This paper indicates hemoglobin expression and regulation in the inner retina and optic nerve head. By providing an intrinsic protective mechanism against hypoxic/oxidative injury, this oxygen-binding protein may have important implications in glaucomatous neurodegeneration.
Abstract
Purpose.
To determine expression, cellular distribution, and regulation of hemoglobin (Hb) in normal and glaucomatous tissues.
Methods.
Proteomic analysis of Hb expression was conducted on protein samples from ocular hypertensive and control rat eyes and human donor eyes with or without glaucoma. Proteomic findings were validated by quantitative (q)RT-PCR, Western blot analysis, immunohistochemistry, and the analysis of new Hb synthesis in culture. Hypoxic regulation of Hb expression was also studied in primary cultures of rat RGCs and macroglia and after transfer of the glia-conditioned medium to RGCs. The role of erythropoietin (EPO) signaling in Hb induction and cell survival was determined by applying recombinant (r)EPO treatment and performing EPO neutralization experiments by using soluble EPO receptor treatment of hypoxic cultures.
Results.
In vivo findings revealed Hb expression in the retina and optic nerve head macroglia and RGCs, suggesting an approximately two-fold upregulation in ocular hypertensive rat eyes and glaucomatous human donor eyes relative to the control eyes. In vitro findings collectively supported that hypoxia boosts glial Hb expression through hypoxia-inducible EPO signaling in an autocrine manner. Based on passive transfer experiments, hypoxia-induced production of glial EPO was also found to upregulate Hb expression in RGCs in a paracrine manner, thereby increasing the hypoxic survival of these neurons.
Conclusions.
Findings of this study provide new insights into tissue oxygen transport in the inner retina and optic nerve head through the regulated expression of Hb in macroglia and RGCs. Upregulation of Hb expression appears to be an intrinsic protective mechanism to facilitate cellular oxygenation and may also provide free radical scavenging.
Dysregulation of blood flow with subsequent tissue hypoxia, secondary to or independent from elevated intraocular pressure (IOP) in glaucoma, has been implicated as a component of the pathogenic mechanisms of optic nerve degeneration and retinal ganglion cell (RGC) loss. Many patients with glaucoma exhibit vascular abnormalities such as vasospasm, systemic hypotension, angiographic vascular perfusion defects, and alterations in blood flow parameters that may result in reduced vascular perfusion in the optic nerve head and retina.1–5 Besides these clinical findings, hypoxic tissue stress is evident in the glaucomatous optic nerve head and retina by increased expression of a hypoxia-induced transcription factor, hypoxia-inducible factor (HIF)-1α.6 HIF-1α is known to activate transcription of a wide variety of genes with products that increase oxygen delivery and represent an adaptive response to hypoxia.7 Of interest, the retinal regions exhibiting increased HIF-1α immunolabeling in some of the donor eyes with glaucoma have been found to exhibit a close concordance with the location of visual field defects recorded in these eyes.6
It has long been known that the vertebrate retina consumes large amounts of oxygen and that the main oxygen consumption in the inner retina takes place in RGCs.8 However, although the maintenance of an adequate oxygen supply is critical for neuronal viability and function, current understanding of RGC oxygenation is incomplete. The oxygen demand of RGCs is supplied mainly by retinal arteries in the retina and short posterior ciliary arteries in the optic nerve head. Despite a heterogeneity in vascular response to increased IOP, oxygen tension in the inner retina is relatively unaffected by IOP changes, owing to effective autoregulation of retinal circulation.8–10 Similarly, optic nerve head perfusion pressure is adequately compensated by vascular autoregulation under normal conditions.11 However, in glaucomatous eyes, several risk factors compromising the autoregulatory control have been proposed to reduce blood flow, particularly in intermittent episodes affected by circadian variations in IOP, systemic blood pressure, and ocular perfusion pressure.1–5 In addition to these vascular factors, another important aspect of tissue oxygen delivery that is not well understood is extravascular oxygen transport. Although tissue perfusion is known to involve gas diffusion depending on the oxygen tension gradient and diffusion distance, it is unclear whether there is a facilitated mechanism for oxygen transport to RGCs from retinal capillaries, which are known to be surrounded by glial cells. This mechanism may be particularly important in glaucomatous conditions in which glial cells are in an activated state and may require increased oxygen consumption.12 Regarding the optic nerve head, the load-bearing connective tissue encasing the lamina cribrosa vasculature possibly makes RGC axons even more dependable for a facilitated oxygen transport. Besides a limited understanding of tissue oxygen transport within the retina and optic nerve head tissues, mechanisms controlling intracellular oxygen transport to mitochondria for oxidative phosphorylation also remain unclear.
Hemoglobin (Hb) is a more than 600 million-year-old respiratory protein that reversibly binds oxygen and mediates oxygen transport function in blood erythrocytes.13 Repeated detection of Hb in our proteomic studies and recent discovery of Hb expression in the retinal pigment epithelium14 stimulated us to determine whether this hemeprotein may also be present in the inner retina and optic nerve head and whether the oxygen transport function of Hb may be involved in RGC oxygenation in normal or glaucomatous eyes. To answer these questions, we performed a series of experiments determining expression and cellular localization of Hb in animal tissues as well as in glaucomatous and nonglaucomatous human donor eyes, with particular attention to RGCs and macroglia. To determine whether this protein is regulated by ambient oxygen concentrations, we also performed in vitro experiments with primary cultures of rat RGCs and macroglia incubated in the absence and presence of hypoxia. Herein, we present new evidence that Hb is expressed by these cell types as repeatedly detected in the retinal proteome and exhibits an upregulation in ocular hypertensive rat eyes and glaucomatous human donor eyes. Our in vitro findings revealed that hypoxia boosts glial Hb expression through glial erythropoietin (EPO), a well-known target of hypoxic HIF-1α signaling. In addition to this autocrine loop, hypoxia also upregulates Hb expression in RGCs in a paracrine manner. These findings support that in addition to tissue gas diffusion, Hb is likely to be involved in tissue oxygen transport in the inner retina and optic nerve head and that, besides vascular autoregulation, this oxygen-binding protein may provide an intrinsic mechanism for regulation of cellular oxygenation. Based on other predicted functions of Hb in free radical scavenging and nitric oxide detoxification, such an intrinsic protective mechanism against hypoxic/oxidative injury may have important implications in glaucomatous neurodegeneration.
Materials and Methods
Experimental Design
Hb expression was determined in different groups of naïve and ocular hypertensive rats (Harlan Sprague-Dawley, Indianapolis, IN) with proteomic analysis techniques. All animals were handled according to the regulations of the Institutional Animal Care and Use Committee, and all procedures adhered to the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Proteomic findings were further validated by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and quantitative Western blot analyses with mRNA and protein samples obtained from the whole retina and enriched cellular fractions, and immunohistochemical analysis determined cellular localization of Hb expression. Enriched RGC mRNA and protein samples were obtained from RGCs selected through the two-step immunomagnetic selection process described later in the article.15 The unselected fraction of retinal cells through this process, which contains macroglial cells, was also used for mRNA and protein sampling.
In addition to rat tissues, proteomic analysis was conducted on retina samples obtained from human donor eyes with or without glaucoma. Cellular localization of Hb was also studied in histologic sections of the retina and optic nerve head obtained from an additional group of glaucomatous and nonglaucomatous human donors. All the human donor eyes were handled according to the tenets of the Declaration of Helsinki.
To determine hypoxic regulation of Hb, in vitro experiments were performed by incubating primary cultures of rat RGCs and macroglia in the presence and absence of hypoxia for up to 48 hours. To determine the role of EPO signaling in Hb regulation and cellular survival, additional culture plates were treated with rat recombinant (r)EPO (R&D Systems, Minneapolis, MN). In addition, EPO neutralization experiments were performed with a recombinant human EPO receptor (EPO-R; R&D Systems).
