat Oy, Helsinki, Finland).
Mesenchymal Stromal Cells
Mesenchymal stem cells (sometimes referred to as mesenchymal stromal cells, or MSCs) were isolated from the bone marrow of adult transgenic Sprague-Dawley rats, genetically engineered to ubiquitously express GFP, as previously described.21 Briefly, bone marrow was aspirated from the tibia and femur and seeded into plastic culture flasks at a density of 5 × 105 cell/cm2 in DMEM (1 g/L glucose; Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen), penicillin (100 U/mL), and streptomycin (100 μg/mL). After 48 hours, plastic-adherent cells were purified by complete medium exchange. The cells were grown until approximately 80% confluent and then passaged as necessary. Previously published assays in which MSCs from the same isolation technique were used have confirmed expression of CD90 and CD44, but not CD34 or CD45.21 Furthermore, we determined these cells to be CD11b negative, but confirmed that they expressed laminin, fibronectin, and collagen IV.
The multipotency of MSCs was verified at passages 5 and 13 by inducing osteogenesis and adipogenesis, as described previously.21 Briefly, osteogenesis was induced by supplementing the culture medium with dexamethasone (0.1 μM), glycerophosphate (10 mM), and ascorbic acid (50 μM), whereas adipogenesis was induced by supplementing the culture medium with 1-methyl-3-isobutylxanthine (0.5 mM), dexamethasone (1 μM), insulin (10 μg/mL), and indomethacin (100 μM). Oil red O or alizarin red S staining was used to confirm differentiation into adipocytes and osteocytes, respectively.
MSCs from passages 10 to 12 were cocultured with retinal explants by suspending them in PBS (750 cells/μL) and placing a 2-μL drop on the inner retinal surface. Cocultures were maintained for 7 days. For transplantation into living rats, MSCs were suspended in PBS (10,000 cells/μL). One week before the induction of ocular hypertension, the left eyes of anesthetized rats were treated with topical anesthetic (tetracaine 1%). Intravitreal injections, proximal to the retina, of 3 μL were administered with a 30-gauge needle on a 5-μL syringe (Hamilton, Reno, NV). Care was taken to ensure that the lens was not damaged.
Immunofluorescence
Retinal explant cultures were fixed by immersion in 4% PFA for 24 hours at 4°C. Rats were deeply anesthetized and then perfused with 4% PFA, after which their eyes were enucleated and the posterior eye cups were immersion fixed for 24 hours at 4°C. All tissue was cryoprotected in 30% sucrose for 24 hours at 4°C, embedded in OCT, frozen on dry ice, and cryosectioned at 14 μm for explants and 40 μm for in vivo tissue.
Tissue was processed for immunohistochemistry on microscope slides (Superfrost-plus; VWR International, Lutterworth, UK). Sections were blocked with 5% normal goat serum (NGS) and permeabilized with 0.2% Triton-100 in 0.1 M PBS for 90 minutes before incubation with the primary antibody (Supplementary Table S1, http://www.iovs.org/cgi/content/full/51/2/960/DC1) diluted in blocking solution overnight at 4°C. Primary antibody binding was detected with appropriate AlexaFluor-conjugated secondary antibodies (Supplementary Table S1) incubated in blocking solution at room temperature for 3 hours. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Inc.).
Tissue was visualized with a standard epifluorescence microscope (model DM6000B; Leica, Wetzlar, Germany) or a laser scanning confocal microscope (model TCS-SPE; Leica). In all figures, the retina is oriented with the retinal ganglion cell side toward the top of the page and the photoreceptor side toward the bottom of the page.
Statistical Analysis
The migratory capacity of MSCs after coculture or transplantation was quantified under direct epifluorescence visualization in sections stained with DAPI plus anti-GFP and anti-laminin antibodies, which permitted simultaneous visualization of grafted MSCs and the ILM while allowing clear discrimination between grafted cells within and outside of the retinal tissue. For retinal explants, the number of MSCs outside of the retina, within the retinal ganglion cell layer, the inner nuclear layer, the inner plexiform layer, and the outer nuclear layer, were quantified for every 10th section, to estimate the total number of MSCs in each retinal layer per explant. For posterior eye cups, MSCs that had clearly migrated into the retina were quantified in every 10th section and averaged to estimate the total number of MSCs in each section. At least four samples were counted per group. Statistical tests were as follows: linear regression analyses and unpaired t-tests (Excel; Microsoft Corp, Redmond, WA); nonlinear regression analyses (SigmaPlot; Systat Software, Inc, San Jose, CA); and nonparametric Mann-Whitney U tests, to compare the percentage of MSC migrating into retinal explants (SPSS; SPSS Inc, Chicago, IL). Data are expressed as the mean ± SEM.
