This study compared the tear epidermal growth factor (EGF) concentration in an asymptomatic control group and patients with dry eye (DE) and determined the correlation between tear EGF levels and tear clearance and severity of ocular disease symptoms and signs of ocular surface disease. A DE subgroup with MGD, delayed tear clearance and normal tear production was found to have significantly elevated tear EGF concentrations and increased prevalence of corneal subepithelial fibrosis and meibomian gland metaplasia. It is possible that elevated tear EGF in this group promotes the corneal subepithelial fibrosis and lid margin changes that are frequently seen in MGD.
Abstract
Purpose.
To compare tear epidermal growth factor (EGF) concentration in dry eye (DE) conditions and determine correlations between EGF levels and severity of symptoms and ocular surface signs.
Methods.
In this prospective case–control study, 35 patients with DE, including subgroups with meibomian gland disease (MGD), Sjögren's syndrome (SS) aqueous tear deficiency, or neurotrophic keratopathy (NK), and 17 asymptomatic control subjects were evaluated. Symptoms, Schirmer test, fluorescein clearance test (FCT), EGF concentration, dye staining, and the presence of corneal subepithelial fibrosis and meibomian gland (MG) orifice metaplasia were recorded. Tear EGF and the severity of irritation and ocular surface signs were correlated.
Results.
Tear EGF was higher in MGD than in the control (P = 0.03) and was lower in SS than in the control (P < 0.0001; MGD (P < 0.05) and NK (P < 0.01) groups. The DE subgroup with results in the FCT > 3 and Schirmer 1 ≥ 8 had higher EGF levels than the group with FCT > 3 and Schirmer 1 < 8 and both groups with good tear clearance (P < 0.01). Tear EGF levels correlated inversely with conjunctival (r = −0.49, P = 0.0032) and corneal (r = −0.39, P = 0.022) dye staining and positively with MG orifice metaplasia (r = 0.36, P = 0.03) and corneal subepithelial fibrosis (r = 0.5, P = 0.0006).
Conclusions.
Tear EGF concentration was increased in eyes with MGD, corneal subepithelial fibrosis, and MG orifice metaplasia. Elevated tear EGF may promote development of corneal subepithelial fibrosis and lid margin changes.
Dry eye syndrome is one of the most common ocular diseases, affecting 14% to 33% of the population worldwide and accounting for a high percentage of patient visits to eye care practitioners.1–3 Evidence suggests that alterations in tear composition are responsible in part for the irritation symptoms and ocular surface disease that develops in DE.4,5 Indeed, a study report showed an association between increased concentrations of inflammatory cytokines in tears and the severity of irritation symptoms and ocular surface disease in DE, regardless of the etiology.6
Epidermal growth factor (EGF), secreted by the lacrimal gland, is one of the most abundant growth factors in human tears. EGF receptors are expressed by the corneal epithelium, keratocytes, and endothelium.7 EGF is a pleiotropic cytokine that can stimulate proliferation, migration, and adhesion of corneal epithelial cells during wound healing.8,9 In conjunction with TGF-β, it stimulates myofibroblastic differentiation and production of extracellular matrix by corneal keratocytes.10 In a survey of tear proteins in patients with DE conditions, the highest concentration of EGF was noted to be in the subset of patients with meibomian gland disease (MGD).6 We have reported that the presence of MG orifice metaplasia is associated with decreased tear clearance.11 Corneal complications, such as epithelial basement membrane disease, Salzmann's nodules, and subepithelial fibrosis are commonly observed in patients with MGD.12 These fibrotic changes are frequently located in the peripheral cornea that is in contact with the upper and lower tear menisci. Based on these findings, we hypothesized that the presence of corneal subepithelial fibrosis and metaplasia of the MG orifice epithelium, two common findings in patients with chronic DE and MG disease, is associated with increased tear EGF concentration.
The purpose of this study was to measure tear EGF concentrations in an asymptomatic control group and in with DE. Furthermore, the correlations between tear EGF concentrations, tear production and clearance, and severity of clinical parameters of DE, including corneal subepithelial fibrosis and MG orifice metaplasia, were determined.