Experimental Rat Glaucoma Model
Similar to previous studies,16,17 IOP elevation was induced in Brown Norway rats by hypertonic saline injections (∼0.1 mL of 1.75 M) into episcleral veins as originally described by Morrison's group.18 The injection was repeated 1 week later if no IOP elevation was detectable. Baseline IOP was obtained before the first saline injection, and the measurements were repeated twice a week with a calibrated tonometer (Tonopen; Medtronic Solan, Jacksonville, FL). Cumulative IOP exposure was estimated by calculating the area under the pressure–time curve in the ocular hypertensive eye, and then subtracting this IOP-time integral from that in the normotensive fellow eye (expressed in units of mm Hg-days). Neuronal damage was determined by comparing the axon count in the ocular hypertensive eye relative to the control fellow eye, as previously described.16,17 RGC mRNA and protein samples were collected by pooling from ocular hypertensive rat eyes matched for cumulative IOP exposure. In this study, we specifically used samples obtained from moderately damaged eyes with a cumulative IOP exposure of 200 to 400 mm Hg-days, which corresponds to a relative axon loss of no more than 50%.16
Human Donor Eyes
Thirty-eight donor eyes with a diagnosis of glaucoma (age, 76.8 ± 11 years) and 30 eyes from donors without glaucoma (age, 71.0 ± 15 years) were used for immunohistochemical analysis. All these donor eyes were fixed within 12 hours after death and processed for 5-μm paraffin-embedded sagittal tissue sections. Detailed information on the donor demographics and clinical data of glaucomatous donor eyes has recently been published.19
Additional human samples that were used in proteomic analyses were obtained from five human donors with glaucoma (age, 88.0 ± 10 years) and five without (age, 86.2 ± 8 years). After retinal dissection under the microscope, 6-mm trephine punches were taken from each quadrant of the retina in a standardized manner and immediately frozen in liquid nitrogen. All these samples were collected within less than 6 hours after death (average postmortem time, 4 hours, 52 minutes for glaucoma; 4 hours 40 minutes for controls). Diagnosis of glaucoma in these donors was well-documented by IOP readings, optic disc assessments, and visual field tests, and control donors had no history of eye disease. The cause of death for all human donors included in this study was acute myocardial infarction or cardiopulmonary failure.
Cell Cultures
Primary cultures of RGCs and macroglia were derived from adult rats, as previously described.15,17,20–22 Briefly, retinas were dissociated in Eagle's minimum essential medium containing 20 U/mL papain, 1 mM L-cysteine, 0.5 mM EDTA, and 0.005% DNase (Worthington, Lakewood, NJ). Immunomagnetic selection of Thy-1.1-positive RGCs was performed with antibody-coated magnetic beads (Dynal, Oslo, Norway) in a two-step process. In the first step, an antibody to macrophage/microglia surface antigens was used. In the second step, the macrophage/microglia-depleted cell suspension was incubated with magnetic beads bound to a monoclonal antibody specific to Thy-1.1 (Millipore/Chemicon, Billerica, MA). Selected cells were seeded on extracellular matrix–coated plates (BD Biosciences, Bedford, MA) and incubated in a serum-free culture medium, as previously described.15,17,20–22 RGCs in these cultures were identified on the basis of retrograde labeling, cell morphology, and the expression of cell markers.15
Cultures of retinal macroglia were prepared with retinal cells depleted for macrophage/microglia and RGCs after the immunomagnetic selection process. After loss of residual neuronal cells by two or three cycles of replating, these cultures contain macroglial cells, including astrocytes and Müller, as previously documented.15
Cultures of optic nerve head astrocytes were also prepared as previously described.22–24 Briefly, after the postlaminar myelinated nerve was removed, the remaining optic nerve head tissue was bisected and the central vessels were removed under a dissecting microscope. The tissues were then cut into small pieces and placed in extracellular matrix–coated flasks, to allow the cells to grow out of the explants in the presence of growth medium (DMEM/F-12 supplemented with 10% serum and antibiotics). Flow cytometric analysis had shown that more than 98% of these cells were glial fibrillary acidic protein (GFAP)–positive astrocytes.24 During the experimental period, cultures of macroglial cells were incubated in a serum-free medium containing DMEM, 1.3% bovine albumin fraction V, 1 μL/mL culture supplement (ITS+ Premix; BD Biosciences), and antibiotics.
Hypoxia was maintained by placing the plates in a dedicated culture incubator with a controlled flow of N2 and CO2 at a setting of 5% CO2 and 5% O2. Control cultures prepared from an identical passage of cells were simultaneously incubated in a regular tissue culture incubator with 95% air/5% CO2 at 37°C.
Cell viability was determined by using a kit containing calcein AM (Live/Dead kit; Molecular Probes, Eugene, OR), as previously described.15,20,22 The survival rate was expressed as the percentage of the total cell number in control wells at each time point.
Because of a relatively limited yield of cell isolation procedures and limited survival rate of RGCs in culture, the number of these postmitotic neurons is not as high as glial cells with mitotic ability. To prevent variations due to different culture preparations, pooled protein samples were used in biochemical analyses. All in vitro experiments were repeated at least four times for each experimental condition, using new samples. Data are presented as the mean ± SD.
Tissue Sampling and Protein Lysation
Retinal tissue punches obtained from human donor eyes were directly subjected to protein lysation. Regarding rat retinal protein samples, after the anterior segments were removed from enucleated rat eyes, the retinas were mechanically dissected under a microscope. In addition to whole retinal protein samples, enriched fractions of retinal cells obtained through the two-step immunomagnetic selection process just described15 were also used for protein sampling. All rats were subjected to transcardiac perfusion to wash the blood out of the blood circulation in these animals. If tissue sampling was for proteomic analysis, only phosphate-buffered saline perfusion was applied, to avoid affecting protein lysation, as opposed to transcardiac perfusion with phosphate-buffered saline followed by paraformaldehyde, as applied in animals with tissues that were used in immunohistochemistry.
Protein lysation used a lysis buffer containing 50 mM HEPES-KOH pH 8.0, 100 mM KCl, 2 mM EDTA, 0.10% NP-40, 2 mM dithiothreitol, 10% glycerol, and protease and phosphatase inhibitors, as previously described.16,17
Mass Spectrometric Analysis
2D-PAGE was performed as previously described.16,17 Gels were stained with a protein gel stain (SYPRO Ruby; Molecular Probes) and excised protein spots were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Peptide sequencing from tryptic digests of the gel pieces and bioinformatic analysis used the previously described criteria.16,17
Protein mixtures were also analyzed through a gel-free LC-MS/MS approach.17,25 The acquired MS/MS spectra were searched against a human protein database (Human RefSeq; www.ncbi.nlm.nih.gov/locuslink/refseq/ provided in the public domain by the National Center for Bioinformatics, Bethesda MD) with a commercial protein identification algorithm (SEQUEST; Sequest Sorcerer; Sage-N Research, Inc., San Jose, CA). Data processing of the output files into a list of proteins has been previously described.25,26 High-probability peptide and protein identifications were assigned from the results using cross-correlation (Xcorr) score cutoffs of 1.5, 2, and 2.5 for +1, +2, and +3 respective charge states and submitted to an in-house informatics platform for comparative quantitative analysis.27 The abundance of each identified protein was determined by normalizing the number of unique spectral counts matching to the protein by its predicted molecular weight. This value has been termed the protein abundance factor (PAF).26,28
As a complementary approach for relative quantification, oxygen isotope labeling was performed with a protocol similar to that previously described.29 Briefly, protein lysates were desalted and dried after digestion with trypsin as described above. Trypsin-catalyzed 16O-to-18O exchange/labeling was performed with a 1:20 enzyme-to-peptide ratio. Equal amounts of samples were dissolved in a solution containing 25 μL 50 mM Tris buffer (pH 7.8), 5 mM CaCl2, and 0.5 μg trypsin prepared either in normal or 18O-labeled water. After their incubation at 37°C for 24 hours, samples were heated to 100°C for 10 minutes and then acidified with 25 μL 5% formic acid. 18O- or 16O-labeled samples were then mixed, desalted on C18 spin columns, and fractionated by using HPLC cartridges (SCX; Waters, Milford, MA) for subsequent mass spectrometric analysis, as just described. Relative abundances for differentially labeled peptides in samples were calculated from their monoisotopic peaks and reported as heavy-to-light 18O/16O ratios for a particular peptide/protein.