Results
Effect of Mechanical Peeling of the ILM on Retinal Graft Migration
The inability of intraocular cell transplants to migrate into adult retinal tissue is well established. Considering that intravitreal cell graft migration appears to be arrested at the ILM,15 we assessed whether removal of the ILM itself, including any inhibitory factors within that microenvironment, would facilitate graft penetration into the neural retina. Using an established organotypic tissue culture system,15 we cultured retinal tissue from adult rats with the RGC side facing up (n = 16). During the dissection process, various proportions of the ILM were mechanically peeled away, along with the vitreous body from some retinal explants (see the Methods section). Attempts were made to peel large amounts of ILM from approximately half of the explants and to preserve most of the ILM in the other half to produce samples with differing ILM preservation. ILM preservation was quantified retrospectively by laminin and/or collagen IV immunohistochemistry (Fig. 1A). Microscopic analysis revealed that removal of the basal lamina (immunoreactive for laminin and collagen IV) could be achieved while consistently preserving GFAP+ and vimentin+ astrocyte/Müller glial endfoot immunoreactivity within the nerve fiber layer of the remaining retinal tissue (Figs. 1C, 1E). As such, the cleavage plane was most likely between the nerve fiber layer and the basal lamina of the ILM. Linear quantification indicated that the retinal explants possessed various levels of residual ILM ranging from 33% to 100% coverage of the retinal surface.
Figure 1.
Mechanical disruption of the ILM enhanced retinal stem cell engraftment. The ILM of retinal explant cultures, visualized by immunoreactivity for laminin, was mechanically peeled (A) or left intact before coculture with MSCs (green) on the vitreal surface. Arrows: intact ILM; arrowheads: areas of the retina where ILM was removed. In the presence of an intact ILM (B, D), MSC migration into the host tissue was minimal, and glial reactivity, visualized by immunoreactivity for GFAP, was generally high (D). Conversely, in the absence of an intact ILM (C, E), MSC migration into the host tissue was robust and, in some cases, glial reactivity was significantly reduced (E). Quantification of total MSCs in each coculture (F), the absolute number of MSCs that had migrated into the host retinal tissue (G), and the percentage of MSCs that had migrated into the host retinal tissue (H) after 7 days of coculture demonstrated a significant correlation with the percentage of intact ILM for each parameter. (I) Explants exhibiting very low levels of glial reactivity throughout the Müller cell bodies (F–H, ♦) were more permissive to cocultured MSC engraftment than explants that exhibited high GFAP reactivity (F–H, •). Blue: DAPI. Scale bars: (A) 500 μm; (B) 100 μm. ***P = 0.001; Mann-Whitney U test.
After 1 day of culture, 1500 MSCs were added to the surface of each retinal explant, and the coculture was maintained for 7 days. At that point, an average of 7363 ± 924 GFP-positive MSCs were present in each coculture, indicating that the grafted cells not only survived, but also proliferated. The total number of remaining MSCs after 7 days in coculture correlated positively (P < 0.05) with ILM preservation (Fig. 1F), suggesting that the presence of ILM promoted MSC survival and/or proliferation. In addition, we found that MSCs predominantly remained outside the ILM where it was preserved (Fig. 1B), whereas the removal of the ILM was associated with significant MSC migration into the retinal tissue (P < 0.05; Figs. 1C, 1G). Normalization of the number of engrafted MSCs to total MSCs per explant improved this correlation (Fig. 1H, P < 0.01). Furthermore, there appeared to be an exponential increase in the percentage of engrafted MSCs as ILM was removed (Fig. 1H).
We also observed that integration of transplanted cells was associated with a general reduction in glial reactivity, as evidenced by a downregulation of GFAP (Fig. 1D, 1E), vimentin, and nestin immunoreactivity in some explants. Most notably, GFAP immunoreactivity was abolished from Müller cell bodies, leaving GFAP exclusively in the nerve fiber layer throughout the entirety of the four explants that underwent extensive ILM peeling (42% ± 5% residual ILM coverage; Fig. 1E, Supplementary Figs. S1E–H, http://www.iovs.org/cgi/content/full/51/2/960/DC1). Reduced GFAP immunoreactivity was seen throughout the tissue, even in areas where ILM was preserved (Supplementary Figs. S1I–L). In contrast, GFAP immunoreactivity was high in Müller cells within the inner plexiform layer, inner nuclear layer, and outer plexiform layer in the other explants that retained more ILM (86% ± 3% residual ILM coverage; Fig. 1D, Supplementary Figs. S1A–D). The reason for reduced GFAP expression in explants that underwent the greatest amount of ILM peeling is unclear. We speculate that this effect may have been caused by the breakage and removal of Müller glial endfeet during ILM peeling, leading to explant trauma in an in vitro system within which glial reactivity and/or health was unable to recover. Note, however, that of the 16 retinal explants assessed, the four that exhibited this highly disrupted pattern of glia-related protein expression were also the most permissive of MSC engraftment (Fig. 1I, P = 0.001). Thus, whereas ILM removal appeared to be associated with improved MSC engraftment in vitro, it is unclear whether this result was due to removal of the inner basal lamina or the effect on cellular components of the ILM which manifested as reduced glial reactivity, or both. Therefore, we then went on to assess the importance of these two factors on stem cell engraftment independently.
Effect of Disruption of the Inner Basal Lamina on Retinal Graft Migration
To determine whether disruption of the basal lamina in the absence of Müller cell injury could facilitate migration of grafted cells into the retina, we used the proteolytic enzyme collagenase, which has been shown to effectively degrade the ILM in the chick retina.22–24 To determine an effective dose, we treated explants with various concentrations of collagenase and then assessed ILM integrity. The effects of 0.2, 0.1, 0.05, 0.02, and 0.01 U collagenase in 2 μL of PBS placed on the inner surface of retinal explants were indistinguishable. At all doses, collagenase caused small disruptions to appear in the basal lamina as early as 2 days (not shown). This result was in contrast to untreated explants in which the ILM was uniformly continuous (Figs. 2A, 2E). By 5 days, laminin and collagen labeling demonstrated irregularities in membrane thickness with multiple small notches, holes, and complete interruptions in the basal lamina that varied in diameter from a few microns to approximately 50 μm (Figs. 2B–D, 2F–H).
