More effective agents have been identified based on an understanding of the chemical mechanisms involved in therapeutic nitroalcohol corneoscleral cross-linking.
Abstract
Purpose.
The recent tissue cross-linking studies indicate that aliphatic β-nitroalcohols (BNAs) may be useful as pharmacologic corneoscleral cross-linking agents. The present study was performed to identify the specific chemistry involved under physiologic conditions, with the intent of identifying more effective agents.
Methods.
The mechanism of chemical cross-linking at pH 7.4 and 37°C was studied using three techniques. The colorimetric Griess assay was used to follow the release of nitrite from three mono-nitroalcohols (2-nitroethanol [2ne], 2-nitro-1-propanol [2nprop]), and 3-nitro-2-pentanol [3n2pent]). Second, the evolution of 2nprop in 0.2 M NaH2PO4/Na2HPO4/D2O was studied using 1H-NMR. Third, thermal shrinkage temperature analysis (Ts), a measure of tissue cross-linking, was used to support information from 1the H-NMR studies.
Results.
A time-dependent release of nitrite was observed for all three mono-nitroalcohols studied. The maximum levels were comparable using either 2ne or 2nprop (∼30%). However, much less (∼10%) was observed from 3n2pent. Using 1H-NMR, 2nprop evolved into a unique splitting pattern. No match was observed with reference spectra from three possible products of denitration. In contrast, 2-methyl-2-nitro-1,3-propanediol (MNPD), a nitro-diol, was identified, implying the formation of formaldehyde from a retro-nitroaldol (i.e., reverse Henry) reaction. In support of this mechanism, Ts shifts induced by the nitro-triol 2-hydroxymethyl-2-nitro-1,3-propanediol (HNPD) were superior to the nitro-diol MNPD which were superior to the mono nitroalcohol 2nprop.
Conclusions.
BNAs function as both formaldehyde and nitrite donors under physiologic conditions to cross-link collagenous tissue. Higher order BNAs are more effective than mono nitroalcohols, raising the possibility of using these agents for therapeutic corneoscleral cross-linking.
In vivo therapeutic corneal collagen cross-linking (CXL) is a new, rapidly expanding area of clinical ophthalmology. The seminal works of Wollensak1 and Spoerl et al.2 over the past 10 years has shown that stabilization of the cornea in vivo through photochemical tissue cross-linking (ultraviolet-A light + riboflavin = UVAR) can halt the progressive thinning and bulging of the cornea seen in keratoconus, an important corneal thinning disorder afflicting mostly younger adults. This therapeutic breakthrough has been a major one for a condition in which many patients eventually must undergo corneal transplantation. To cross-link the cornea, riboflavin is used as a photosensitizer and UVA (λmax = 370 nm) irradiation is applied to a de-epithelialized corneal surface for 30 minutes. The impressive clinical results which began in Germany have ignited widespread use of this new treatment throughout the world, which includes the United States where the first FDA-approved clinical trial is under way. Clinical indications for this procedure are growing and include postsurgical ectasia, a previously unrecognized long-term complication of excimer laser keratorefractive surgery (i.e., LASIK), as well as corneal melting, pathologic swelling (i.e., bullous keratopathy), and difficult-to-treat infections (i.e., acanthamoeba). Despite the effectiveness of UVAR therapy, however, this treatment method poses attendant risks (particularly those related to ultraviolet irradiation), provides only partial cross-linking of the cornea, is not suitable for corneas thinner than 400 μm, requires painful epithelial debridement, and cannot be used in the peripheral cornea.2
Using a topical, pharmacologic, self-administered compound to produce a comparable (or superior) degree of cross-linking to the UVAR therapy could provide numerous benefits. First, if no UVA irradiation is necessary, it would remove any long- or short-term risk of UVA exposure. Second, patients would not be subjected to painful epithelial debridement. In addition to these two clear advantages, there are several other hypothetical benefits that have yet to be confirmed. These are mentioned here, not as a matter of proven fact but rather in an effort to elaborate on the rationale for pursuing this approach. Third, patients would benefit from the ease of application (i.e., perhaps using an eye drop). Fourth, a more complete cross-linking could be possible, particularly if water soluble cross-linking agents (such as nitroalcohols) that can diffuse freely through the corneal stroma are used. The UVAR method cross-links only the anterior 200 μm of cornea which correlates with the depth of penetration of UVA light into the riboflavin-soaked cornea.3 Fifth, a dose modulation could have the effect of controlling the magnitude rather than the single effect currently produced with the UVAR procedure. Finally, it may be possible to treat the peripheral cornea, which is not possible with UVAR (i.e., concern regarding UVA induced limbal stem cell damage). This possibility could be particularly relevant to treating pellucid marginal degeneration (a related peripheral corneal thinning disorder).