Quantitative RT-PCR
Expression of α- and β-globin genes was determined by using protocols similar to those previously described.21 Briefly, mRNA was extracted (TRIzol kit; Invitrogen, Carlsbad, CA), and any possible DNA contamination was removed by incubation with DNase I (Invitrogen). Oligo-dT-primed first-strand cDNA was synthesized (Superscript 3 First Strand Synthesis System; Invitrogen) with 1 μg of total RNA per reaction. SYBR green PCR amplification of cDNAs was performed with Taq DNA polymerase (Promega, Madison, WI, on an Mx3000P system; Stratagene, La Jolla, CA). PCR primers included rat α-globin 5′-AGAACTGCTGGGGGAAGATT-3′ and 5′-AGGCATCAGCAACCTTCTTG-3′ (GenBank accession number BC160818); rat β-globin 5′-GGAAAGGTGAACCCTGATGA-3′ and 5′-TGTTTCAGGCCATCATTGAA-3′ (GenBank accession number NM_033234.1); and rat β-actin as a control 5′-TGTCACCAACTGGGACGATA-3′ and 5′-GGGGTGTTGAAGGTCTCAAA-3′ (GenBank accession number NM_031144). In addition, primer sets of rat GFAP 5′-AGGATGACCCTCGTGTATCG-3′ and 5′-CCCCTCTCGACTCATCCATA-3′ (GenBank accession number NM_017009) and Thy-1 5′-CTGGTCAAGTGTGGTGGCAT-3′ and 5′-ATGAAGTCCGTGGCTTGGAG-3′ (GenBank accession number NM_012673; http://www.ncbi.nlm.nih.gov/Genbank; provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD) were used to validate RGC and macroglia samples. Denaturation was started initially at 95°C for 4 minutes, followed by 40 amplification cycles of 20 seconds at 95°C to denature the DNA, and 20 seconds at 60°C and 20 seconds at 72°C for primer annealing and extension. In addition to control reactions with primers for β-actin, negative control reactions for each primer pair were also performed without reverse transcriptase and with water instead of the cDNA template. All reactions were performed in triplicate and repeated at least three times with different samples. Cycle threshold numbers (Ct values) were obtained by computer (MxPro-Mx3000P; Stratagene); dissociation curve analysis was performed for each reaction, to ensure amplification of the specific product; and serial dilution curves were obtained to verify amplification efficiencies (R2 > 0.990). The relative expression was determined by normalizing the Ct values to β-actin with the 2ΔΔCt method.30
Quantitative Western Blot Analysis
Immunoblot analysis was performed as previously described.16,17 Primary antibodies used included specific antibodies against Hb. We used Hb antibodies from different sources (AbD; Serotec, Raleigh, NC; Bethyl Laboratories, Montgomery, TX; Novus Biologicals, Littleton, CO; and Santa Cruz Biotechnology [H-80], Santa Cruz, CA). However, the presented rat data only include those obtained with the AbD antibody (1:300; Serotec) for consistency. Additional antibodies used include those against EPO (N-19), EPO-R (H-194; 1:500; Santa Cruz Biotechnology), HIF-1α (1:500, H1α67; Novus Biologicals), or erythrocyte-specific proteins, including glycophorin A and B and blood group AB and H antigens (1:500; Abcam, Cambridge, UK). In addition, a β-actin antibody (Sigma-Aldrich, St. Louis, MO) was used to reprobe the stripped immunoblots for loading and transfer control. The secondary antibody incubation was performed with a specific IgG conjugated with horseradish peroxidase (1:2000; Sigma-Aldrich). The primary antibody was omitted to serve as a control. At least four immunoblots were obtained by using new protein samples, and protein bands were analyzed (Axiovision imaging system; Carl Zeiss, Thornwood, NY). After normalization to β-actin, average values obtained from ocular hypertensive and control samples were used to calculate the change in protein expression.
Detection of New Protein Synthesis
An alkyne protein analysis detection kit (Click-iT TAMRA; Invitrogen) was used to determine new protein synthesis in cultured cells. An amino acid analogue of methionine containing azido moiety (Click-iT l-aziodohomoalanine; Invitrogen), was incorporated into newly synthesized proteins of cells cultured in a methionine-free culture medium. Hb antibody immunoprecipitation of the lysed protein samples was performed on spin columns (Pierce, Rockford, IL), as previously described.17 Azide-labeling in the eluted fraction was then detected by SDS/PAGE with a tetramethylrhodamine alkyne detection kit (Click-iT; Invitrogen) that utilizes the click reaction between azide and alkyne with a detection sensitivity of 0.5 to 3 femtomoles.
Enzyme-Linked Immunosorbent Assay
Specific kits were used to measure Hb (Immmunology Consultants Laboratory, Newberg, OR) and EPO (R&D Systems) levels in the conditioned culture medium by the quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique as previously described.22
Immunohistochemical Analysis
To determine the extent and cellular localization of Hb, immunoperoxidase labeling and double immunofluorescence labeling were performed in histologic sections from rat and human eyes. All procedures were similar to those that have been published.6,17,19 Primary antibodies were the same as described for Western blot analysis. In addition to these primary antibodies, rabbit antibodies against Brn-3 (C-13) or GFAP (1:200; Santa Cruz Biotechnology) were used to identify RGCs and macroglia during double-immunofluorescence labeling, respectively. A biotinylated IgG (1:400; Millipore/Chemicon) was used as the secondary antibody for immunoperoxidase labeling and hematoxylin for counterstaining. For double immunofluorescence labeling, a mixture of Alexa Fluor 488- or 568-conjugated species-specific IgGs (1:400; Molecular Probes) was used for secondary antibody incubation. Negative controls were performed by replacing the primary antibody with serum. In addition, slides used for double-immunofluorescence labeling were incubated with each primary antibody followed by an inappropriate secondary antibody to determine that each secondary antibody was specific to the species it was made against.
For each procedure, at least four histologic sections were used from each eye, including those obtained from the superior and inferior halves of the retina. All slides subjected to immunohistochemical analysis were masked for the identity and diagnosis of donors or the status of experimental rat eyes, and numbered by a technician unfamiliar with the retina and optic nerve head disease before their immunolabeling. To determine the extent of immunolabeling, it was quantitatively graded on digitized images in a masked fashion by measuring the specific immunolabeling areas (Axiovision imaging system; Carl Zeiss) as previously described.19 A percentage value was expressed for each slide as the average ratio of the measurement to the total area analyzed, multiplied by 100. The mean extent of immunolabeling was calculated for each eye after the value obtained from the negative control slide was subtracted.
Results
Hemoglobin Expression in the Retina and Optic Nerve Head Macroglia and Retinal Ganglion Cells in Rat and Human Eyes
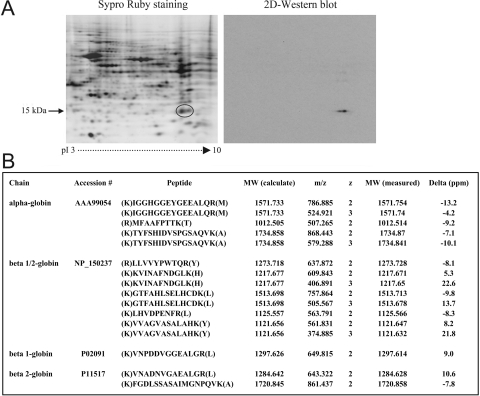
Proteomic analyses of rat retinal protein samples repeatedly detected Hb. This erythrocyte protein is not unexpected in the retinal proteome, because the retina contains blood. However, Hb was detectable after transcardiac perfusion of the blood circulation to wash the blood out of the circulation of these animals. As shown in Figure 1A, 2D-PAGE followed by mass spectrometric analysis revealed Hb components clustered at ∼15 kDa and a pI zone of 6.5 to 8.0. Within the Hb cluster, α (molecular mass, 15.9 kDa; pI 6.8) and β (molecular mass, 15.9 kDa; pI 6.7) side chains were identified by mass spectrometry. Similar to these findings of the 2D-PAGE-based proteomic analysis, a gel-free proteomic technique involving LC-MS/MS analysis of protein mixtures also identified Hb in the rat retinal proteome. Figure 1B summarizes the mass spectrometric data, which exhibited significant matches with the Hb side chains α and β. Although other rare Hb side chains, including γ and ε, were also repeatedly identified by mass spectrometry, they are not presented in the table, since the mass spectral data did not meet the criteria of more than two confirmed peptides. However, despite repeated detection of Hb in the rat retinal proteome, proteomic analyses did not detect any other erythrocyte markers in these samples. It should also be noted that our studies using proteomic analysis techniques similarly detected Hb expression in retinal protein samples obtained from mouse and monkey eyes; however, only the rat data are presented herein.
Figure 1.
Proteomic analysis of rat retinal Hb expression. (A) A 2D-gel obtained from a control rat retinal protein sample. Mass spectrometric analysis of the circled gel spot identified the Hb side chains, α and β. Consistent with the results of the 2D-PAGE-based proteomic analysis, a gel-free proteomic technique involving LC-MS/MS analysis of protein mixtures also identified Hb in the rat retinal proteome. (B) Summary of the mass spectrometric data. To further validate the proteomic data, Western blot analysis was performed with a specific Hb antibody. The 2D-Western blot shown in (A) demonstrates specific immunolabeling in a similar location as the indicated cluster of Hb side chains on the 2D-gel. Rat retinal protein samples were obtained after transcardiac perfusion to wash the blood out of the blood circulation. Data represent four independent experiments using different samples.
For further validation of the proteomic findings, additional experiments used qRT-PCR and Western blot analyses to determine retinal Hb expression. qRT-PCR analysis with mRNA samples obtained from enriched fractions of retinal cells supported the expression of α and β side chains of Hb (Fig. 2A). As shown in Figure 1A, 2D-Western blot analysis with a specific Hb antibody also revealed immunolabeling at similar locations with the indicated spots of Hb side chains on 2D-gels. Additional experiments incorporating Western blot analysis of mixed retinal protein samples or enriched fractions also supported Hb expression in the retina (Fig. 2B).