Figure 2.
In vitro digestion of the ILM with collagenase did not enhance retinal stem cell engraftment. Retinal explants were treated in vitro with the indicated collagenase doses. Immunohistochemical analysis 5 days later revealed that collagenase treatment caused significant disruption of the ILM (arrowheads: discontinuities; red: laminin, A–D; collagen IV, E–H). Coculture of MSCs (green) on the RGC surface of explants demonstrated no difference in proliferation (M) or retinal engraftment (N) induced by collagenase treatment (J, L) compared to PBS-treated explants (I, K). However, collagenase pretreatment followed by MSC coculture resulted in the protrusion of Müller cell processes (stained for nestin in blue) through the ILM (stained for laminin [I, J] or collagen IV [K, L] in red), forming a continuous barrier against the MSC graft. (A–H) Blue, DAPI; (I–L) white, DAPI. Scale bar, 100 μm.
The effect of basal lamina disruption on MSC migration was investigated in vitro by coculturing MSCs on retinal explants 4 days after collagenase treatment. Compared with the controls, treatment with 0.2 U collagenase did not affect MSC proliferation or viability and did not enhance MSC migration into the retina (Table 1, Figs. 2M, 2N). Laminin and collagen IV labeling revealed greater disruption of basal laminar integrity in collagenase-treated explants (Figs. 2J, 2L), compared with control the (Figs. 2I, 2K). In addition, nestin/laminin double-labeling (blue/red, respectively) confirmed that collagenase treatment compromised the integrity of the ILM to the extent that Müller cell and/or astrocyte processes were able to breach the structure and make contact with the grafted MSCs directly, such that Müller glia endfeet formed a continuous barrier against the MSC bolus, and the ILM was no longer in contact with the graft (Figs. 2J, 2L). This clearly demonstrates that the basement membrane is unnecessary for blockade of graft migration into the retina and, instead, appears to suggest that Müller glia constitute the primary barrier.
We also tested plasmin as an alternative proteolytic enzyme in vitro, as it has been shown to cleave laminin and fibronectin at the ILM in human cadaveric eyes.25 This enzyme produced less discernible changes in the integrity of the ILM and also had no effect on the proliferation or migration of cocultured MSCs (Table 1).
Finally, we investigated whether collagenase could modulate the integrity of the basal lamina in vivo. After intravitreal injection, collagenase digested components of the ocular blood vessels causing hemorrhage, an effect that does not occur in the avascular chick retina but which is known to occur after intracerebral injection and has been used to model stroke in rodents.26,27 A dose of 0.2 U collagenase produced widespread vitreal and subretinal hemorrhaging within 24 hours (Figs. 3A, 3B), whereas doses of 0.05 and 0.02 U caused occasional, localized subretinal bleeding (not shown). All doses produced similar disruption of ILM integrity with occasional discontinuities observed in the basement membrane, as confirmed by both collagen IV and laminin immunohistochemistry (Figs. 3C–E). To test whether this ILM disruption was sufficient to permit integration of stem cells, explants were made from retinas 7 days after intravitreal collagenase injection and cocultured with MSCs. Despite some observed disruption to the ILM after in vivo collagenase, no change in either MSC proliferation/survival or retinal integration was observed (Table 1; Figs. 3F, 3G).
Figure 3.
In vivo digestion of the ILM with collagenase did not enhance retinal stem cell engraftment. Profuse intraocular hemorrhaging was observed in eyes 24 hours after intravitreal injection of 0.2 U collagenase (B). The posterior eye cup of a PBS-treated eye is shown for comparison (A). Immunohistochemistry for laminin (red, C–E) demonstrated the disruption of ILM structure in collagenase-treated eyes (arrowheads: discontinuities). Coculture of MSCs on the RGC surface of explants demonstrated no difference in MSC proliferation (F) or retinal engraftment (G) induced by prior in vivo collagenase treatment. (F, G) Representative of similar results obtained with three different doses of collagenase. Blue: DAPI. Scale bar, 100 μm.
Effect of Attenuation of Glial Reactivity on Retinal Graft Migration In Vitro
α-Aminoadipic acid (AAA) is a glutamate analogue that is selectively gliotoxic. Within the retina, AAA has been used for the specific destruction or transient impairment of Müller cell function without direct effects on neuronal populations. To determine whether targeted disruption of glial activity would enhance the integration of cocultured cells, we treated explants with 2 μL of 100 μg/μL AAA (6 hours after isolation), and 24 hours later MSCs were cocultured on the inner retinal surface. After 7 days of coculture, AAA-treated explants demonstrated a complete loss of nestin immunoreactivity, strong downregulation of GFAP with expression limited to the ILM, and structural disruption of vimentin expression (Fig. 4). These changes have been associated with reduced glial reactivity in the retina.28 No obvious changes in β-III-tubulin or NeuN immunoreactivities were observed (data not shown), indicating that AAA did not adversely affect the RGCs. AAA did not disrupt laminin or collagen immunoreactivity in the ILM (Figs. 5A, 5B)
Figure 4.