Nitroalcohols have been used extensively in a wide variety of industrial and commercial applications, ranging from rocket fuels and explosives to toilet deodorizers and plasticizers.4 They have also served as convenient starting compounds as well as chemical intermediates for the synthesis of various classes of organic derivatives.5 The number and scope of industrial applications is vast and includes polymerization chemistry, where nitroalcohols can act as formaldehyde donors to cross-link compounds such as urea, melamine, phenols, and resorcinol, in the production of resins, plastics, polyesters, and polyurethane products.6 In addition, because of their widespread industrial use, the health and safety effects of nitroalcohols have been studied extensively for acute toxicity, teratogenicity, and mutagenicity/carcinogenicity where their profile is quite favorable,4 in stark contrast to formaldehyde, which has widely publicized unfavorable toxicity and carcinogenicity profiles.7
Although nitroalcohols have been used for many purposes in the past, to the best of our knowledge, they have not been proposed for in vivo therapeutic purposes. Our recent tissue cross-linking studies have raised the possibility of using aliphatic β-nitroalcohols (BNAs) for pharmacologic, therapeutic corneoscleral cross-linking. In those initial studies, we were interested in observing the cross-linking effects induced under physiologic pH and temperature, since these are the conditions present in living tissue. The results of those studies showed that aliphatic mono-nitro alcohols, such as 2-nitroethanol (2ne), 2-nitro-1-propanol (2nprop), and 3-nitro-2-pentanol (3n2pent), can induce cross-linking effects in both a time- and concentration-dependent manner.8,9 The present study was performed to identify the specific chemistry involved under these conditions, with the intent of identifying more effective agents based on mechanism.
Materials and Methods
2ne, 2-nitroethane, 2nprop, and 3n2pent [mixture of (±) threo and (±) erythro], propylene oxide (Fluka, Buchs, Switzerland), propylene glycol, propionaldehyde, formaldehyde, Dextran T500, NaH2PO4, Na2HPO4, penicillin (5000 IU/mL)/streptomycin (5000 μg/mL), deuterium oxide (D2O), EDTA, sulfanilic acid, N-ethylenediamine hydrochloride, and HCl, were all obtained from the Sigma-Aldrich Chemical Co. (St. Louis, MO). 2-Methyl-2-nitro-1,3-propanediol (MNPD; MW = 135) and 2-hydroxymethyl-2-nitro-1,3-propanediol (HNPD; MW = 151) were obtained from TCI America (Portland, OR). Purified water (Millipore, Billerica, MA) was used in all the experiments.
Colorimetric Nitrite Assay Studies
The spontaneous liberation of free nitrite was monitored with a modification of the colorimetric Griess assay. Briefly, 20 μL of supernatant was applied to a 400 μL well (96-well microtiter plate). Each sample was assayed in triplicate. Fifty microliters of 2N HCl and 50 μL of sulfanilic acid (1 mg/mL) were added to the sample and incubated at room temperature for 10 minutes, followed by 50 μL of N-ethylenediamine hydrochloride (2 mg/mL) and with an additional incubation for 25 minutes. The plate was read at 546 nm (purple color) on a kinetic microplate spectrophotometer (Benchmark Microplate Reader; Bio-Rad Laboratories, Hercules, CA). A 1-mM solution of sodium nitrite was used to create a standard curve that was developed on each day of sampling.
Three different short chain aliphatic BNAs were studied for spontaneous release of nitrite. The concentration of 10 mM was used for each compound. The incubation solution was identical with that which we have previously used for in vitro ocular tissue cross-linking efficacy studies and included 20% dextran (T500) and 0.2 M NaH2PO4/Na2HPO4 (pH 7.4). The incubation solution was sampled serially over the course of 14 days to monitor the liberation of free nitrite from the solution.
BNA (2nprop) Evolution Studies Using 1H-NMR
This method was chosen for the present study because it is particularly useful for studying the mechanism of a chemical reaction. By observing the evolution of a compound over time, changes in the NMR spectrum corresponding to reactive intermediates and/or reaction products can be identified. In other words, any change in molecular structure either through chemical decomposition and/or addition will affect the electron shielding of protons and register as changes in the NMR signal through chemical shifts, disappearance of peaks, or changes in the splitting pattern. Comparison of unknown spectra to that of reference (i.e., standards) spectra can be especially helpful in this regard and was used extensively in this study.
In the present study, 2nprop was chosen to focus on because of the favorable signal observed in initial experiments. That is, the number and location of peaks observed represented a relatively uncomplicated signal to follow, yet was sufficient in quantity to enable observance of changes over time. This method allowed for easier identification of products (through the unique splitting and the shifts of the multiple product peaks). 2ne produced few peaks, which may have hindered our ability to identify potential products and thus was not pursued in the 1H-NMR studies.