Materials and Methods
Patients
This study was approved by the Baylor College of Medicine, Institutional Review Board (IRB). After informed consent, 35 consecutive patients with newly diagnosed DE meeting the inclusion and exclusion criteria were enrolled for the study at the Ocular Surface Center at Baylor College of Medicine, Houston, Texas. The enrollment procedure and study protocol were in compliance with the Declaration of Helsinki. Patients completed the Ocular Surface Disease Index (OSDI) symptom severity questionnaire,13 followed by a complete ocular surface examination of both eyes by one of the investigators (SCP) in the following sequence: tear collection, biomicroscopic examination of the lid margins, meibomian glands and cornea, fluorescein tear break-up time (TBUT), corneal fluorescein staining, fluorescein clearance test, conjunctival lissamine green staining, and Schirmer 1 test. The presence of corneal subepithelial fibrosis (gray subepithelial tissue with elevation of the overlying epithelium) was noted. The TBUT was evaluated 1 minute after instilling 5 μL of 1% sodium fluorescein (Greenpark Pharmacy, Houston, TX) onto the inferior tarsal conjunctiva. After a blink, the precorneal tear film was examined using a biomicroscope with a 10× objective under blue-light illumination. The interval between the blink and the appearance of the first dark spot or discontinuity in the precorneal fluorescein-stained tear layer was recorded. Three separate readings were taken in each eye, and the results were averaged. To evaluate corneal fluorescein staining, the ocular surface was examined with a biomicroscope 10× objective through a yellow filter (Wratten 12, Boston 7503; Essilor, Omega, Dallas, TX) under blue-light illumination 2 minutes after instillation of 1% sodium fluorescein. The cornea was viewed through an acetate template containing five zones (central, temporal, nasal, superior, and inferior) with the outer circumference aligned with the limbus and the intensity of corneal fluorescein staining was graded according to the Baylor grading scheme.14 Briefly, the number of dots of fluorescein staining was graded in each of five zones on a standardized 5-point scale: 0 dots, 0; 1–5 dots, 1; 6–15 dots, 2; 16–30 dots, 3; and >30 dots, 4. One point was added to the score if there was one area of confluent staining, and 2 points were added if there were two or more. Tear clearance was performed with a modification of a previously reported technique.15 Fifteen minutes after instillation of 1% sodium fluorescein, the color of the tear meniscus in the outer one third of the lower lid was visually compared with one of the colors of a standardized visual scale,15 and the tear clearance score was graded from 0 to 6. If the color of the tear meniscus was judged to be between two of the six standard scale colors, then the score was graded between these two standard colors. Lissamine green staining of the nasal and temporal bulbar conjunctiva (graded 0–3 in each area based on the density of dots) was evaluated 1 minute after instillation of lissamine green from a strip (Wilson Ophthalmic, Mustang, OK). Schirmer 1 test was performed by placing Schirmer test strips (Eagle Vision, Memphis, TN) over the lower lid margin, at the junction of the lateral and middle thirds, for 5 minutes. The strip wetting was measured and recorded in millimeters.
Inclusion criteria for diagnosis of DE included a symptom severity score >20 and TBUT ≤ 7 seconds.6 Patients were subclassified by the presence of MGD, SS aqueous tear deficiency, or NK. MGD was diagnosed by evidence of dysfunction (lack of expressible meibum from ≥ 75% of five digitally expressed glands on the central upper and lower lid)6 and the presence of one or more morphologic changes of the meibomian glands, including ≥50% acinar atrophy of the glands on the medial half of the lower lid, vascular dilation on or scalloping of the posterior lid margin and MG orifice metaplasia. Inclusion criteria for diagnosis of NK included punctate keratopathy in the central or inferior cornea in patients with a history of diabetic neuropathy, ophthalmic zoster, scleral buckling procedure or surgical trigeminal ganglion ablation that persisted for ≥2 weeks with corneal sensitivity measured by the Cochet-Bonnet esthesiometer ≤1cm with no signs of active infection or improvement with preservative free artificial tears (Optive Sensitive; Allergan Inc., Irvine, CA) instilled at least 4 times a day.16 The United States European Study Group consensus criteria were used for diagnosis of SS.17
The subjects with DE were stratified into four levels of clinical severity based on the signs and symptoms according to the Dry Eye Workshop (DEWS) criteria (Table 1).18 If all criteria for a severity group were not met, severity grading was based on the worst parameter (e.g., severity grade 3 was assigned in subject with corneal fluorescein staining was ≥ 6 and conjunctiva staining = 2). The patients were excluded if they were using any topical medications other than nonpreserved artifical tears, wearing contact lenses, had undergone ocular surgery in the past year, had evidence of other ocular surface diseases. Seventeen subjects were recruited as the group. Inclusion criteria for the control subjects were absence of corneal and conjunctival dye staining and a symptom severity score ≤20.