Treatment of retinal explants with AAA suppressed glial reactivity elicited by stem cell coculture. MSCs (green) were placed on the RGC surface 24 hours after treatment of the explant with either PBS (A, C, E) or AAA (B, D, F), maintained in coculture for 7 days and subsequently processed for immunohistochemistry. Compared with controls, AAA treatment resulted in the downregulation of GFAP (A, B, red), abolition of nestin expression (C, D, red), and disruption of the pattern of vimentin expression (E, F, red). Blue, DAPI. Scale bar, 100 μm.
Figure 5.
Suppression of glial reactivity enhanced retinal engraftment of cocultured stem cells in vitro. MSCs (green) were cocultured on the vitreal surface of retinal explants for 7 days, beginning 24 hours after treatment with PBS (A) or AAA (B). Despite the persistence of an intact basal lamina (visualized by immunolabeling of laminin, red), AAA treatment dramatically enhanced the migration of MSCs into the retinal tissue. (C) Quantification of MSC migration, with or without AAA treatment (200 μg). Dark-shaded bars: PBS-treated explants; light-shaded bars: AAA-treated explants. Blue: DAPI. ***P < 0.01 by Mann-Whitney U test with Bonferroni adjustment for multiple comparisons. Scale bar, 100 μm.
AAA-mediated suppression of glial reactivity was associated with a marked increase in the percentage of MSCs that migrated into the retinal explant tissue (39.3% ± 11.4% in AAA-treated explants versus 0.8% ± 0.4% in control explants, P < 0.001, Table 1, Fig. 5). Of interest, there were approximately twice as many MSCs in both the retinal ganglion cell layer and the inner nuclear layer compared with the inner plexiform layer (Fig. 5C). In contrast, almost no MSCs could be found in the outer nuclear layer. In fact, in many instances an abrupt discontinuation of MSC migration was observed at the inner boundary of the ONL (Fig. 4). In addition, we noted a preference for MSCs to establish residence directly adjacent to blood vessels within the inner retina, which are strongly immunoreactive for laminin (Fig. 5B). Treatment with a lower dose of AAA (2 μL of 10 μg/μL) produced similar results (Table 1).
Effect of Attenuation of Glial Reactivity on Retinal Graft Migration In Vivo
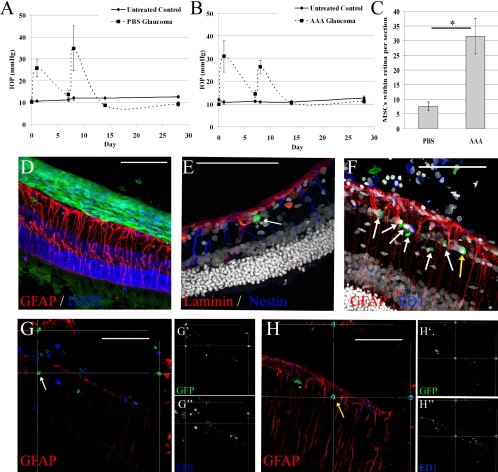
Having demonstrated that glial reactivity is associated with poor retinal integration of cocultured cells in vitro, we investigated the role of glial reactivity in blocking migration of engrafted cells into the retina in vivo. As diseased retinas are more receptive to cell integration than healthy retinas,12 we transplanted MSCs intravitreally and then induced ocular hypertension, an experimental model of glaucoma. The ocular hypertension profiles are shown in Figures 6A and 6B. Nine days before the induction of ocular hypertension, 5 μL of AAA (100 μg/μL) or PBS was injected intravitreally. Two days after AAA or PBS injection, MSCs were transplanted intravitreally, and integration was assessed 5 weeks later. Unlike in retinal explants, we were able to assess glial reactivity 5 weeks after AAA treatment, at which point, the expression of GFAP and nestin was strong, indicating that the Müller cells had, at least partially, recovered from the AAA treatment (Figs. 6D–F).
Figure 6.
Intravitreal AAA enhanced in vivo retinal engraftment of intravitreally transplanted stem cells in glaucoma. Ocular hypertension was experimentally induced unilaterally after intravitreal transplantation of MSCs and treatment with AAA or PBS. Bilateral IOP profiles are shown for animals treated with PBS (A) and AAA (B), with laser treatments occurring on days 0 and 7. Five weeks after transplantation, the number of MSCs that had migrated into the retinal tissue was quantified for eyes treated with PBS or AAA (C, *P < 0.05). In all eyes, most of the transplanted cells remained in the vitreous cavity and formed a multilayered sheet on the surface of the ILM (D). However, individual GFP+ MSCs also migrated into the retinal tissue and were observed beneath the ILM (E, red for laminin). (F) A retinal section with numerous GFP+ cells within the retinal tissue. Most GFP+ cells were transplanted MSCs (white arrows), whereas rare ED1+/GFP+ cells represented macrophages that had phagocytosed GFP (yellow arrows). Orthogonal projections, of the same confocal z-stack shown in (F), clearly demonstrate the noncolocalization of GFP and ED1 in MSCs (G) and the co-localization of these markers in some macrophages (H). (E, F) White: DAPI. Scale bars, 100 μm.