A 100-mM solution of 2nprop was made up in 0.2 M NaH2PO4/Na2HPO4/D2O (pH 7.4), covered, and placed in a heating block at 37°C over the course of 10 days. The sample was monitored daily by 1H-NMR, to follow the evolution of products. Control experiments were conducted in 0.2 M NaH2PO4/Na2HPO4 (pH 7.4) using both D2O and H2O. Identification of structural unknowns was pursued using available standards and included three possible products of denitration (i.e., propylene oxide, propionaldehyde, and propylene glycol), and one product of a nitroaldol condensation, MNPD. These reference 1H-NMR spectra were then compared with spectra from the 2nprop evolution studies. All 1H-NMR spectra were obtained on a 400- or 500-MHz NMR instrument (Bruker Instruments, Billerica, MA).
Thermal Shrinkage Temperature (Ts) Studies
The assay of thermal shrinkage temperature, a measure of tissue cross-linking, was used to support mechanistic information gathered from the 1H-NMR studies. The compounds tested using the Ts assay included 2nprop, a mono-nitroalcohol; MNPD, a nitro-diol; and HNPD, a nitro-triol. In each case, 8 × 4-mm porcine scleral strips were used as a collagenous tissue substrate for cross-linking. The cross-linking reaction and analysis was performed as described elsewhere.8 Briefly, 1 mL of incubation solution was added to a 2-mL tube (Eppendorf, Fremont, CA) and reacted in a water bath for 12 to 24 hours at 37°C. The solution contained 20% dextran (T500; to prevent tissue swelling), 0.2 M NaH2PO4/Na2HPO4 (pH 7.4), penicillin/streptomycin (10 μL/mL), and EDTA (1 mM), to prevent endogenous proteolysis. The β-nitroalcohol compounds were added at 10 mM. Each condition was run in triplicate. After incubation, the samples were evaluated for cross-linking effect with the Ts assay.
Results
Spontaneous Nitrite Release from BNAs
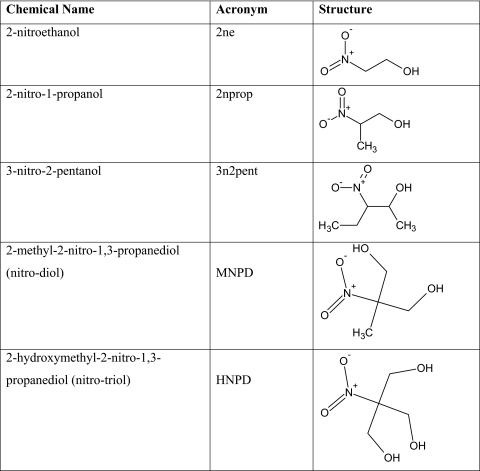
Under a condition of physiologic pH and temperature, free nitrite was liberated from all three BNAs studied. The release was time dependent and occurred maximally for 2ne and 2nprop. As shown in Figure 1, the range of nitrite release on a molar basis for both 2ne and 2nprop approached 30% by day 7. By contrast, the amount of nitrite released from 3n2pent was significantly less (∼10%) over the same time course.
Figure 1.
Spontaneous generation of nitrite from BNAs under conditions of physiologic pH and temperature. Incubations using 10 mM BNAs were performed at pH 7.4 and 37°C. Over the course of 14 days, the supernatant was sampled daily and nitrite determined using the colorimetric Griess assay. Each point represents the average of three independent determinations. All three compounds showed time-dependent increases in nitrite release with 2ne and 2nprop having levels significantly higher than 3n2pent. The samples reached equilibrium at about day 7 and approached maximal levels of ∼30% for 2ne and 2nprop and ∼10% for 3n2pent.
Because formaldehyde was identified as an evolution product of 2nprop in the 1H-NMR studies (described later), the possibility that a false-positive signal (from formaldehyde) from the Griess assay was considered. However, no positive color change was observed when using formaldehyde alone (10 mM) under identical conditions, ruling out this possibility. As well, based on the 1H-NMR evolution studies showing that formaldehyde was being liberated from 2nprop, the possibility that there was a denitration of 2-nitroethane (the other product of the decomposition of 2nprop in the reverse Henry reaction discussed later) was considered as well. However, under the same incubation conditions, 2-nitroethane did not release nitrite, suggesting that a different pathway gives rise to the free nitrite.
BNA Evolution Studies with 1H-NMR
Deuterium Exchange.
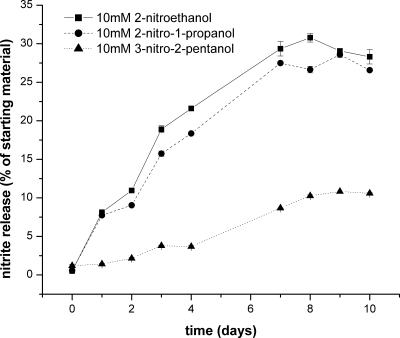
Two observations were made regarding the evolution of 2nprop under physiological conditions of 37°C and pH 7.4. First, as shown in Figure 2, there was a rapid loss of proton signal from the nitro-containing β-carbon. This loss occurred in phosphate-buffered solution but not in D2O. The loss of signal at that site indicated that deuterium exchange had occurred, suggesting that the nitro-containing β-carbon proton is labile. Such proton lability indicates an enhanced acidity. These findings of deuterium exchange were confirmed using HPLC/MS in which the starting material was noted to increase mass by 1 AMU (data not shown).