Table 1.
Severity Grading Criteria for DE Conditions
| Group | Symptom Severity Score* | Tear Break-up Time (s) | Conjunctival Staining Score† | Corneal Staining Score‡ |
|---|---|---|---|---|
| No DE | ≤20 | >7 | 0 | 0 |
| DE 1 | >20 | ≤7 | ≤3 | ≤2 |
| DE 2 | >20 | ≤7 | ≥3 | <6 |
| DE 3 | >20 | ≤7 | ≥3 | ≥6, including central cornea or filaments |
| DE 4 | >20 | ≤7 | ≥3 | ≥12 |
DE 1–4, dry eye severity levels.
Symptom severity score measured by ocular surface disease index (OSDI) questionnaire.13
Lissamine green staining of the nasal and temporal bulbar conjunctival was graded 0–3 in each area based on the density of the dots.
Corneal fluorescein staining was graded according to the Baylor grading scheme14 in each of five zones on a standardized 5-point scale: 0 dots, 0; 1 to 5 dots, 1; 6 to 15 dots, 2; 16 to 30 dots, 3; >30 dots, 4. One point was added to the score if there was one area of confluent staining, and 2 points were added if there were two or more.
Tear Collection
Minimally stimulated tear fluid was collected from the inferior tear meniscus of each eye using a 0.5-μL glass capillary micropipette (Drummond, Broomall, PA). The tear samples from both eyes (1.0 μL total) were eluted into one tube (Eppendorf, Fremont, CA) containing 9 μL of assay buffer for a final dilution of 1:10, centrifuged for 2 minutes, and immediately transported in an insulated cooler to a −80°C freezer where they remained frozen until they were used for the immunoassay.
EGF Analysis
EGF levels in these samples were analyzed in a masked fashion with an immunobead assay (Beadlyte; Millipore Corp., Billerica, MA) according to the manufacturer's protocol. In brief, 10 μL of each tear sample, 25 μL of the 1× bead solution, and 10 μL of assay buffer (for blanks) were incubated at 4°C overnight in wells of a microtiter plate precoated with assay buffer. Plates were washed two times with 200 μL of wash buffer containing 0.05% preservative (Proclin; Sigma-Aldrich, St. Louis, MO). Human cytokine detection antibody (25 μL) was added to each well, and the samples were incubated at 37°C for 1 hour. Streptavidin-phycoerythrin (25 μL) was added to each well, and the samples were incubated at 37°C for 30 minutes. The plates were washed two times with 200 μL of wash buffer, and 150-μL sheathing fluid was added to all wells and the beads suspended on the plate shaker for 5 minutes. The plate was then read (100 IS; Luminex, Austin, TX) system and the results were analyzed (Bead View Analysis software; Millipore). The concentration of EGF was calculated from a standard curve of known concentrations of recombinant human EGF.
Statistical Analysis
Clinical parameters and tear cytokine concentrations in the study groups were compared by analysis of variance (ANOVA) with Tukey post hoc testing (Prism; GraphPad, La Jolla, CA). Reported probabilities for multiple comparisons made for the same group were lower than the Bonferroni-corrected threshold. The mean of measured clinical parameters of both eyes was used for statistical comparison.
Correlations between clinical parameters including, symptom severity, Schirmer 1 scores, FCT, conjunctival and corneal staining scores, presence of meibomian gland orifice metaplasia (0, absent; 1, present), and corneal subepithelial fibrosis (0, absent; 1, present) with tear EGF concentrations were determined by Spearman correlations.
Results
Demographic and Clinical Data
The demographic data for control and patients with DE are presented in Table 2. There were no significant differences in age and gender between the patients with DE and the asymptomatic control group (P ≥ 0.5). The clinical findings in the control and DE groups are presented in Table 3.
Table 2.