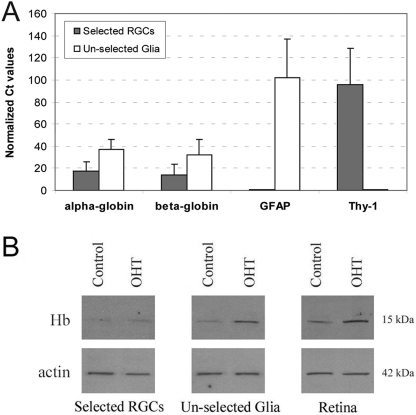
Figure 2.
Additional validation of rat retinal Hb expression by qRT-PCR and Western blot analyses. Whole retina or enriched cellular fractions were used for sampling. Enriched RGC mRNA and protein samples were obtained using RGCs selected from dissociated retinal cells through the two-step immunomagnetic selection process. Unselected fraction of retinal cells through this process, which contains macroglial cells, was also used for mRNA and protein sampling. (A) Summary of the results of qRT-PCR analysis using 40 amplification cycles, which support mRNA expression of Hb α and β side chains in the rat retina. All reactions were performed in triplicate and repeated three times using different samples. Presented are relative expression values (mean ± SD) calculated by normalizing the Ct values to β-actin using the 2ΔΔCt method. In addition to control reactions using primers for β-actin, the specific cell markers GFAP and Thy-1 were also tested to validate enriched cellular fractions in samples. The actin-normalized Ct values for GFAP in the selected RGCs fraction (0.51 ± 0.4) and Thy-1 in the unselected glial fraction (0.64 ± 0.3) were too small to be seen in the graph, indicating little or no gene expression. (B) Western blot analysis further supporting Hb expression in rat retinal protein samples obtained from ocular hypertensive (OHT) or control eyes. Immunoblots were obtained using four different samples, and after normalization to β-actin, average band intensities were compared between control and ocular hypertensive samples. This comparison detected a less than two-fold (1.96 ± 0.2) increased expression of Hb in ocular hypertensive retinas relative to control retinas (Mann-Whitney rank sum test, P = 0.054). Quantitative mass spectrometric analysis similarly detected an approximate two-fold increase in expression of Hb in ocular hypertensive samples (2.02 ± 0.3-fold; Mann-Whitney rank sum test, P = 0.006).
To provide a comparison between ocular hypertensive and control samples, quantitative Western blot and quantitative mass spectrometric analyses were performed. Quantitative analyses of protein expression detected an increase in the expression level of Hb in ocular hypertensive retinas relative to normotensive controls. However, this increase was not found to be more than two-fold by either quantitative Western blot analysis (1.96 ± 0.2; Mann-Whitney rank sum test, P = 0.054) or quantitative mass spectrometric analysis (2.02 ± 0.3; Mann-Whitney rank sum test, P = 0.006).
When histologic sections of rat eyes were analyzed, ocular hypertensive rat retinas also exhibited a greater extent of Hb immunolabeling compared with normotensive controls. Because the pattern of Hb immunolabeling was similar between rat and human tissues, only human findings are presented herein.
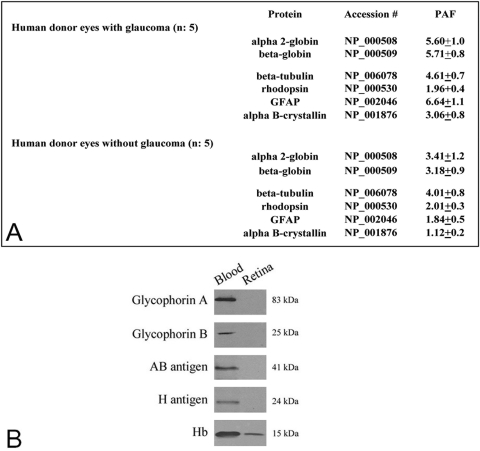
Consistent with the findings in rat samples, proteomic analysis of retinal protein samples obtained from human donor eyes with or without glaucoma repeatedly detected Hb expression. Figure 3A summarizes the findings of mass spectrometric analysis, which identified α and β side chains of Hb in the human retinal proteome. Other rare Hb side chains, including γ and ε, were identified in some of the samples. Similar to ocular hypertensive rats, retinal Hb expression in the glaucomatous human donors exhibited a less than two-fold (1.72 ± 0.9) increase relative to nonglaucomatous control eyes (Mann-Whitney rank sum test, P < 0.001).
Figure 3.
Hb expression in the human retina. (A) Summary of the findings of quantitative mass spectrometric analysis, which identified Hb α and β side chains in the human retinal proteome. Based on PAF values representing the protein abundance, retinal Hb expression exhibited a less than two-fold (1.72 ± 0.9) increase in the glaucomatous human donor eyes relative to nonglaucomatous control eyes (Mann-Whitney rank sum test, P < 0.001). In addition to Hb side chains, PAF values for additional proteins are presented as the control. There was prominent increase in the expression of GFAP (a glial activity marker) and αB-crystallin (a stress response protein), although the expression of β-tubulin (a microtubule protein) and rhodopsin (an outer neuronal marker) was constant in glaucomatous samples. (B) Western blot analysis of human blood or retinal protein samples using antibodies to different erythrocyte-specific proteins. Other than Hb, no retinal immunoreactivity was detectable for erythrocyte markers, including glycophorin A and B, and blood group AB and H antigens. Immunoblots were repeated four times with similar results.
Although Hb was repeatedly detected in the human retinal proteome, proteomic analyses did not detect any other erythrocyte markers in the human retinal protein samples. In addition, no immunoreactivity was detectable by Western blot analysis of retinal protein samples with antibodies to erythrocyte-specific proteins, such as glycophorin A and B and blood group AB and H antigens (Fig. 3B).
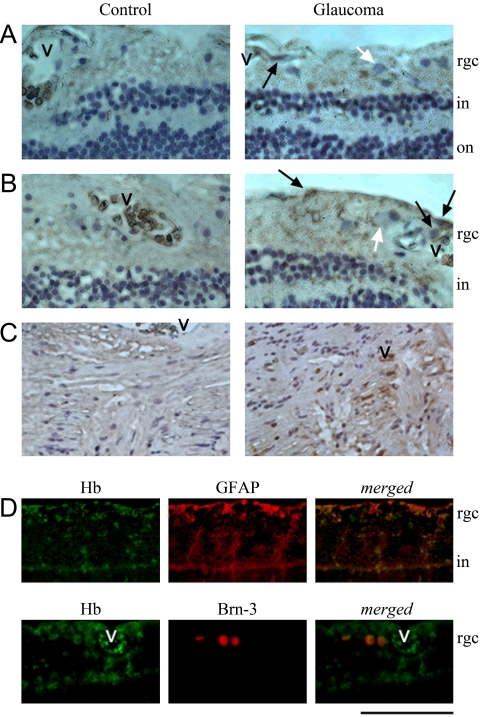
To determine the extent and cellular localization of Hb expression in the retina and optic nerve head, histologic sections of the retina and optic nerve head obtained from human donor eyes were also used in immunohistochemical analysis. Consistent with proteomic findings, histologic sections of the human retina and optic nerve head tissues exhibited immunolabeling with a specific Hb antibody (Fig. 4). In addition to erythrocytes within blood vessels, intracellular Hb immunolabeling was also detectable in the inner retina and optic nerve head cells. Based on morphologic features of cell types, Hb immunolabeling most prominently included retina and optic nerve head astrocytes, which are located close to vascular structures. In addition, some Müller cells, whose cell bodies are located in the inner nuclear layer and processes extend through the retina, exhibited Hb immunolabeling in glaucomatous eyes. Double immunofluorescence labeling supported the localization of Hb immunolabeling mainly in macroglial cells, although some RGCs also exhibited faint immunolabeling for Hb (Fig. 4D).
Figure 4.