As described previously, we observed that many of the transplanted MSCs lined the ILM and did not migrate into the retinal tissue (Fig. 6D). However, AAA treatment produced a more than 300% increase in the number of MSCs that had migrated into the retina (31.4 ± 6.0 cells/section for AAA-treated eyes vs. 7.6 ± 1.5 cells/section for vehicle-treated eyes, Figs. 6C, 6E–G; P < 0.05). This result equated to retinal engraftment of approximately 10% of the intravitreally transplanted cells in AAA-treated glaucomatous eyes. Colabeling of GFP and ED1, a marker of macrophages/monocytes, confirmed that the GFP+ cells were not macrophages falsely identified as MSCs, because of GFP phagocytosis (Figs. 6G, 6H).
Discussion
In the present study, we used in vitro and in vivo methods to investigate the barriers to the migration of intravitreal cell grafts. We used MSCs in these experiments, as these cells are currently attracting much attention as a potential therapy for CNS diseases, because of their neuroprotective properties,29–31 their ability to home to degenerating tissue,32 and their possible, though controversial, neural transdifferentiation potential.33 Since migration from the vitreous cavity into the neural retina is an early event that is likely to be important in stem cell therapy that provides both neural regeneration and neuroprotection, we focused the present study on cellular migration rather than on more specific events such as neural differentiation. We found that the ECM of the inner basal lamina is neither necessary nor sufficient to prevent retinal engraftment of stem cells but that reactive glial processes appear to play a dominant role in this process. Furthermore, we found that exogenous manipulation of the inhibitory environment can overcome inhibition of transplant migration and propose that suppression of glial reactivity will be a necessary component of intraocular stem cell transplantation therapies in the future. This is a major step forward in the development of cell therapies for retinal disease, as suboptimal graft integration has been a major stumbling block to date. As glial reactivity is a ubiquitous phenomenon throughout the CNS, our results are likely also to apply to potential cell-based therapies for a range of other CNS conditions.
Intraocular transplantation of stem cells for retinal therapy can be achieved via two approaches, either subretinally or intravitreally, with each technique possessing advantages and disadvantages for particular applications. Subretinal injections leave cells physically constrained adjacent to the outer retina and near to rich blood supply, whereas intravitreal injections are technically simpler and provide direct access to the inner retina. Most research into improving the outcome of intraocular grafts has focused on subretinal injections, in part because of an initial focus on diseases of the photoreceptors. However, we have an interest in applying stem cell therapies to glaucoma, a common neurodegenerative disease of the inner retina that is the leading cause of irreversible blindness worldwide.3 In the context of inner retinal disease, intravitreal injections are likely to be more applicable than subretinal injections. Although studies involving subretinal transplantation have identified both ECM molecules and cellular factors as inhibitory to graft migration, it is unclear whether these elements play the same role, if any, when the graft is placed intravitreally. Besides providing useful information for developing treatments for inner retinal disease, determining the commonality of barriers to cell transplantation in different regions of the retina may provide insights that will aid in developing cell-based therapies in other CNS compartments.
Components of the ECM have been identified as potential barriers to the integration of transplanted stem cells in the CNS. For example, enzymatic degradation of chondroitin sulfate proteoglycans has been shown to enhance stem cell engraftment in the spinal cord34 and brain35 and also to augment the integration of neural stem cells after intraocular transplantation, although the effects have been modest.16,17 Matrix metalloproteinase-2 has a similar effect in vitro.18 In the present study, we focused on degradation of proteins concentrated at the retinal ILM, as this appears to be the site of blockade for intravitreally transplanted cells. Although our enzymatic treatments effectively digested the inner basal lamina ECM proteins laminin and collagen, they did not enhance the migration of cells into the retina. This contrasts with data from subretinal approaches where destruction of physical impairments to cell integration has proven beneficial at the outer limiting membrane.19 This effect may be due to fundamental differences in the microenvironment of the inner and outer retina. It is possible that glial obstacles are more prominent in the inner retina, rather than inhibitory ECM factors and physical barriers as in the outer retina, such that enzymatic ECM digestion has a negligible effect on intravitreal graft migration. Morphologic localization of glial intermediate filaments supports this view, given that immunoreactivity of these proteins is much higher in the inner retina, compared with the outer retina, under both normal and pathologic circumstances.15 Indeed, this concurs with the data presented by West et al.,19 who studied the effects of AAA on subretinal transplantation. They noted that AAA treatment led to an approximately threefold increase in the number of photoreceptor progenitors that integrated into the outer nuclear layer 3 weeks after injection. Of importance, the authors of this study attributed the effect to a structural disruption of the outer limiting membrane, which is composed primarily of heterotypic and homotypic adherence junctions between Müller glia and photoreceptors. Furthermore, that report indicated that GFAP immunoreactivity in healthy eyes that had not received transplants was localized exclusively to the inner retina and was not affected by AAA treatment; however, GFAP immunoreactivity in transplanted eyes was not investigated. In contrast, the present study demonstrated a dramatic increase in reactive gliosis after retinal explant culture, the onset of ocular hypertension, and intravitreal transplantation. The effects of AAA on highly reactive retinal glial cells appear to be different from normal control retinal tissue,19,36 as we demonstrated a dramatic downregulation in reactive intermediate filaments after treatment in the current models. That we also demonstrated improvement in retinal engraftment of intravitreally transplanted cells after AAA treatment indicates that glial reactivity appears to predominate over ECM-mediated effects on cell graft migration in the context of inner retinal disease. However, it is possible that combinatorial treatments would produce an even more robust effect on intravitreal transplant migration than suppressing glial reactivity alone.