Demographic Features of Control Subjects and Patients with DE
| Group | n | Mean Age ± SD (y) | % Female |
|---|---|---|---|
| Control | 17 | 45.1 ± 14.7 | 70 |
| All DE | 35 | 57.5 ± 13.2 | 86.1 |
| MGD | 20 | 55.3 ± 16.4 | 89 |
| SS | 9 | 58.7 ± 18.3 | 100 |
| NK | 6* | 61.6 ± 6.0 | 67 |
Diabetic neuropathy, n = 2; ophthalmic zoster, n = 2; scleral buckling procedure, n = 1; surgical trigeminal ganglion ablation, n = 1.
Table 3.
Clinical Features of Normal Subjects and Patients with DE
| Group | n | Symptom Severity Score* | Schirmer 1 Score (mm) | Conjunctival Staining Score† | Corneal Staining Score‡ | Corneal Subepithelial fibrosis§ | Meibomian Gland Orifice Metaplasia‖ | FCT¶ |
|---|---|---|---|---|---|---|---|---|
| Normal | 17 | 11.9 ± 2.3 | 27.0 ± 7.8 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 1.5 ± 0.7 |
| All DE | 35 | 34.8 ± 9.4 | 13.0 ± 11.0 | 1.7 ± 2.0 | 4.6 ± 4.4 | 0.4 ± 0.49 | 0.47 ± 0.5 | 4.3 ± 1.0 |
| P < 0.00011 | P = 0.00011 | P < 0.00011 | P < 0.00011 | P < 0.00011 | ||||
| MGD | 20 | 33.2 ± 8.7 | 18.5 ± 11.9 | 0.5 ± 1.01 | 3.2 ± 2.7 | 0.47 ± 0.51 | 0.78 ± 0.41 | 3.7 ± 2.8 |
| P < 0.00011 | P = 0.00012 | P < 0.00012 | P = 0.0013 | P < 0.00011,2,3 | P < 0.0001a | |||
| P = 0.0012 | ||||||||
| SS | 9 | 36.8 ± 9.6 | 4.79 ± 2.0 | 4.9 ± 2.01 | 6.33 ± 5.37 | 0 ± 0 | 0.2 ± 0.4 | 5.1 ± 0.8 |
| P < 0.00011 | P = 0.00051 | P < 0.00011 | P < 0.00011 | P < 0.00011 | ||||
| NK | 6 | 41.2 ± 15.6 | 8.4 ± 3.0 | 1.67 ± 2.66 | 7.93 ± 4.8 | 0.6 ± 0.4 | 0 ± 0 | 3.77 ± 1.7 |
| P < 0.00011 | P = 0.00011 | P < 0.00011 | P < 0.00011 | P < 0.00011 |
Normal, normal control group. The mean score of both eyes was used for the Schirmer 1 test, corneal fluorescein staining, conjunctival staining scores and BCVA. P, 1vs. normal control; 2vs. SS; and 3vs. NK.
Symptom severity score measured by OSDI questionnaire.13
Lissamine green staining of the nasal and temporal bulbar conjunctiva was graded 0–3 in each area based on the density of dots.
Corneal fluorescein staining was graded according to the Baylor grading scheme14 in each of five zones on a standardized 5-point scale 0 dots, 0; 1–5 dots, 1; 6–15 dots, 2; 16–30 dots, 3; and >30 dots, 4. One point was added to the score if there was one area of confluent staining, and 2 points were added if there were two or more.
0, absent; 1, positive.
0, absent; 1, positive.
Tear clearance score was graded from 0 to 6.15
Compared with the control group, the symptom severity scores were significantly greater in the DE group as a whole, as well as in the MGD, SS, and NK subgroups (P < 0.0001). The Schirmer 1 score were significantly lower (P = 0.0001), and conjunctival the lissamine green and corneal fluorescein staining scores were significantly greater (P < 0.0001) in the DE group and the SS and NK subgroups than in the control group. However, corneal subepithelial fibrosis was more prevalent in the MGD and NK subgroups than in the SS subgroup and the control group. MG orifice metaplasia was significantly greater in the MGD subgroup than in the SS and NK subgroups and the control group (P < 0.0001). Tear fluorescein clearance was delayed in the DE group and in the MGD, SS and NK subgroups compared with that in the control group (P < 0.0001).
The severity parameters for each of the four levels of DE severity are presented in Table 4. Corneal fluorescein staining was significantly greater in the level 4 severity group than in the level 1 and 2 groups (P < 0.001). Conjunctival dye staining was significantly greater in level 4 than in levels 1, 2, and 3 (P < 0.005). MG orifice metaplasia was greater in levels 1 and 2 than in levels 3 and 4 (P < 0.001).
