Abstract
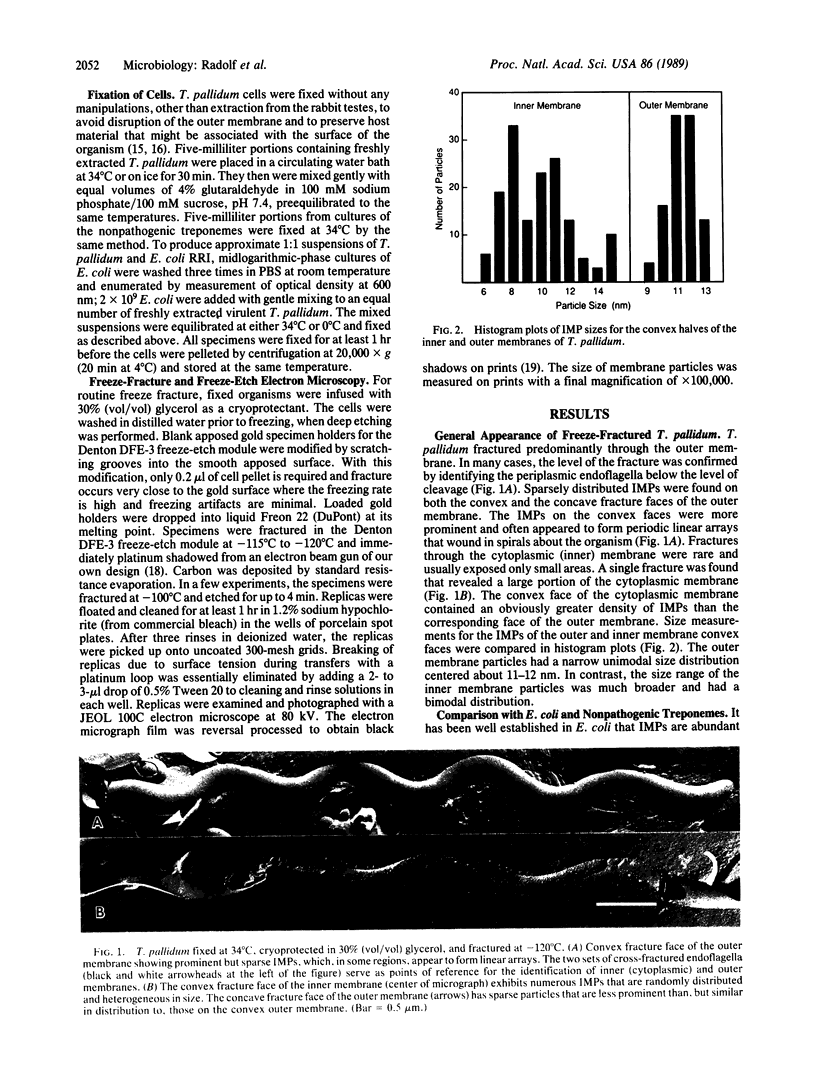
Freeze fracture and deep etching were used to investigate the ultrastructural basis for the observation that anti-treponemal antibodies bind poorly to the surface of virulent Treponema pallidum. Fractures of T. pallidum outer membranes contained scarce, uniformly sized intramembranous particles (IMPs). IMPs on the convex faces often appeared to form linear arrays that wound in spirals about the organism. In contrast to the outer membrane, IMPs of the cytoplasmic membrane were randomly distributed, numerous, and heterogeneous in size. In Escherichia coli and T. pallidum cofractures, IMPs of the E. coli outer membranes were densely packed within the concave fracture faces, while the T. pallidum fractures were identical to the experiments lacking the E. coli internal controls. Outer membranes of two representative nonpathogenic treponemes, Treponema phagedenis biotype Reiter and Treponema denticola, contained numerous IMPs, which segregated preferentially with the concave halves. Examination of apposed replicas and deep-etched specimens indicated that at least some of the IMPs extend through the T. pallidum outer membrane and are exposed on the surface of the organism. The outer membrane of intact T. pallidum appears to contain a paucity of integral membrane proteins that can serve as targets for specific antibodies. These findings appear to represent an unusual parasitic strategy for evasion of host humoral defenses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Koplow J., Goldfine H. Alterations in envelope structure of heptose-deficient mutants of Escherichia coli as revealed by freeze-etching. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5145–5149. doi: 10.1073/pnas.72.12.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovind-Hougen K., Birch-Andersen A., Nielsen H. A. Electron microscopy of treponemes subjected to the Treponema pallidum immobilization (TPI) test. II. Immunoelectron microscopy. Acta Pathol Microbiol Scand C. 1979 Aug;87C(4):263–268. [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier L., Moudden H., Shechter E. Lipid and protein segregation in Escherichia coli membrane: morphological and structural study of different cytoplasmic membrane fractions. Proc Natl Acad Sci U S A. 1977 Feb;74(2):452–456. doi: 10.1073/pnas.74.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985 Jan;134(1):585–592. [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMPSON F. A., Jr, McLEOD C. P. Relationship between treponemal immobilizing antibodies and acquired immunity in experimental syphilis. J Immunol. 1951 Jul;67(1):41–48. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- NELSON R. A., Jr, DIESENDRUCK J. A., ZHEUTLIN H. E. C., STACK P. S., BARNETT M. Studies on treponemal immobilizing antibodies in syphilis. I. Techniques of measurement and factors influencing immobilization. J Immunol. 1951 Jun;66(6):667–685. [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Cockayne A., Bailey M. J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985 Sep;131(9):2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Fehniger T. E., Silverblatt F. J., Miller J. N., Lovett M. A. The surface of virulent Treponema pallidum: resistance to antibody binding in the absence of complement and surface association of recombinant antigen 4D. Infect Immun. 1986 May;52(2):579–585. doi: 10.1128/iai.52.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect Immun. 1988 Jul;56(7):1825–1828. doi: 10.1128/iai.56.7.1825-1828.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz W. W., Reynolds R. C. Enhancement of three-dimensional appearance of freeze-fracture images by reversal processing of electron microscopy sheet film. J Microsc. 1978 Mar;112(2):249–252. doi: 10.1111/j.1365-2818.1978.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Schwarz H., Sonntag I., Henning U. Mutational change of membrane architecture. Mutants of Escherichia coli K12 missing major proteins of the outer cell envelope membrane. Biochim Biophys Acta. 1976 Oct 19;448(3):474–491. doi: 10.1016/0005-2736(76)90301-1. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Robards A. W. Plastic deformation during freeze-cleavage: a review. J Microsc. 1977 May;110(1):1–25. doi: 10.1111/j.1365-2818.1977.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Bacterial virulence and pathogenesis: an overview. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S637–S646. doi: 10.1093/clinids/5.supplement_4.s637. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]