Figure 2.
2nprop undergoes deuterium exchange in neutral phosphate buffer. 1H-NMR spectra are shown for 2nprop in D2O (top) and D2O/phosphate buffer pH 7.4 (bottom) kept at room temperature for 3 days. Top spectrum (taken in D2O): the presence of three proton signals (a, b, c) as expected from 2nprop. Bottom spectrum: disappearance of the b proton signal, corresponding to the position occupied on the nitro-carrying β-carbon. Also, because the NMR medium was water (i.e., D2O), hydrogen bonding and proton exchange eliminates the hydroxyl proton peak in this spectrum.
Lability of this proton, as indicated by the finding of deuterium exchange, could, in theory, lead to the formation of a corresponding nitronate anion HOCH2(C NO2–)CH3. Thus, early in the study, the possibility that the nitronate form could have been a reactive intermediate in the cross-linking reaction was considered. We tested this hypothesis by synthesizing the nitronate salt of 2nprop and used it for tissue cross-linking. The conditions for incubation were identical with those used for mono-nitroalcohol cross-linking. However, the thermal shrinkage temperature shift induced by the nitronate form of 2nprop was identical with that induced by 2nprop, indicating that the nitronate form was not a key reactive intermediate in the cross-linking reactions (data not shown).
NO2–)CH3. Thus, early in the study, the possibility that the nitronate form could have been a reactive intermediate in the cross-linking reaction was considered. We tested this hypothesis by synthesizing the nitronate salt of 2nprop and used it for tissue cross-linking. The conditions for incubation were identical with those used for mono-nitroalcohol cross-linking. However, the thermal shrinkage temperature shift induced by the nitronate form of 2nprop was identical with that induced by 2nprop, indicating that the nitronate form was not a key reactive intermediate in the cross-linking reactions (data not shown).
Diol Formation.
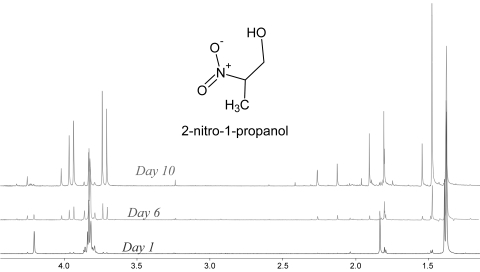
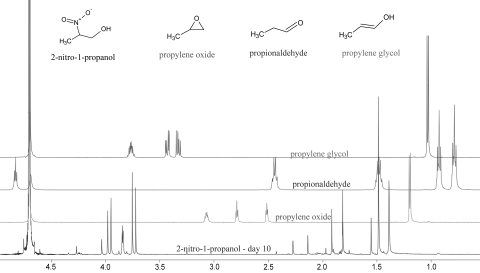
As shown in Figure 3, over the course of several days under physiologic conditions, the 2nprop signal evolved significantly. First, the multiplet at ∼3.8 ppm transformed into a pair of strongly coupled doublets, whereas the doublet at 1.4 ppm became a singlet. Over the course of days, a pair of doublets began to appear on either side of the peaks at 3.8 ppm and the singlet at 1.4 ppm shifted downfield to ∼1.5 ppm. This evolution continued from days 1 to 10, at which time, only a trace of the original peak was seen at 3.8 ppm.
Figure 3.
Evolution of 2nprop over the course of 10 days at pH 7.4 and 37°C. Three spectra are overlaid and show the day 1, 6, and 10 signals from 2nprop. Under these conditions, the 2nprop 1H-NMR signal evolved indicating the formation of a new structure. There was a unique splitting pattern that developed over time at ∼3.7–4.0 ppm corresponding to the c protons as well as a chemical shift to higher frequency at ∼1.4–1.5 ppm corresponding to the a protons. The b proton signal is not seen and has undergone deuterium exchange as shown in Figure 2.
Next, based on initial experiments that showed that free nitrite is formed spontaneously under these conditions, we postulated that denitration (or an elimination of nitrous acid, HONO) could result in the formation of products with cross-linking efficacy. Three possible products of denitration were obtained commercially and their 1H-NMR signal compared with the evolution product of 2nprop. The three compounds included an epoxide (propylene oxide), which has been proposed to form from 2-ne,10 an aldehyde (propionaldehyde), and an alkene glycol (propylene glycol). Any of these three compounds could have accounted for the observed cross-linking effect. As shown in Figure 4, none of these signals were identified as an evolution product of 2nprop. In contrast, however, the nitro-diol, MNPD, was positively identified (Fig. 5) accounting for a majority of the largest unknown evolution peaks. This observation suggests that formaldehyde forms from the 2nprop starting material and reacts with the parent molecule to form the nitro-diol. Scheme 1 is adopted from Shvekhgeimer5 and outlines the reactions that plausibly lead to the formation of the nitro-diol identified. The formaldehyde release occurs through a base-catalyzed, thermally driven retro-nitroaldol (reverse Henry), reaction. This reaction is known to occur under alkali conditions.5
Figure 4.