Table 4.
Clinical Features of Patients with DE Stratified by Severity
| Severity Level | n | Symptom Severity Score* | Schirmer 1 Score (mm) | Conjunctival Staining Score† | Corneal Staining Score‡ | Corneal Subepithelial Fibrosis§ | Meibomian Gland Orifice Metaplasia‖ | FCT¶ |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 30.5 ± 11.2 | 18 ± 12 | 0.25 ± 0.46 | 0.75 ± 1 | 0.57 ± 0.53 | 0.71 ± 0.48 | 4.25 ± 1.13 |
| P < 0.0011,4,5 | P < 0.0011 | |||||||
| 2 | 16 | 32.6 ± 10.3 | 14 ± 12 | 1.5 ± 2.3 | 3.6 ± 2 | 0.25 ± 0.44 | 0.62 ± 0.5 | 4.16 ± 1.5 |
| P < 0.0011 | P < 0.0011 | P < 0.001a,d | P < 0.0011 | |||||
| 3 | 7 | 36.3 ± 13.5 | 9 ± 8 | 2.3 ± 2.14 | 9.3 ± 1.5 | 0.7 ± 0.4 | 0.14 ± 0.41 | 3.43 ± 1.66 |
| P < 0.0011 | P < 0.0011,2,3 | |||||||
| 4 | 4 | 41 ± 10.0 | 4.8 ± 1.3 | 5.63 ± 0.75 | 13.7 ± 2.3 | 0.25 ± 0.5 | 0 ± 0 | 5.5 ± 0.41 |
| P < 0.0011 | P < 0.0051,2,3,4 | P < 0.0011,2,4 | P < 0.0011 | |||||
| Control | 17 | 11.9 ± 2.3 | 27.0 ± 7.8 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 1.5 ± 0.7 |
Control, normal control group. The mean score of both eyes was used for the Schirmer 1 test, corneal fluorescein staining. conjunctival staining scores, and BCVA. P, 1vs. control; 2vs. level 1; 3vs. level 2; 4vs. level 3; and 5vs. level 4. Symboled footnotes are described in Table 3.
EGF Concentrations
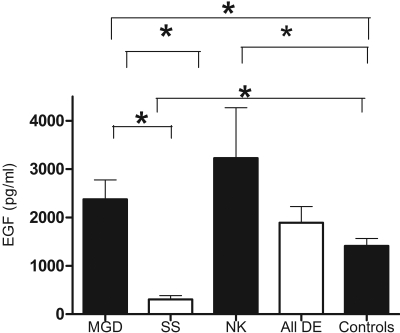
The measured tear EGF concentrations were in the linear portion of the standard curve. The EGF levels in control subjects and patients with DE are presented in Figure 1. The EGF level was 1412.9 ± 595.0 pg/mL in the control group and 2016.8 ± 1977 pg/mL in the entire DE group. The lowest EGF levels were seen in the SS subgroup (310.9 ± 232.34 pg/mL), significantly lower than in the control group (P < 0.0001) and the MGD (P < 0.05) and NK (P < 0.01) subgroups. EGF levels were significantly higher in the DE subgroup with MGD compared to the control group (P = 0.03). The highest EGF levels were seen in patients with NK (3227.5 ± 2950 pg/mL), significantly higher than those in the control group (P = 0.02).
Figure 1.
Tear EGF concentration in asymptomatic control subjects, all patients with DE, and the SS, MGD, and NK DE subgroups. *P < 0.05.
Clinical Parameters and Tear EGF Concentrations Stratified by FTC and Schirmer 1 Scores
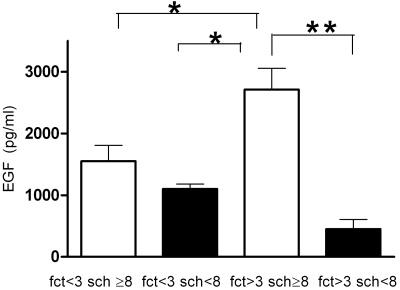
The control subjects and patients with DE were stratified into four subgroups, according to their FCT and Schirmer test scores (Table 5). The subgroup with FCT > 3, Schirmer 1 ≥ 8 included 14 patients with MGD and 4 with NK. This subgroup had the highest EGF levels, significantly higher than those in the group with FCT > 3, Schirmer 1 < 8 (P < 0.001) and both the groups had a good tear clearance (FCT < 3; P < 0.01; Fig. 2). Corneal subepithelial fibrosis and MG orifice metaplasia were also significantly higher in this subgroup than the other three subgroups (P < 0.0001). The subgroup with FCT > 3 and Schirmer 1 < 8 included all the patients with SS and two patients with NK. This subgroup had significantly higher conjunctival and corneal dye staining scores than did the other three groups. Symptom severity scores were significantly greater in the both the groups with delayed FCT than in the group with FCT < 3 and Schirmer ≥ 8.