In contrast to the lack of intraretinal migration of MSCs observed after enzymatic disruption of the retinal ILM, suppression of glial reactivity using a selective toxin greatly potentiated retinal integration of intravitreally transplanted cells. Similar results have been found previously using transgenic techniques to knockout glial expression of the proteins GFAP and vimentin.13 The authors reported that both subretinal and intravitreal transplantation of neural stem cells into adult mice resulted in minimal retinal engraftment, as has been well documented previously. However, they observed a more than sixfold increase in stem cell migration into the retina from subretinal transplantation after knockout of GFAP and vimentin while simultaneously preserving retinal structure and function. Suppression of Müller cell expression of GFAP and vimentin has been associated with a reduction in their reactivity.28 The data presented in our study indicate that a relatively comparable increase in the number of engrafted stem cells can be attained by a transient, rather than permanent, disruption of glial cell function. A return of GFAP expression by Müller cells after in vivo AAA treatment confirmed the transient nature of our intervention. Such acute environmental manipulation is preferable, given that permanent loss of filament protein expression by Müller cells increases retinal vulnerability to mechanical damage.37
In contrast to these results, Nishida et al.14 demonstrated a high degree of retinal integration by intravitreally transplanted neural stem cells after mechanical retinal injury. In that paper, transplanted cells integrated near the injury site and also in regions of structurally intact retina, which also exhibited reactive gliosis, up to 1200 μm away from the injury site. They suggested that glial reactivity facilitated graft integration, possibly by local production of growth factors and/or chemokines. However, other reports have suggested that reactive gliosis, or components thereof, constitutes a barrier to the retinal integration of numerous transplanted cell types in a variety of circumstances.10,13,15,17,18,38–40 An interesting hypothesis to explain this apparent discrepancy was proposed by Zhang et al.,41 when they observed that neurites from abutting retinas in culture would cross-integrate only when certain glial-associated structures were disrupted, but when this did occur, it tended to take place near areas of high GFAP expression. They admitted that neurite integration may have triggered GFAP upregulation, but suggested that, alternatively, reactive glial cells facilitated neurite integration as long as separate glial barriers at the interface of the abutting retinas were disrupted. Thus, they proposed that glial cells possess both inhibitory and facilitative components. If this is the case, then it is possible that the AAA treatment used in the present study suppressed beneficial glial mechanisms that could further enhance integration of grafted cells, such as chemokine secretion. As such, future research should identify methods of blocking the inhibitory effects of reactive gliosis while preserving any potential facilitative effects. Nonetheless, the current results demonstrate that reactive gliosis is a significant component of the inhibitory barrier to retinal integration of intravitreally transplanted stem cells. Moreover, suppression of glial reactivity had a net overall benefit for grafted cell integration.
It should also be noted that the present study was conducted with MSCs used as the transplanted cell type. Although reactive glial processes have been implicated in blocking the integration of numerous classes of transplanted stem cells in the retina,10,13,15,17,18,38–40 it is unclear whether AAA would have the same effect for other stem cell types of interest. If different classes of cells respond to different migratory cues within the host retina or have different intrinsic migratory potentials, then it is possible that protocols aimed at enhancing engraftment would be met with various levels of success, depending on the cell type transplanted.
Although we have clearly shown that disruption of glial reactivity enhances transplanted MSC engraftment in the retina, we observed remarkable differences in the effects of AAA treatment in vitro and in vivo. Most notable is that AAA was at least 10 times more effective in the culture system than in vivo, perhaps because of differences in the duration of glial reactivity suppression, as reactivity remained low in the explant cultures for the duration of the experiment yet reverted to a high level in vivo, presumably after AAA clearance. It is likely that, in contrast to the eyes of living animals, the artificial culture conditions did not permit glial cell recovery after AAA treatment, thereby providing a longer window of opportunity for the migration of cells into the retina in vitro. Thus, it is conceivable that suppression of glial reactivity for a longer period in vivo produces a stronger effect. Furthermore, transplants in vivo are exposed to an active immune system that is mostly absent in vitro. Indeed, even though the present study used pseudoautologous grafts (cells from different animals of the same strain), we observed a strong degree of macrophage/monocyte infiltration and identified some inflammatory cells that co-labeled for GFP, implying phagocytosis of engrafted cells by host cells. Immune activity has been identified as a process that limits cell transplant efficacy in the CNS,42 and immunosuppression with indomethacin in conjunction with ECM modulation has been shown to improve subretinal graft survival and integration.17 Thus, protocols to safely and efficiently suppress immune responses against intraocular grafts should be investigated.