Immunohistochemical analysis of the cellular localization of Hb expression in the human retina and optic nerve head. Consistent with proteomic findings, histologic sections of the retina and optic nerve head from human donor eyes exhibited immunolabeling with a specific Hb antibody. (A, B) Immunoperoxidase labeling of retina sections for Hb in glaucomatous donor eyes with moderate (A) or more advanced (B) damage and age-matched controls. (C) Immunoperoxidase labeling of optic nerve head sections in a glaucomatous donor with moderate damage and the age-matched control. The level of glaucomatous damage in these donor eyes was assessed based on the available clinical data, including optic disc assessments and visual field tests.19 An increase was detectable in the extent of Hb immunolabeling and the number of immunolabeled cells in most (31/38 eyes with glaucoma) but not all glaucomatous eyes, which also exhibited regional differences with an indistinct pattern. In addition to erythrocytes within blood vessels (v), intracellular Hb immunolabeling was most prominent in macroglial cells. Based on known morphologic features and retinal distributions of macroglial cell types, retinal Hb immunolabeling mainly corresponded to astrocytes (black arrow) characterized by smaller and oval shape nuclei and close localization to retinal vasculature in the RGC (white arrow) layer. In addition, some cells in the inner nuclear layer, likely corresponding to Müller cells, exhibited Hb immunolabeling. (C) Optic nerve head astrocytes also exhibited immunolabeling for Hb. (D) Double immunofluorescence labeling of the glaucomatous human retina using specific antibodies to Hb (green) and GFAP or Brn-3 (red) as marker for retinal macroglia or RGCs. In merged images, most of the Hb immunolabeling (yellow) was localized to GFAP-positive macroglial cells. However, some Brn-3-positive RGCs also exhibited Hb immunolabeling. rgc, retinal ganglion cell layer; in, inner nuclear layer; on, outer nuclear layer. Scale bar: (A, B) 100 μm; (C) 250 μm; (D) 150 μm.
Findings of tissue immunolabeling for Hb were also consistent with an upregulation of Hb expression in human donor eyes with glaucoma compared with nonglaucomatous control eyes (Figs. 4A–C). Although some macroglial cells and their processes exhibited faint immunolabeling for Hb in the control human retina and optic nerve head, an increase was detectable in the intensity of Hb immunolabeling and the number of immunolabeled cells in human donor eyes with glaucoma relative to control eyes without glaucoma. Based on digital image analysis, the extent of Hb immunolabeling was 12.16% ± 0.3% in the glaucomatous retina, but less than 5% in nonglaucomatous controls. However, it should also be noted that the increased Hb immunolabeling was more prominent in 31 of 38 glaucomatous donor eyes examined, and that Hb immunolabeling in these eyes exhibited regional differences, and not all RGCs or macroglia were prominently immunolabeled for Hb.
Thus, proteomic analysis, gene expression analysis, and immunohistochemical analysis collectively demonstrate Hb expression in the inner retina and optic nerve head, which exhibits a relative increase in ocular hypertensive rat eyes and glaucomatous human donor eyes.
Regulation of Hemoglobin Expression by Hypoxia-Inducible EPO Signaling
When primary cultures of rat RGCs and retina and optic nerve head macroglia were used in Western blot analysis, Hb expression was found to be prominently detectable in glial cells, while in vitro expression of Hb was subtle in RGCs (Fig. 5). In vitro expression of Hb, which persisted for serial passages of glial cell cultures, excludes the possibility of blood contamination of protein samples or phagocytosed erythrocytes by glia. An additional piece of in vitro evidence supporting the local synthesis of Hb comes from gel analysis of the Hb antibody immunoprecipitates obtained after methionine incorporation (Fig. 5A).
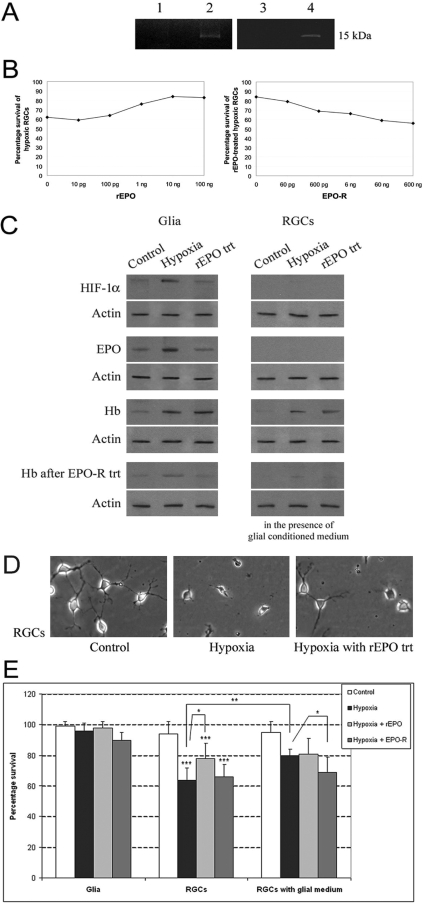
Figure 5.
In vitro experiments determining Hb expression and hypoxic regulation in primary cultures of rat retinal cells. (A) Hb antibody immunoprecipitates obtained after methionine incorporation into cellular proteins using the click reaction. Eluted proteins were separated by SDS/PAGE. Lanes 2 and 4: 15-kDa bands corresponding to newly synthesized Hb by retinal macroglial cells and ONH astrocytes, respectively, and thereby supporting local synthesis of Hb by these cells. Lanes 1 and 3: control immunoprecipitates of same samples obtained after replacing the Hb antibody with IgG. Experiments were repeated four times with similar results. The role of EPO signaling in Hb induction and cellular survival was determined by treating the cultured cells with rEPO (10 ng/mL) or EPO-R (60 ng/mL) based on the dose-effect curves shown in (B). (C) Western blot analysis of Hb expression in retinal cell cultures. Retinal macroglial cells exposed to hypoxia exhibited a prominent increase in Hb expression (3.64 ± 0.7-fold; Mann-Whitney rank sum test, P < 0.001). This hypoxia-induced upregulation of Hb in glial cells was found parallel to increased expression of HIF-1α and EPO in these cells. RGCs cultured alone did not express a significant amount of HIF-1α or EPO under normoxic condition or hypoxia. However, Hb expression of RGCs exhibited an upregulation after exposure to hypoxia in the presence of hypoxia-conditioned glial medium as well as after rEPO treatment (2.42 ± 0.6-fold; Mann-Whitney rank sum test, P < 0.029). Treatment with EPO-R resulted in a neutralization of these effects. Data are representative of four experiments with new protein samples. (D) Phase-contrast images of RGCs incubated under normal conditions or incubated under hypoxia in the absence or presence of rEPO treatment. Hypoxic RGCs exhibited characteristics of cell death, shrinkage of the cell body and dendrites, and cell debris. (E) Used level of hypoxia or any of the treatments did not prominently affect the glial cell survival. However, incubation of RGCs under hypoxia resulted in a prominent decrease (over 30%) in the number of surviving cells. Addition of the conditioned medium obtained from hypoxic glial cultures or treatment with rEPO provided an approximate 25% protection to these hypoxic RGCs (Mann-Whitney rank sum test: *P = 0.04; **P = 0.002; ***P < 0.001). Data represent results in four independent experiments and are presented as the mean ± SD.
To determine whether Hb expression is regulated by ambient oxygen concentrations, a series of in vitro experiments were performed by incubating cell cultures in the absence and presence of hypoxia. In vitro studies were also conducted to determine the role of EPO signaling in Hb induction and cell survival. Therefore, rEPO treatment was applied and EPO neutralization experiments were performed with EPO-R treatment of hypoxic cultures. In initial treatment experiments, we used increasing concentrations of rEPO to determine the optimal effective dose to improve RGC survival in our in vitro model. The treatment dose for EPO-R was then determined that would neutralize this effect. Based on the dose–effect curves shown in Figure 5B, 10 ng/mL rEPO and 60 ng/mL EPO-R doses were selected for the following in vitro treatment experiments.
Exposure of cell cultures to hypoxia resulted in a prominent increase in Hb expression relative to control cultures incubated under normal conditions. Glial cells exposed to hypoxia exhibited an approximately four-fold (3.64 ± 0.7) increase in Hb expression (Mann-Whitney rank sum test, P < 0.001). ELISA titers of Hb measured in the conditioned medium were similarly greater (increase, 2.2 ± 0.3-fold) in hypoxic cultures of glial cells relative to control cultures. This hypoxia-induced upregulation of Hb in glial cells was found to parallel the increased expression of HIF-1α and EPO in these cells (Fig. 5C). Conditioned medium collected from hypoxic glial cell cultures was also used to measure EPO levels by ELISA. Consistent with the findings of Western blot analysis, exposure of glial cells to hypoxia resulted in an approximately two-fold increase in EPO-ELISA titers in the culture medium.
RGCs cultured alone did not express a significant amount of HIF-1α or EPO under normoxic conditions or hypoxia; however, their Hb expression exhibited a more than two-fold increase (2.42 ± 0.6) after exposure to hypoxia in the presence of hypoxia-conditioned glial medium, as well as after rEPO treatment (Mann-Whitney rank sum test, P < 0.029). Treatment with EPO-R resulted in a neutralization of these effects (Fig. 5C). Phase-contrast images shown in Figure 5D exemplify morphologic characteristics of RGCs incubated under normal conditions or incubated in the presence of hypoxia with or without rEPO treatment.