Table 5.
Clinical Parameters and Tear EGF Levels in Control Subjects and Patients with DE Grouped According to FCT and Schirmer 1 Scores
| Group | Cutoffs | n | Symptom Severity Score* | Conjunctival Staining Score† | Corneal Staining Score‡ | Corneal Fibrotic Changes§ | Meibomian Gland Orifice Metaplasia‖ | EGF Levels¶ |
|---|---|---|---|---|---|---|---|---|
| 1 | FCT ≤ 3 | 19 | 15.1 ± 7.97 | 0 ± 0 | 0.6 ± 1.9 | 0 ± 0 | 0.12 ± 0.3 | 1335 ± 602 |
| Schirmer ≥ 8 | ||||||||
| 2 | FCT ≤ 3 | 4 | 26.3 ± 12.3 | 0 ± 0 | 2.6 ± 2.0 | 0.25 ± 0.5 | 0.25 ± 0.5 | 1101 ± 154.4 |
| Schirmer < 8 | ||||||||
| 3 | FCT > 3 | 18 | 35.1 ± 11.13 | 0.8 ± 1.1 | 4.0 ± 3.0 | 0.56 ± 0.51 | 0.87 ± 0.34 | 3101 ± 2173 |
| Schirmer ≥ 8 | P < 0.00011 | P < 0.00011 | P < 0.00021,4 | P < 0.00011,2,4 | P < 0.011,2 | |||
| P < 0.0014 | ||||||||
| 4 | FCT > 3 | 11 | 37.55 ± 10.72 | 4.7 ± 2.4 | 7.18 ± 4.7 | 0.09 ± 0.3 | 0.18 ± 0.4 | 433.5 ± 465 |
| Schirmer < 8 | P < 0.00011 | P < 0.00011,2,3 | P < 0.00011,2,3 | P < 0.0011,3 |
P, 1vs. group 1; 2vs. group 2; 3vs. group 3; and 4vs. group 4. Symboled footnotes are described in Table 3.
Figure 2.
Tear EGF concentration in asymptomatic control subjects and all patients with DE subgrouped according to FCT and Schirmer 1 (sch) scores. *P < 0.001, **P < 0.0001.
Correlation Analyses: EGF Levels and Clinical Parameters
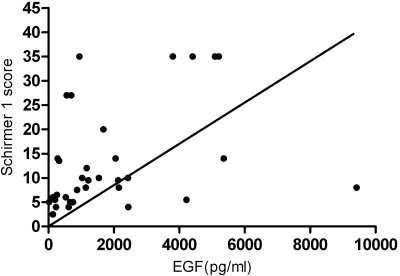
The rank (Spearman) correlation coefficients between tear epidermal growth factor concentration and clinical parameters are presented in Table 6. No correlation was found between the tear EGF concentration and symptom severity scores. A significant inverse correlation was found between the tear EGF levels and the conjunctival dye staining (r = −0.49, P = 0.0032) and corneal dye staining scores (r = −0.39, P = 0.022). MG orifice metaplasia (r = 0.36, P = 0.03), as well as corneal subepithelial fibrosis (r = 0.5, P = 0.0006) correlated significantly with tear EGF concentrations. Furthermore, there was a significant correlation between tear EGF levels and the Schirmer 1 scores (r = 0.47, P = 0.0042; Fig. 3).
Table 6.
Correlation between Tear EGF Level and Dry DE Clinical Severity Parameters
| Clinical Parameter | Spearman r | P |
|---|---|---|
| Symptom severity score | 0.09 | 0.59 |
| Conjunctival staining score | −0.49 | 0.0032 |
| Corneal staining score | −0.39 | 0.022 |
| Meibomian gland orifice metaplasia | 0.36 | 0.03 |
| Corneal subepithelial fibrosis | 0.5 | 0.0006 |
| Schirmer 1 score | 0.47 | 0.0042 |
| FCT score | −0.1 | 0.34 |
Significant correlations are in bold. Symptom severity score measured by OSD1 questionnaire.