Although the results presented herein demonstrated for the first time that inner retinal stem cell engraftment may be facilitated by acute downregulation of glial reactivity, the compound used is unlikely to be of clinical interest. In mice, 100 μg/μL AAA injected intravitreally has been shown to produce a transient disruption of Müller cells, with recovery observed 2 weeks after treatment and a peak effect at 72 hours.19 An important finding was that AAA was toxic to Müller glia exclusively, whereas the rest of the retina appeared normal. However, AAA is a gliotoxin and besides suppressing glial reactivity, it also disrupts normal physiological function of the Müller cells, which is likely to severely affect vision. Moreover, it is likely to suppress the production of a variety of chemokines (such as stromal cell-derived factor-1) that are produced by glial cells, which have been shown to be important in guiding the migration of transplanted cells in the brain after ischemic insult, and may play a role in cell therapy for other neurodegenerative conditions.43–45 Instead of such a general approach, more targeted efforts are needed. This may be approached from at least two directions. First, it may be better to block reactive glial changes without disrupting other physiological functions. The JAK/STAT3 signaling pathway has been implicated in upstream signaling of glial activation,46,47 and modulation of this pathway may overcome glial inhibition to retinal engraftment without affecting visual function. Second, specific downstream processes that occur in reactive glial cells must be identified. Glial reactivity is a blanket term that is associated with a wide variety of changes that occur in stressed glial cells, including hypertrophy; upregulation of intermediate filament expression; alterations in the production of neurotrophins, cytokines, chemokines, and reactive oxygen species; and changes in buffering properties for extracellular ions and molecules.48–50 It is unlikely that all these changes contribute equally to the poor integration of intraocular grafts. Instead, it may be that the effect noted in our study was mediated primarily by a physical blockade of cell migration by hypertrophic Müller cell processes and, therefore, targeted reduction of hypertrophy may be helpful. In addition, reactive glial cells may produce inhibitory molecules that block the integration of grafted cells and suppressing either the production or activity of these molecules may allow for a high level of stem cell engraftment in the CNS.
In summary, we have demonstrated for the first time that the predominate block to retinal integration of intravitreally transplanted stem cells is glial cell reactivity, as opposed to physical barriers contained within the ILM. An important finding was that even a transient reduction in glial reactivity could significantly enhance engraftment of stem cells into the retina in vivo. These findings have direct implications for the development of stem cell therapies for common irreversible neurodegenerative retinal diseases such as glaucoma. However, given that glial reactivity also inhibits the integration of stem cells in the brain,51–53 these findings are also applicable to the CNS as a whole. Finally, although identification of the major barrier to stem cell integration in the mature inner retina is a major step forward, it is unlikely to be sufficient for clinical therapy. Therefore, further research is necessary to characterize other inhibitory factors of interest, as it is likely that a combinatorial approach will be necessary for optimization of transplanted cell engraftment.
Supplementary Material
Acknowledgments
The authors thank David Hunt, Peter Connick, and Siddharthan Chandran for advice and assistance with cell isolation and culture.
Footnotes
Presented in part at the 2008 Cambridge Ophthalmological Symposium and included in the Cambridge Ophthalmological Symposium Proceedings issue of the journal Eye. [Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye. 2009;23(10):1980–1984.]
Supported by the Glaucoma Research Foundation, the Richard Norden Glaucoma Research Fund, Fight for Sight, and an NC3Rs prize for reducing the use of animals in vision research. TVJ holds a Gates-Cambridge Scholarship and a National Institutes of Health Graduate Partnerships Program Fellowship. KRM holds a GlaxoSmithKline Clinician-Scientist Award.
Disclosure: T.V. Johnson, None; N.D. Bull, None; K.R. Martin, None
References
- 1.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006; 444: 203–207 [DOI] [PubMed] [Google Scholar]
- 2.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in crx-deficient mice. Cell Stem Cell 2009; 4: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol 2007; 245: 414–422 [DOI] [PubMed] [Google Scholar]
- 5.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells 2006; 24: 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Na L, Xiao-Rong L, Jia-Qin Y. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol 2009; 247: 503–514 [DOI] [PubMed] [Google Scholar]
- 7.Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye 2009; 23(10): 1980–1984 [DOI] [PubMed] [Google Scholar]
- 8.Bull ND, Johnson TV, Martin KR. Stem cells for neuroprotection in glaucoma. Prog Brain Res 2008; 173: 511–519 [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 2006; 344: 1071–1079 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci 2007; 27: 4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charalambous P, Hurst LA, Thanos S. Engrafted chicken neural tube-derived stem cells support the innate propensity for axonal regeneration within the rat optic nerve. Invest Ophthalmol Vis Sci 2008; 49: 3513–3524 [DOI] [PubMed] [Google Scholar]
- 12.Chacko DM, Das AV, Zhao X, James J, Bhattacharya S, Ahmad I. Transplantation of ocular stem cells: the role of injury in incorporation and differentiation of grafted cells in the retina. Vision Res 2003; 43: 937–946 [DOI] [PubMed] [Google Scholar]
- 13.Kinouchi R, Takeda M, Yang L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci 2003; 6: 863–868 [DOI] [PubMed] [Google Scholar]
- 14.Nishida A, Takahashi M, Tanihara H, et al. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Invest Ophthalmol Vis Sci 2000; 41: 4268–4274 [PubMed] [Google Scholar]