Comparison of 2nprop evolution spectra with three possible products of denitration. Based on the initial finding that a denitration (i.e., loss of an –NO2 group) occurs under these conditions (i.e., see Fig. 1), an attempt was made to identify the 2nprop evolution product. Four 1H-NMR spectra are overlaid and show the 2nprop evolution signal (bottom) and three possible products of denitration: propylene oxide, propionaldehyde, and propylene glycol. None of these three reference signals showed a match.
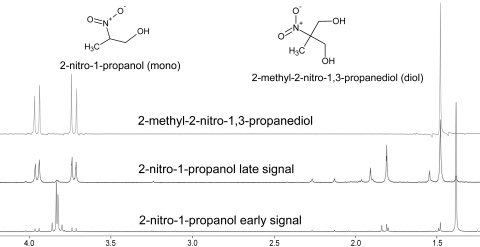
Figure 5.
The formation of MNPD from 2nprop. Comparison of the 1H-NMR evolution spectrum of 2nprop with its corresponding nitro-diol confirms the presence of this compound in the evolution of the mono-nitroalcohol on heating at 37°C at pH 7.4 in buffer. This finding implies the formation of formaldehyde from 2nprop as shown in Scheme 1.
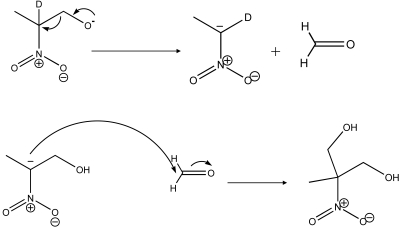
Scheme 1.
2nprop can undergo a base-catalyzed, thermally driven retro-nitroaldol reaction (i.e., reverse Henry) to give formaldehyde and its nitroalkane. Subsequent reaction of formaldehyde with the starting material gives MNPD the nitrodiol. D, deuterium. Adopted from Shvekhgeimer MGA. Rus Chem Rev. 1998;67(1):35–68.
Thermal Shrinkage Temperature Studies
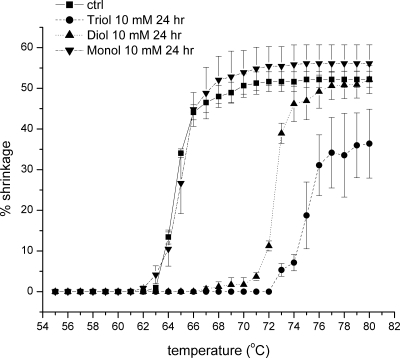
Ts temperature analysis was performed on porcine scleral tissue that had been cross-linked with 2nprop, MNPD, and HNPD. Previous studies have been performed, predominately using a 96-hour incubation period.8,9 In the present studies, the time of incubation was decreased to evaluate whether the higher order nitroalcohols would produce greater cross-linking effects than the mono-nitroalcohol. As shown in Figure 6, little to no shift in Ts was noted for 2nprop after this shortened incubation time. This was significantly less than the shift observed when in the 96 hour reaction. On the other hand, the nitro-diol MNPD, produced significantly greater shifts in Ts than the mono-nitroalcohol. The Ti and T50 and were shifted by 0.2 and 0.4°C for 2nprop and 8.4 and 7.9°C for MNPD at 24 hours.
Figure 6.
Thermal shrinkage temperature effects comparing the cross-linking efficacy of the mono-nitroalcohol (2nprop) with the nitro-diol (MNPD) and the nitro-triol (HNPD). After incubation (24 hours; pH 7.4, 37°C), the cross-linking effects were evaluated by thermal shrinkage temperature analysis and were greatest for the triol, followed by the diol. Using this shortened incubation time, the mono-nitroalcohol 2nprop showed no effect. Ts shifts were also noted for the diol and triol after 12 hours of incubation (see Table 1) indicating a much higher efficacy than any of the other mono-nitroalcohols studied previously. These findings support the concept that the mono-alcohol can donate 1 mole of formaldehyde per molecule, the diol 2 moles, and the triol 3 moles.
The cross-linking effects were also compared between the nitro-diol MNPD and the nitro-triol HNPD. Again, similar reagent concentrations (10 mM) were used for the comparison to the mono-nitroalcohol, 2nprop. After 24 hours of reaction, the nitro-triol shifted the Ts (Ti and T50 = 10.4) to a greater extent than that which was observed with the nitro-diol. These findings support the concept proposed in the Swedo patent6 that 2nprop gives 1 mole of formaldehyde per molecule, the diol gives 2 moles, and the triol gives 3 moles. Finally, the reaction was conducted for an even shorter time period (i.e., 12 hours). Again, it was observed that the triol was superior to the diol after the 12-hour incubation. In addition, the Ts shifts were comparable to those observed after 24 hours, indicating that much of the reaction had already occurred within the first 12 hours (Table 1).