These biochemical and morphologic findings were consistent with the surviving cell counts in cultures. The level of hypoxia or any of the treatments did not prominently affect the glial cell survival. However, incubation of RGCs under hypoxia resulted in a prominent decrease (over 30%) in the number of surviving cells (Mann-Whitney rank sum test; P < 0.001). Addition of the conditioned medium obtained from hypoxic glial cultures (P = 0.002) or treatment with rEPO (P = 0.04) provided an approximately 25% protection to these hypoxic RGCs (Fig. 5E). These in vitro findings support that parallel to increased glial expression of HIF-1α, hypoxia boosts Hb expression through a well-known target of the HIF-1α signaling, EPO. In addition to this autocrine upregulation in glia, hypoxia also upregulates Hb expression in RGCs through EPO signaling in a paracrine manner, thereby increasing RGC survival.
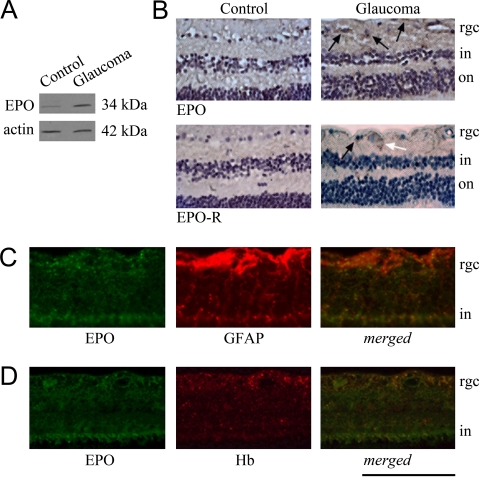
To better determine the relevance of these in vitro findings to in vivo conditions in human eyes, retinal protein samples from human donor eyes were also used in Western blot analysis of EPO expression. As shown in Figure 6A, retinal EPO expression was found to exhibit a more than two-fold increase in glaucomatous human samples relative to control samples (Mann-Whitney rank sum test, P < 0.01). Consistent with the findings of Western blot analysis, immunohistochemical analysis of human tissues with EPO and EPO-R antibodies detected a more intense immunolabeling in glaucomatous retinas relative to nonglaucomatous control retinas (Fig. 6B). Immunolabeling pattern was consistent with predominant macroglial localization of EPO, including astrocytes and Müller cells, whereas EPO-R was detectable on both macroglia and RGCs. Findings of double immunofluorescence labeling also supported that the increased EPO immunolabeling in the glaucomatous human retina is prominently co-localized with Hb immunolabeling (Figs. 6C, 6D).
Figure 6.
Association of EPO and EPO-R with glaucoma. (A) Western blot analysis obtained with human retinal protein samples supports an over two-fold increased expression of EPO in glaucoma (Mann-Whitney rank sum test, P < 0.01). Data represent repeated immunoblots with protein samples from five human donor eyes with glaucoma and five control eyes without glaucoma. β-Actin serves as a loading and transfer control. Immunohistochemical analysis also determined EPO and EPO-R immunolabeling in the glaucomatous human retina. (B) Immunoperoxidase labeling of the retina sections obtained from human donor eyes with or without glaucoma. Based on morphologic assessment of cell types, retinal EPO immunolabeling predominantly localized to macroglial cells (black arrow), whereas EPO-R immunolabeling was detectable on both macroglia (black arrow) and RGCs (white arrow). (C, D) Double-immunofluorescence labeling of the glaucomatous human retina using specific antibodies to EPO or EPO-R (green) and GFAP or Hb (red). Merged images (yellow) in (C) and (D) support co-localization of EPO with GFAP and Hb in glaucomatous eyes. rgc, retinal ganglion cell layer; in, inner nuclear layer; on, outer nuclear layer. Scale bar, (B) 250 μm; (C, D) 150 μm.
Thus, the findings of in vitro experiments support that hypoxic stress upregulates Hb expression in macroglial cells and RGCs through hypoxia-inducible EPO signaling. Immunohistochemical analysis of human donor eyes provides supportive evidence for a similar activation of the EPO/Hb axis in human glaucoma.
Discussion
Findings of this study involving proteomics, gene analysis, and immunohistochemistry revealed that Hb is expressed in the retina and optic nerve head, including macroglial cells and RGCs. The Hb expression was found to exhibit an approximately two-fold upregulation in ocular hypertensive rat eyes and glaucomatous human donor eyes. Our in vitro findings also revealed that hypoxia boosts glial Hb expression through hypoxia-inducible EPO signaling. In addition to this autocrine regulation in glial cells exposed to hypoxia, Hb expression is also upregulated in hypoxic RGCs through the EPO signaling in a paracrine manner. As discussed in the following text, findings of this study provide important links to many previous findings associated with glaucomatous neurodegeneration.
It has become clear through studies of Hb for over a century that this important oxygen transport protein of blood erythrocytes is widely distributed in diverse organisms, even in those that do not possess a bloodstream.31–33 It is also possible to find many studies reporting Hb expression in normal and diseased brain, including its neurons.34–37 Increasing applications of microarray analysis and proteomic analysis techniques over the past decade, which have produced large lists of molecules, have detected Hb in brain and retina samples. Blood components are not unexpected in the brain or retina because these tissues contain blood. It is for this reason that detection of Hb in previous models was mostly unappreciated and its functional importance remained unstudied. Notably however, some studies have specifically focused on neuronal Hb expression.38 Other mammalian cell types with nonerythroid lineage have also been reported to express Hb, including macrophages39 and pulmonary epithelium, where a high-rate of oxygen trafficking occurs.40 Similarly, the most obvious option for the physiological function of Hb in the retina and optic nerve head appears to be facilitating oxygen transport to meet high metabolic activities.
Thus, Hb expression in the retina and optic nerve head may constitute an intrinsic mechanism to increase oxygen availability and facilitate cellular oxygenation to provide protection under hypoxic conditions (Fig. 7). In adults, the major form of Hb is HbA, which is a tetramer consisting of two α and β polypeptide chains as we have repeatedly detected in the retinal proteome. Hb with its quaternary structure is an ideal molecule for oxygen transport due to allosteric control of oxygen binding and unloading capacity without the need for any other cellular process.33,41 Based on known oxygen-binding abilities, Hb may serve as an oxygen store enabling continued neuronal activity under intermittent episodes of hypoxia as a highly preserved evolutionary mechanism.42,43 Since tissue oxygen delivery by diffusion depends on the oxygen tension gradient, it is expected that oxygen delivery to RGCs via the proposed Hb-facilitated transport becomes even more important in glaucomatous eyes suffering from intermittent episodes of reduced vascular perfusion due to circadian variations in IOP, systemic blood pressure, and ocular perfusion pressure.5 Tissue oxygen diffusion is also known to be critically dependent on the diffusion distance. This intensifies the importance of a native mechanism facilitating oxygen transport to RGCs, since RGCs are not located in as close proximity to retinal blood vessels as are astrocytes. In respect to the load-bearing connective tissue sheathing the lamina cribrosa vasculature, a facilitated oxygen transport should also be important for RGC axons as they pass through the optic nerve head. In addition to oxygen storage and cellular delivery, Hb may also function as a sensor for gaseous ligands such as oxygen, nitric oxide, or carbon monoxide, thereby leading to the activation of counteracting mechanisms. Another putative function of Hb in the retina and optic nerve head may similarly be associated with scavenging free radicals and detoxifying nitric oxide, both of which are increased during glaucomatous injury.20,44
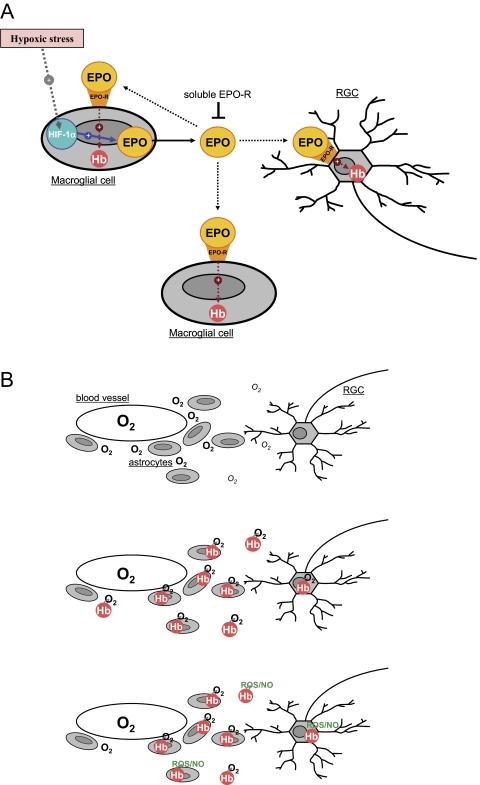
Figure 7.