Figure 3.
Correlation between tear EGF concentration and the Schirmer 1 score.
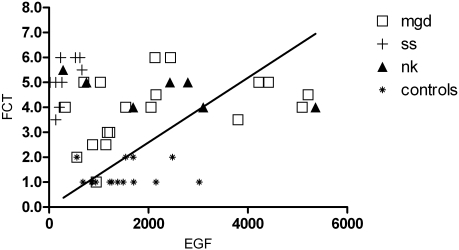
The tear EGF level and FCT showed an inverse but not significant correlation (r = −0.1, P = 0.34). The correlation of tear EGF levels and FCT in all DE subgroups and control subjects is shown in Figure 4. All the patients in the SS group had delayed FCT but low EGF levels compared with the control subjects. Most patients in the MGD subgroup had delayed tear clearance and high EGF levels than did the control subjects. Similarly, the NK subgroup shows delayed tear clearance and high EGF levels.
Figure 4.
Plot of tear EGF concentration and FCT at the end of 15 minutes. FCT scores ≤3 are considered normal. All Sjögren's syndrome samples had tear EGF concentrations <700 pg/mL.
Discussion
DE is a common ocular surface disease that has a variety of clinical presentations that include eye irritation, increased sensitivity to air drafts, and blurred vision.1–4 A previously reported study found a link between compositional changes in tears and the severity of irritation symptoms and ocular surface disease that develops in DE.6 Our group has previously reported that tear EGF concentration is increased in patients with DE with MGD compared with that in those without MGD.6 In the present study, we found the tear EGF was significantly higher in the DE subgroup with MGD than in the control group and the subgroup with SS. EGF levels were significantly lower in the SS group than the control group and DE subgroups with MGD and NK. An interesting and unexpected finding was the elevated EGF concentration in patients with NK compared with the control group.
EGF was measured by a sensitive bead immunoassay in a minute volume (1 μL) of tears collected from the inferior meniscus with a microcapillary pipette. It is likely that the tear volume was greater in subjects with normal tear production and delayed tear clearance compared with those with reduced aqueous tear production; however, we were able to collect the same volume of tears from all subjects and the measured tear EGF concentrations should represent the actual concentration the cells on the surface of the eye are exposed to. Care was taken to minimally stimulate reflex tearing during tear collection by using dim illumination and avoiding contact with the lid margin or ocular surface. Our group has previously reported that tear EGF concentrations decrease with induction of reflex tearing.19 It is well recognized that the ability to reflex tear is diminished in SS and NK.20,21 Therefore, in the event reflex tearing was induced in the subjects who retained the ability to reflex tear, it would have resulted in a decrease in tear EGF concentration, not the increased concentration we observed.
On subgroup of patients with DE based on tear clearance and Schirmer 1 scores, the subgroup with delayed tear clearance and Schirmer 1 scores ≥ 8 had tear EGF concentrations significantly higher than both subgroups with normal tear clearance, and the subgroup with delayed tear clearance and decreased Schirmer 1 score. Corneal fibrotic changes, as well as MG orifice metaplasia were significantly higher in this group with delayed tear clearance and normal tear production than the other three subgroups. This group with delayed tear clearance, and normal Schirmer 1 score also had significantly higher symptom severity and corneal dye staining scores than the group with normal tear clearance and Schirmer 1 score.
Most of the patients with MGD (14/20) were found in the subgroup with FCT > 3 and Schirmer 1 ≥ 8, and it is probable that the high tear EGF concentrations in this group coupled with the delayed tear clearance are responsible for the significantly higher corneal fibrotic changes and MG orifice metaplasia seen in this group. The altered corneal permeability found in DE may facilitate diffusion of tear EGF to basal corneal epithelial cells and subepithelial keratocytes, which may then be stimulated to produce the extracellular matrix proteins that are responsible for the fibrotic changes.22
Similar to previously reported studies, tear EGF concentration showed significant correlation with Schirmer 1 score, indicating it is a marker of lacrimal gland function. A statistically significant positive correlation was noted between tear EGF concentration and clinical severity parameters, including corneal subepithelial fibrosis and MG orifice metaplasia. The high tear EGF concentrations observed in the DE subgroups with MGD and NK may play a role in the corneal fibrotic changes and neovascularization found in both these conditions. Corneal and conjunctival dye staining scores showed a significant negative correlation with tear EGF concentration. This finding may explain the increased corneal and conjunctival dye staining in SS aqueous tear deficiency, the group with the lowest tear EGF levels.