Table 1.
Ts Temperature Shifts Induced by Higher Order β-Nitroalcohols in Porcine Sclera
| Compound/Condition | Reagent Concentration and Time | Ti (°C) ± SE | TiΔ (°C) | T50 (°C) ± SE | T50Δ (°C) | P (for Ti) |
|---|---|---|---|---|---|---|
| 2nprop (mono nitroalcohol) | 10 mM 24 h | 62.6 ± 0.4 | 0.3 | 65.0 ± 0.3 | 0.4 | 0.383 |
| MNPD (nitro-diol) | 10 mM 24 h | 70.8 ± 0.0 | 8.5 | 72.5 ± 0.1 | 7.9 | <0.001 |
| 10 mM 12 h | 68.3 ± 0.6 | 6.0 | 72.1 ± 0.6 | 7.5 | <0.001 | |
| HNPD (nitro-triol) | 10 mM 24 h | 72.7 ± 0.5 | 10.4 | 75.0 ± 0.3 | 10.4 | <0.001 |
| 10 mM 12 h | 69.8 ± 0.2 | 7.5 | 75.8 ± 0.5 | 11.4 | <0.001 | |
| Control porcine sclera | 62.3 ± 0.2 | 64.6 ± 0.1 |
Ti, temperature at 1% absolute shrinkage; TiΔ, change in Ti as compared with control; T50, temperature at 50% of maximum shrinkage (or maximum rate of shrinkage change); T50Δ, change in T50 as compared with the control.
Discussion
In the present study, nitroalcohols functioned as formaldehyde donors under physiologic pH and temperature to cross-link collagenous tissue. This result was determined by the evolution of 2nprop by 1H-NMR, as well as by evaluating the cross-linking efficacy of two higher-order nitroalcohols, a nitro-diol and a nitro-triol. Nitroalcohols have been shown previously to function as formaldehyde donors under alkali conditions in a reverse Henry reaction. Hall11 studied the dissociation of 2,2-dinitropropanol in aqueous buffers at pH 6.2 and at 10°C, 25°C, and 40°C. His kinetic data suggested that there is a rapid ionization of the carbinol hydroxyl group followed by a slow dissociation to give formaldehyde and the nitroalkane. Consistent with these findings was a report by Guanti et al.12 who performed a kinetic study on the decomposition of β-nitro alcohols containing an aromatic function at the hydroxyl-containing carbon. The investigators studied the decomposition at 25°C in a pH range of 6.5 to 8.4. Their findings supported the findings by Hall with the dinitro-carbinols, that there is an initial fast deionization of the hydroxyl proton, followed by a rate-limiting C-C bond cleavage to give the aldehyde and nitroalkane. In his patent application from 2003 related to industrial use of formaldehyde donating compounds, Swedo6 includes a table of “formaldehyde equivalents” representing the theoretical number of moles per pound of formaldehyde available using various compounds. The table includes three nitroalcohols, a mono-nitroalcohol, a nitro-diol, and a nitro-triol. The level of formaldehyde available reflects the concept that 1, 2, and 3 moles of formaldehyde are released per molecule, respectively. Our shrinkage temperature data comparing the mono nitroalcohol, nitro-diol, and nitro-triol are in agreement with such a concept. That is, the shrinkage temperature shifts were progressively greater using the higher order nitroalcohols.
The issue of formaldehyde release, as it pertains to toxicity and safety matters requires particular discussion. There is a significant discrepancy between the toxicity/mutagenicity of formaldehyde, per se, and formaldehyde donating compounds, such as the nitroalcohol class of compounds. Formaldehyde is a well-known compound that has widespread applications. As such, the potential for toxicity and carcinogenicity to humans through various exposure routes has been of significant interest to governmental regulatory agencies.13 Formaldehyde is mutagenic to mammalian and bacterial cells and is carcinogenic in animal models. Although there is longstanding controversy regarding the carcinogenic potential in humans, the International Agency for Research on Cancer has recently changed its designation of formaldehyde from a class 2A (probably carcinogenic to humans) to class 1 (carcinogenic to humans).14 In stark contrast to formaldehyde, mono-nitroalcohols have a favorable safety profile, making them particularly attractive for in vivo use. The oral LD50 of 2nprop to chicks was reported to be >1300 mg/kg body weight15 and has been fed to cattle without any apparent adverse effects.16 When studied using the Ames mutagenicity assay,17 neither 2nprop nor 3n2pent showed mutagenicity in three strains of Salmonella. The nitro-triol HNPD has been studied extensively due to its widespread industrial usage. It has been placed in toxicity category III for acute oral, dermal, and inhalation effects (category I is highest and category IV is the lowest) and is not mutagenic under a battery of tests.4,18 Table 2 is included to provide a general appreciation of the differences in in vivo toxicity between BNAs and formaldehyde. Unfortunately, most formaldehyde toxicity data are related to inhalational exposure, for which no BNA data are available. However, oral and intraperitoneal toxicity data are available for both formaldehyde and BNAs in mice and underscore the dramatic differences that exist between BNAs and formaldehyde regarding toxicity. As shown in Table 2, the difference in LD50 between formaldehyde and several BNAs for mice by either intraperitoneal injection or oral exposure is approximately 100×. That being said, although BNAs are clearly less toxic than formaldehyde when administered systemically, it remains to be determined whether these agents will ultimately be proven safe for ocular applications. The reason for the difference in safety between formaldehyde and nitroalcohols (as formaldehyde donors) is not clear based on a review of the existing literature. However, this difference presents a major advantage to using nitroalcohols for in vivo clinical uses. It may be that the slow, controlled release of formaldehyde from BNAs allows for rapid consumption by protein reactions in the local extracellular milieu. This prevents the accumulation of unreacted formaldehyde while maintaining low, effective concentrations. In this way, untoward cellular effects, such as that which occurs when using formaldehyde directly may be avoided.