- 15.Johnson TV, Martin KR. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Invest Ophthalmol Vis Sci 2008; 49: 3503–3512 [DOI] [PubMed] [Google Scholar]
- 16.Bull ND, Limb GA, Martin KR. Human Müller stem cell (MIO-M1) transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci 2008; 49: 3449–3456 [DOI] [PubMed] [Google Scholar]
- 17.Singhal S, Lawrence JM, Bhatia B, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem Cells 2008; 26: 1074–1082 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Mandai M, Akimoto M, Yoshimura N, Takahashi M. The simultaneous treatment of MMP-2 stimulants in retinal transplantation enhances grafted cell migration into the host retina. Stem Cells 2006; 24: 2406–2411 [DOI] [PubMed] [Google Scholar]
- 19.West EL, Pearson RA, Tschernutter M, Sowden JC, Maclaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res 2008; 86: 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci 2002; 43: 402–410 [PubMed] [Google Scholar]
- 21.Hunt DP, Irvine KA, Webber DJ, Compston DA, Blakemore WF, Chandran S. Effects of direct transplantation of multipotent mesenchymal stromal/stem cells into the demyelinated spinal cord. Cell Transplant 2008; 17: 865–873 [DOI] [PubMed] [Google Scholar]
- 22.Halfter W, Winzen U, Bishop PN, Eller A. Regulation of eye size by the retinal basement membrane and vitreous body. Invest Ophthalmol Vis Sci 2006; 47: 3586–3594 [DOI] [PubMed] [Google Scholar]
- 23.Halfter W, Dong S, Schurer B, Ring C, Cole GJ, Eller A. Embryonic synthesis of the inner limiting membrane and vitreous body. Invest Ophthalmol Vis Sci 2005; 46: 2202–2209 [DOI] [PubMed] [Google Scholar]
- 24.Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest Ophthalmol Vis Sci 2005; 46: 1000–1009 [DOI] [PubMed] [Google Scholar]
- 25.Li X, Shi X, Fan J. Posterior vitreous detachment with plasmin in the isolated human eye. Graefes Arch Clin Exp Ophthalmol 2002; 240: 56–62 [DOI] [PubMed] [Google Scholar]
- 26.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke 1997; 28: 2296–2302 [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke 1990; 21: 801–807 [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa T, Takeda M, Lewis GP, et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Invest Ophthalmol Vis Sci 2007; 48: 2760–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavin S, Kurkalli BG, Karussis D. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg 2008; 110: 943–946 [DOI] [PubMed] [Google Scholar]
- 30.Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 2008; 265: 131–135 [DOI] [PubMed] [Google Scholar]
- 31.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007; 40: 609–619 [DOI] [PubMed] [Google Scholar]
- 32.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009; 4: 206–216 [DOI] [PubMed] [Google Scholar]
- 33.Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells: a critical review. APMIS 2005; 113: 831–844 [DOI] [PubMed] [Google Scholar]
- 34.Ikegami T, Nakamura M, Yamane J, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci 2005; 22: 3036–3046 [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Nakanishi K, Hayakawa M, et al. Reduction of brain injury in neonatal hypoxic-ischemic rats by intracerebroventricular injection of neural stem/progenitor cells together with chondroitinase ABC. Reprod Sci 2008; 15: 613–620 [DOI] [PubMed] [Google Scholar]
- 36.Rich KA, Figueroa SL, Zhan Y, Blanks JC. Effects of Muller cell disruption on mouse photoreceptor cell development. Exp Eye Res 1995; 61: 235–248 [DOI] [PubMed] [Google Scholar]
- 37.Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M. Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. J Cell Sci 2004; 117: 3481–3488 [DOI] [PubMed] [Google Scholar]
- 38.Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ. Long-term photoreceptor transplants in dystrophic and normal mouse retina. Invest Ophthalmol Vis Sci 1994; 35: 3145–3153 [PubMed] [Google Scholar]
- 39.Zhang Y, Arner K, Ehinger B, Perez MT. Limitation of anatomical integration between subretinal transplants and the host retina. Invest Ophthalmol Vis Sci 2003; 44: 324–331 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Akimoto M, Imai H, et al. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant 2007; 16: 493–503 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Kardaszewska AK, van Veen T, Rauch U, Perez MT. Integration between abutting retinas: role of glial structures and associated molecules at the interface. Invest Ophthalmol Vis Sci 2004; 45: 4440–4449 [DOI] [PubMed] [Google Scholar]
- 42.Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx 2004; 1: 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 2008; 1195: 104–112 [DOI] [PubMed] [Google Scholar]
- 44.Shyu WC, Lin SZ, Yen PS, et al. Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther 2008; 324: 834–849 [DOI] [PubMed] [Google Scholar]
- 45.Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res 2007; 1183: 138–147 [DOI] [PubMed] [Google Scholar]
- 46.Herrmann JE, Imura T, Song B, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 2008; 28: 7231–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Na YJ, Jin JK, Kim JI, Choi EK, Carp RI, Kim YS. JAK-STAT signaling pathway mediates astrogliosis in brains of scrapie-infected mice. J Neurochem 2007; 103: 637–649 [DOI] [PubMed] [Google Scholar]
- 48.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005; 50: 427–434 [DOI] [PubMed] [Google Scholar]
- 49.Correa-Cerro LS, Mandell JW. Molecular mechanisms of astrogliosis: new approaches with mouse genetics. J Neuropath Exp Neur 2007; 66: 169–176 [DOI] [PubMed] [Google Scholar]
- 50.McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res 2001; 63: 109–115 [DOI] [PubMed] [Google Scholar]
- 51.Larsson A, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(−/−)Vim(−/−) mice. Neurochem Res 2004; 29: 2069–2073 [DOI] [PubMed] [Google Scholar]
- 52.Menet V, Gimenez y Ribotta M, Chauvet N, et al. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J Neurosci 2001; 21: 6147–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menet V, Gimenez YRM, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia 2000; 31: 267–272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.