It is known that topical application of EGF to the corneal epithelium leads to epithelial hypertrophy and enhances the division of corneal epithelial cells.22 Furthermore, EGF has been associated with development of corneal vascularization.23 Tear EGF is essential for maintaining homeostasis of the ocular surface epithelia and to promote epithelial healing after trauma; however; it is likely that when tear EGF concentration increases beyond a certain level, it may promote hypertrophy of the corneal and MG ductal epithelium resulting in the MG orifice metaplasia that is frequently observed in patients with MGD. Additional research is needed to establish this link firmly and to develop strategies to prevent these corneal and lid margin changes in patients with DE with decreased tear clearance.
Footnotes
Supported by National Institutes of Health Grant EY11915, an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, The William Stamps Farish Fund, and The Hamill Foundation.
Disclosure: K. Rao, None; W.J. Farley, None; S.C. Pflugfelder, None
References
- 1.Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol 1997; 124: 723–728 [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000; 118: 1264–1268 [DOI] [PubMed] [Google Scholar]
- 3.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne. Aust Ophthalmol 1998; 105: 1114–1119 [DOI] [PubMed] [Google Scholar]
- 4.Research in dry eye: report of the Research Subcommittee of the International Dry Eye Work Shop (review). Ocul Surf 2007; 5(2): 179–193 [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am J Ophthalmol 2004; 137: 337–342 [DOI] [PubMed] [Google Scholar]
- 6.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 2009; 147: 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 2000; 19: 113–129 [DOI] [PubMed] [Google Scholar]
- 8.Brazzell RK, Stern ME, Aquavella JV, Beuerman RW, Baird L. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci 1991; 32: 336–340 [PubMed] [Google Scholar]
- 9.Maldonado BA, Furcht LT. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Invest Ophthalmol Vis Sci 1995; 36: 2120–2126 [PubMed] [Google Scholar]
- 10.He J, Bazan HE. Epidermal growth factor synergism with TGF-beta1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci 2008; 49: 2936–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology 1999; 106: 803–810 [DOI] [PubMed] [Google Scholar]
- 12.Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol 1996; 40: 343–367 [DOI] [PubMed] [Google Scholar]
- 13.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000; 118: 615–621 [DOI] [PubMed] [Google Scholar]
- 14.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol 2004; 137: 109–115 [DOI] [PubMed] [Google Scholar]
- 15.Macri A, Rolando M, Pflugfelder SC. A standardized visual scale for evaluation of tear fluorescein clearance. Ophthalmology 2000; 107: 1338–1343 [DOI] [PubMed] [Google Scholar]
- 16.Goins K. New insights into the diagnosis and treatment of neurotrophic keratopathy. Ocul Surf 2005: 2: 96–110 [DOI] [PubMed] [Google Scholar]
- 17.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjögren syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjögren Syndrome. Ann Rheum Dis 1996; 55: 116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Report of the Management and Therapy Subcommittee of the International Dry Eye Workshop. Ocul Surf 2007; 5: 163–178 [DOI] [PubMed] [Google Scholar]
- 19.Jones DT, Monroy D, Pflugfelder SC. A novel method of tear collection: comparison of glass microcapillary pipettes with porous polyester rods. Cornea 1997; 16: 450–458 [PubMed] [Google Scholar]
- 20.Tsubota K, Kaido M, Yagi Y, Fujihara T, Shimmura S. Diseases associated with ocular surface abnormalities: the importance of reflex tearing. Br J Ophthalmol 1999; 83: 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heigle TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea 1996; 15: 135–138 [DOI] [PubMed] [Google Scholar]
- 22.Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci 1992; 33: 3292–3301 [PubMed] [Google Scholar]
- 23.Nezu E, Ohashi Y, Kinoshita S, Manabe R. Recombinant human epidermal growth factor and corneal neovascularization. Jpn J Ophthalmol 1992; 36: 401–406 [PubMed] [Google Scholar]