Table 2.
Comparison of Toxicity of BNAs to Formaldehyde
| LD50 Mice by Intraperitoneal Injection | LD50 Mice by Oral Ingestion | |
|---|---|---|
| Formaldehyde | 16 mg/kg (Ref. 19) | 42 mg/kg (Ref. 20) |
| 2-ne (mono-nitroalcohol) | 2100 mg/kg (1779–2478) (Ref. 21) | NA |
| 2nprop (mono-nitroalcohol) | NA | NA |
| MNPD (nitro-diol) | 1600 mg/kg (1103–2320) (Ref. 21) | 4000 mg/kg (Ref. 4) |
| HNPD (nitro-triol) | 4000 mg/kg (Ref. 21) | 1900 mg/kg (Ref. 4) |
NA, data not available.
Based on the results of the present study, it is unclear as to the significance of the nitrite release to the cross-linking reaction. Free nitrite can induce non-enzymatic covalent cross-linking in collagen, albeit very slowly and at much higher concentrations.22 Under the conditions described in this study, free nitrite (as sodium nitrite) does not induce a shift in Ts temperature. Thus, it is unlikely to have contributed significantly to the cross-linking effect, although the possibility of a catalytic effect has yet to be explored in depth. From a chemical mechanisms perspective, there are a few possibilities to explain the release of nitrite. First, a false-positive Griess test (limit of detection = 0.1 μM) was considered. However, we tested the assay by using formaldehyde and found no signal (i.e., color change) arguing against the possibility of a false-positive test. Another possibility is related to the products of the reverse Henry reaction. The normal scenario is that formaldehyde is released from the 2nprop, leaving behind a nitroalkane salt. It could be that the nitroalkane salt is then releasing nitrite. However, this possibility was tested by examining levels of nitrite released from 2-nitroethane, a product from the 2nprop retro-nitroaldol reaction (reverse Henry). In this case, under the same conditions, 2-nitroethane did not release free nitrite. Thus, this pathway is unlikely to be involved. Finally, we can speculate that the reactions that result in the denitration are competing with formaldehyde release, since both occur only under alkaline conditions. Additional studies are needed to delineate the origins of nitrite release in these reactions.
We recently reported on Ts shifts induced by three mono-nitroalcohols, 2ne, 2nprop, and 3n2pent using both porcine cornea9 and sclera.8 In the corneal paper, we observed modest increases in Ts using a 96 hours incubation time. The shifts induced using 10 mM concentrations were 3.3°C, 2.9°C, and 3.8°C, for 2ne, 2nprop, and 3n2pent, respectively. Similarly, in scleral tissue the shifts induced using 10 mM concentrations for 96 hours were 4.3°C, 1.5°C, and 2.4°C, for 2ne, 2nprop, and 3n2pent, respectively. By comparison, the higher order nitroalcohols used in this study were found to induce cross-linking faster and to a greater extent, on a mole-for-mole basis that the mono-nitroalcohols. The nitro-diol shifted the Ts by 6.0 and 8.5°C at 12 and 24 hours, respectively, and the nitro-triol shifted the Ts by 7.5 and 10.4°C at 12 and 24 hours, respectively. No shift was observed for 2nprop after 24 hours. The significantly faster and improved efficacy shown in this study (compared with the effects reported previously using mono-nitroalcohols) using the higher order nitroalcohols increases the likelihood that a topical pharmacologic corneoscleral cross-linking approach will be possible.
In summary, nitroalcohols act as both formaldehyde and nitrite donors under physiologic pH and temperature to induce tissue cross-linking. Higher order nitroalcohols are more effective in vitro than previously studied β-nitro alcohols raising the possibility of using these agents for therapeutic corneoscleral cross-linking. Studies using live rabbit eyes are under way and will ultimately determine their therapeutic value.