The regulation and predicted functions of Hb in the retina and optic nerve head. (A) Hypoxic stress leads to upregulation of HIF-1α and increased production and secretion of EPO by macroglial cells. EPO induces Hb synthesis through EPO-R signaling in autocrine and paracrine fashions. (B) Regulated expression of Hb in macroglial cells and RGCs likely provides an intrinsic protective mechanism against hypoxic/oxidative injury. Hb may facilitate tissue oxygen transport in the inner retina and optic nerve head to improve the oxygenation of RGCs and their axons. This oxygen-binding protein may also function in free radical (ROS) scavenging and nitric oxide (NO) detoxification.
As an alternative view, we wonder whether glaucomatous tissues exhibit an insufficiency in the Hb-mediated intrinsic mechanism proposed based on in vitro hypoxia experiments. In support of this possibility, increased Hb immunolabeling was not prominent in all glaucomatous donors examined. Even in donor eyes exhibiting increased Hb immunolabeling, not all RGCs or macroglia were prominently immunolabeled for Hb. Parallel to this observation, quantitative proteomic analysis of retinal Hb expression in glaucomatous donors detected a less than two-fold increase relative to nonglaucomatous control eyes. It seems quite possible that any insufficiency and/or dysfunction in oxygen transport to RGCs could lead to RGC hypoxia even in the existence of normal vascular perfusion and normal oxygen delivery to the tissue. For example, potentially increased oxygen consumption by activated astrocytes, which are located in closer proximity to blood vessels than are RGCs, may lead to a decrease in the oxygen level available for RGCs in glaucoma. It is also tempting to determine in future studies whether binding of Hb by increased free radicals in glaucomatous tissues may negatively affect the oxygen binding ability of this protein and thereby RGC oxygenation. Although the clinical evidence of vascular abnormalities is not present in all patients with glaucoma, an increased retinal immunolabeling for HIF-1α was detectable in a diverse sample of glaucomatous donor eyes exhibiting different levels of glaucomatous damage.6 This observation may support the possibility that independent from any potential insufficiency in vascular perfusion, there may also be a deficiency in tissue oxygen transport resulting in RGC hypoxia in glaucomatous eyes. Findings of this study warrant further studies using in vivo models to better simulate in vivo conditions of oxygen requirement and delivery to RGCs. In addition to tissue oxygenation, mechanisms controlling the intracellular transport of oxygen to mitochondria should be further studied, since mitochondrial energy generation is compromised in RGCs and the resultant energy deficits may lead to RGC death in glaucoma.45
It is a challenge of studies of human donor eyes that their findings can be difficult to interpret mainly due to perimortem tissue alterations. However, in addition to donor age and cause of death, glaucomatous and control eyes included in this study were matched for the postmortem period. The death-to-fixation time was less than 12 hours for donors whose tissues were used in immunohistochemical analysis, whereas the human tissue samples used in proteomic analysis were collected within less than 6 hours after death. An excellent maintenance of the retinal structure and the absence of any evidence for autolysis in retinal cells also support that perimortem tissue alterations should not confound the interpretation of immunolabeling differences between donor eyes with or without glaucoma. Most important, complementary findings of immunohistochemical and proteomic analyses of human tissues are further supplemented by in vivo and in vitro studies of rat tissues.
Our in vitro findings support that similar to the brain,46 retina and optic nerve head macroglia under hypoxic stress provide autocrine and paracrine signals to provide protection against hypoxic injury. As summarized in Figure 7, after exposure to hypoxia, induction of glial HIF-1α may result in an upregulated synthesis of EPO. Although EPO secreted by glial cells may act on glia in an autocrine manner, this glycoprotein may also act on RGCs in a paracrine manner. The EPO secreted by macroglial cells can activate EPO-R on RGCs, thereby upregulating Hb expression and increasing hypoxic survival of these neurons. EPO has traditionally been viewed as a hematopoietic cytokine and an obligatory growth factor for erythrocyte production. Several recent reports have also indicated that EPO and its receptor are expressed in many other tissues, including the central nervous system and retina. It is now clear that this multifunctional protein has erythropoietic, neuroprotective, and angiogenic activities. There are numerous preclinical studies demonstrating a neuroprotective potential for EPO in a variety of nervous system disorders including glaucoma.47–49 Our in vitro findings suggest that Hb-mediated intrinsic protective mechanism(s) against hypoxic/oxidative injury are associated, in part, with the proposed neuroprotective ability of EPO treatment in glaucoma. Supportive evidence for this suggestion includes that EPO is a major target for the transcriptional activity of HIF-1α, which is upregulated in glaucoma,6 and that the retinal distribution of Hb expression in glaucomatous eyes is very similar to that of HIF-1α6 and EPO.48
It is also challenging to determine whether the RGC protection detected in our in vitro treatment experiments is merely associated with Hb upregulation or is associated with other downstream effects of EPO signaling. A difficulty in determining Hb-related cellular protection is intrinsic to the in vitro experimental model. This is because any protective effect of Hb that can be detected in an in vitro model may be limited to subcellular oxygen transport or other predicted functions like free radical scavenging or oxygen sensing to activate counteracting mechanisms. However, such an in vitro model carries a limitation in determining the predicted function of Hb in tissue oxygen transport, since the oxygen is equally distributed to monolayered cells in a culture plate. Further studies in in vivo models are therefore warranted to better understand Hb function in tissue oxygenation. Our ongoing studies using an in vivo hypoxia model are expected to provide further clarification.
Another oxygen-binding globin, neuroglobin, initially discovered in the brain50 and later in the retina, has also been suggested to protect against hypoxic/ischemic or oxidative stress,51,52 including that in glaucomatous eyes.53 However, rather than involvement in oxygen transport, the proposed protective ability of neuroglobin has been attributed to its function as a reactive oxygen and nitrogen scavenger,54,55 as well as a stress-responsive sensor for signal transduction.56 Cytoglobin, predominantly expressed in connective tissue fibroblasts, is also expressed in the brain and retina at low levels,52 and has been implicated in oxygen-requiring reactions, such as those catalyzed by hydroxylases.55 In addition, due to its nuclear localization, cytoglobin has been proposed to have possible functions as transcriptional regulator.57
A different aspect of retinal Hb expression may be the potential neurotoxicity of its breakdown products as occurs after brain hemorrhage.58,59 For example, although it is essential for normal functioning of cells, when abnormally accumulated during aging or neurodegenerative disorders, iron can mediate oxidative stress. Astrocytes, largely responsible for distributing iron in the brain, are ideally positioned around the vasculature to regulate the transport of iron to other brain cells and protect them against iron toxicity. Although astrocytes do not appear to have a high metabolic requirement for iron, they possess iron-transporting proteins, store iron efficiently in ferritin, and can export iron by a mechanism that involves ferroportin and ceruloplasmin.60 An increased expression of iron-regulating proteins in glaucomatous eyes (which is localized to astrocytes in the RGC layer and Müller cell bodies in the inner nuclear layer) suggests an upregulation of iron metabolism during glaucomatous neurodegeneration.61,62 It would be interesting to know whether the upregulated iron metabolism in glaucoma is associated with increased synthesis of Hb and/or an intrinsic effort against the toxicity of its iron content. We also wonder whether hemeoxygenase, also upregulated in the glaucomatous retina,63 is associated with Hb breakdown.
In summary, by revealing the regulated expression of Hb by retina and optic nerve head macroglia and RGCs, findings of this study provide new insights into oxygen homeostasis in the inner retina and optic nerve head with important implications in glaucoma. By proposing a new dimension of tissue oxygenation, besides vascular perfusion, these findings stimulate further research to better determine the functional importance of Hb in facilitating oxygen transport to RGCs and perhaps providing free radical scavenging. In addition, whether such an intrinsic protective mechanism is associated with the neuroprotective ability of EPO treatment in glaucoma should be clarified. Future studies should also enlighten us as to whether there is an insufficiency and/or dysfunction in this intrinsic mechanism in glaucoma. This possibility could open a new perspective for selective RGC hypoxia during intermittent episodes of reduced vascular perfusion caused by circadian variations in IOP and systemic blood pressure, or even in the presence of sufficient vascular perfusion.
Footnotes
Supported in part by National Eye Institute Grants 2R01 EY013813, 1R01 EY017131, R24 EY015636; an unrestricted grant to the University of Louisville Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness Inc., New York, NY; the Office of Science Financial Assistance Programs, U.S. Department of Energy; and National Institutes of Health Grant DK176743.
Disclosure: G. Tezel, None; X. Yang, None; C. Luo, None; J. Cai, None; A.D. Kain, None; D.W. Powell, None; M.H. Kuehn, None; W.M. Pierce, None
References
- 1.Hayreh SS. Pathogenesis of optic nerve damage and visual field defects. In: Heilman K, Richardson KT. eds. Glaucoma, Conceptions of a Disease Philadelphia: WB Saunders; 1978; 104–180 [Google Scholar]
- 2.Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol 2001; 85: 1252–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cioffi GA. Three common assumptions about ocular blood flow and glaucoma. Surv Ophthalmol 2001; 45(suppl 3): S325–S331; discussion S332–S334 [DOI] [PubMed] [Google Scholar]
- 4.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21: 359–393 [DOI] [PubMed] [Google Scholar]
- 5.Werne A, Harris A, Moore D, BenZion I, Siesky B. The circadian variations in systemic blood pressure, ocular perfusion pressure, and ocular blood flow: risk factors for glaucoma? Surv Ophthalmol 2008; 53: 559–567 [DOI] [PubMed] [Google Scholar]
- 6.Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 2004; 122: 1348–1356 [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999; 15: 551–578 [DOI] [PubMed] [Google Scholar]
- 8.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 2003; 121: 547–557 [DOI] [PubMed] [Google Scholar]
- 9.Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci 1981; 21: 34–38 [PubMed] [Google Scholar]
- 10.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 2001; 20: 175–208 [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nerve head. Can J Ophthalmol 2008; 43: 308–312 [DOI] [PubMed] [Google Scholar]
- 12.Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci 2003; 44: 3025–3033 [DOI] [PubMed] [Google Scholar]
- 13.Edsall JT. Understanding blood and hemoglobin: an example of international relations in science. Perspect Biol Med 1986; 29: S107–S123 [DOI] [PubMed] [Google Scholar]
- 14.Tezel TH, Geng L, Lato EB, et al. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Invest Ophthalmol Vis Sci 2009; 50: 1911–1919 [DOI] [PubMed] [Google Scholar]
- 15.Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci 2000; 20: 8693–8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci 2005; 46: 3177–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Luo C, Cai J, Pierce WM, Tezel G. Phosphorylation-dependent interaction with 14–3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci 2008; 49: 2483–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res 1997; 64: 85–96 [DOI] [PubMed] [Google Scholar]
- 19.Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci 2007; 48: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci 2004; 45: 4049–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tezel G, Yang X. Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res 2005; 81: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci 2007; 48: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci 1999; 19: 1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci 2001; 42: 365–371 [PubMed] [Google Scholar]
- 25.Uriarte SM, Powell DW, Luerman GC, et al. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol 2008; 180: 5575–5581 [DOI] [PubMed] [Google Scholar]
- 26.Powell DW, Weaver CM, Jennings JL, et al. Cluster analysis of mass spectrometry data reveals a novel component of SAGA. Mol Cell Biol 2004; 24: 7249–7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAfee KJ, Duncan DT, Assink M, Link AJ. Analyzing proteomes and protein function using graphical comparative analysis of tandem mass spectrometry results. Mol Cell Proteomics 2006; 5: 1497–1513 [DOI] [PubMed] [Google Scholar]
- 28.Cummins TD, Powell DW. Use of quantitative mass spectrometry analysis in kidney research. Semin Nephrol 2007; 27: 574–583 [DOI] [PubMed] [Google Scholar]
- 29.Heller M, Mattou H, Menzel C, Yao X. Trypsin catalyzed 16O-to-18O exchange for comparative proteomics: tandem mass spectrometry comparison using MALDI-TOF, ESI-QTOF, and ESI-ion trap mass spectrometers. J Am Soc Mass Spectrom 2003; 14: 704–718 [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi S, Matsubara H, Webster DA. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature 1986; 322: 481–483 [DOI] [PubMed] [Google Scholar]
- 32.Bogusz D, Appleby CA, Landsmann J, Dennis ES, Trinick MJ, Peacock WJ. Functioning haemoglobin genes in non-nodulating plants. Nature 1988; 331: 178–180 [DOI] [PubMed] [Google Scholar]
- 33.Poyart C, Wajcman H, Kister J. Molecular adaptation of hemoglobin function in mammals. Respir Physiol 1992; 90: 3–17 [DOI] [PubMed] [Google Scholar]
- 34.Blalock EM, Chen KC, Sharrow K, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 2003; 23: 3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohyagi Y, Yamada T, Goto I. Hemoglobin as a novel protein developmentally regulated in neurons. Brain Res 1994; 635: 323–327 [DOI] [PubMed] [Google Scholar]
- 36.Slemmon JR, Hughes CM, Campbell GA, Flood DG. Increased levels of hemoglobin-derived and other peptides in Alzheimer's disease cerebellum. J Neurosci 1994; 14: 2225–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CW, Liao PC, Yu L, et al. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol Dis 2004; 17: 367–377 [DOI] [PubMed] [Google Scholar]
- 38.He Y, Hua Y, Liu W, Hu H, Keep RF, Xi G. Effects of cerebral ischemia on neuronal hemoglobin. J Cereb Blood Flow Metab 2009; 29(3): 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A 1999; 96: 6643–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem 2006; 281: 5668–5676 [DOI] [PubMed] [Google Scholar]
- 41.Royer WE., Jr Structures of red blood cell hemoglobins: oxygen carriers in blood and tissues. In: Mangum CE. ed. Advances in Comparative and Environmental Physiology Vol. 13 Berlin Heidelberg: Springer Verlag; 1992: 87–116 [Google Scholar]
- 42.Kraus DW, Colacino JM. Extended oxygen delivery from the nerve hemoglobin of tellina alternata (bivalvia). Science 1986; 232: 90–92 [DOI] [PubMed] [Google Scholar]
- 43.Vandergon TL, Riggs CK, Gorr TA, Colacino JM, Riggs AF. The mini-hemoglobins in neural and body wall tissue of the nemertean worm, Cerebratulus lacteus. J Biol Chem 1998; 273: 16998–17011 [DOI] [PubMed] [Google Scholar]
- 44.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 2006; 25: 490–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res 2008; 173: 339–352 [DOI] [PubMed] [Google Scholar]
- 46.Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci 2002; 22: 10291–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai JC, Wu L, Worgul B, Forbes M, Cao J. Intravitreal administration of erythropoietin and preservation of retinal ganglion cells in an experimental rat model of glaucoma. Curr Eye Res 2005; 30: 1025–1031 [DOI] [PubMed] [Google Scholar]
- 48.Fu QL, Wu W, Wang H, Li X, Lee VW, So KF. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue Retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol 2008; 28(2): 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong L, Bradley J, Schubert W, et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci 2007; 48: 1212–1218 [DOI] [PubMed] [Google Scholar]
- 50.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature 2000; 407: 520–523 [DOI] [PubMed] [Google Scholar]
- 51.Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe?—evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem 2003; 278: 1932–1935 [DOI] [PubMed] [Google Scholar]
- 52.Ostojic J, Sakaguchi DS, de Lathouder Y, et al. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest Ophthalmol Vis Sci 2006; 47: 1016–1023 [DOI] [PubMed] [Google Scholar]
- 53.Rajendram R, Rao NA. Neuroglobin in normal retina and retina from eyes with advanced glaucoma. Br J Ophthalmol 2007; 91: 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem 2004; 279: 22841–22847 [DOI] [PubMed] [Google Scholar]
- 55.Fago A, Hundahl C, Malte H, Weber RE. Functional properties of neuroglobin and cytoglobin: insights into the ancestral physiological roles of globins. IUBMB Life 2004; 56: 689–696 [DOI] [PubMed] [Google Scholar]
- 56.Brunori M, Vallone B. A globin for the brain. FASEB J 2006; 20: 2192–2197 [DOI] [PubMed] [Google Scholar]
- 57.Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L. A globin in the nucleus! J Biol Chem 2003; 278: 30417–30420 [DOI] [PubMed] [Google Scholar]
- 58.Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med 2003; 35: 872–881 [DOI] [PubMed] [Google Scholar]
- 59.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006; 5: 53–63 [DOI] [PubMed] [Google Scholar]
- 60.Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR. The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 2007; 32(11): 1884–1890 [DOI] [PubMed] [Google Scholar]
- 61.Farkas RH, Chowers I, Hackam AS, et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci 2004; 45: 1410–1417 [DOI] [PubMed] [Google Scholar]
- 62.Stasi K, Nagel D, Yang X, Ren L, Mittag T, Danias J. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Invest Ophthalmol Vis Sci 2007; 48: 727–732 [DOI] [PubMed] [Google Scholar]
- 63.Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci 2007; 48: 4580–4589 [DOI] [PubMed] [Google Scholar]