Footnotes
Supported in part by Research to Prevent Blindness, National Institutes of Health/National Center for Research Resources Grant UL1RR024156, and National Institutes of Health/National Eye Institute Grant R21EY018937 (DCP). Statistical assistance was provided by the Biostatistics Consulting Service in the Department of Biostatistics of the Mailman School of Public Health at Columbia University Medical Center.
Disclosure: D.C. Paik, None; M.R. Solomon, None; Q. Wen; None; N.J. Turro, None; S.L. Trokel, None
References
- 1.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Cur Opin Ophthalmol 2006; 17: 356–360 [DOI] [PubMed] [Google Scholar]
- 2.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007; 26: 385–389 [DOI] [PubMed] [Google Scholar]
- 3.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg 2006; 32: 279–283 [DOI] [PubMed] [Google Scholar]
- 4.Bollmeier AF. Nitroalcohols. Kirk-Othmer Encyclopedia of Chemical Technology New York: John Wiley & Sons, Inc., 1996 [Google Scholar]
- 5.Shvekhgeimer MGA. Aliphatic nitro alcohols: synthesis, chemical transformations and applications. Russian Chem Rev 1998; 67(1): 35–68 [Google Scholar]
- 6.Swedo RJ. inventor; Dow Chemical, assignee Phenolic resin systems for fiber reinforced composite manufacture. U.S. Patent 10,383,272, March 7, 2003 [Google Scholar]
- 7.Heck HD, Casanova M, Starr TB. Formaldehyde toxicity: new understanding (review). Crit Rev Toxicol 1990; 20(6): 397–426 [DOI] [PubMed] [Google Scholar]
- 8.Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Aliphatic β-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res 2008; 87: 279–285 [DOI] [PubMed] [Google Scholar]
- 9.Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Initial studies using aliphatic β-nitro alcohols for therapeutic corneal cross-linking. Invest Ophthalmol Vis Sci 2009; 50: 1098–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balazy M, Iesake T, Park JL, Jiang H, Kaminski PM, Wolin MS. Vicinal nitrohydroxyeicosatrienoic acids: vasodilator lipids formed by reaction of nitrogen dioxide with arachidonic acid. J Pharm Exp Therap 2001; 299(2): 611–619 [PubMed] [Google Scholar]
- 11.Hall TN. Reaction of nitro alcohols. II. The kinetics of dissociation of 2,2,-dinitropropanol in aqueous buffers. J Org Chem 1965; 30(9): 3157–3160 [Google Scholar]
- 12.Guanti G, Petrillo G, Thea S, Cevasco G. Carbonyl-forming elimination reactions: a kinetic study of the decomposition of β-nitroalcohols in water. Tetrahedron Lett 1980; 21: 4735–4738 [Google Scholar]
- 13.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Formaldehyde Bethesda, MD: U.S. Department of Health and Human Services Public Health Service, July1999 [PubMed] [Google Scholar]
- 14.Hauptmann M. Epidemiological studies of formaldehyde and cancer: recent results and their interpretation. Occup Environ Med 2004; 61(e17): M1.1 [Google Scholar]
- 15.Jung YS, Anderson RC Edrington TS, et al. Experimental use of 2-nitro-1-propanol for reduction of Salmonella typhimurium in the ceca of broiler chicks. J Food Prot 2004; 67: 1945–1947 [DOI] [PubMed] [Google Scholar]
- 16.Majak W. Further enhancement of nitropropanol detoxification by ruminal bacteria in cattle. Can J Anim Sci 1992; 72: 863–870 [Google Scholar]
- 17.Conaway CC, Hussain NS, Way BM, Fiala ES. Evaluation of secondary nitroalkanes, nitrocarbinols, and other aliphatic nitro compounds in the Ames Salmonella assay. Mut Res 1991; 261(3): 197–207 [DOI] [PubMed] [Google Scholar]
- 18.R.E.D. Facts. Tris(hydroxymethyl)-nitromethane. Washington, DC: U.S. Environmental Protection Agency, Publication EPA-738-F-93-016, September1993 [Google Scholar]
- 19.Bingham E, Cohrssen B, Powell CH. Patty's Toxicology Vol. 5 5th ed.New York: John Wiley & Sons; 2001: 967 [Google Scholar]
- 20.Lewis RJ, Sr, ed. Sax's Dangerous Properties of Industrial Materials 11th ed.Hoboken, NJ: Wiley-Interscience, Wiley & Sons, Inc.2004; 1814 [Google Scholar]
- 21.Fridman AL, Kremleva OB, Zalesov VS, et al. Synthesis and physiological activity of aliphatic nitro compounds. XII. Relationship between structure, toxicity, and bacteriostatic activity in a series of β-nitroalcohols (in Russian). Khimiko-Farmatsevticheskii Zhurnal 1977; 11(1): 73–75 [Google Scholar]
- 22.Paik DC, Saito LY, Sugirtharaj DD, Holmes JW. Nitrite induced cross-linking alters remodeling and mechanical properties of collagenous engineered tissues. Connect Tis Res 2006; 47: 163–176 [DOI] [PubMed] [Google Scholar]