Retinal ganglion cells can be protected by a caspase-substrate binding peptide, IQACRG, which protects proteins from caspase cleavage.
Abstract
Purpose.
This study investigated whether the enzymatically inactive caspase mimetic IQACRG protects rat retinal ganglion cells (RGCs) from excitotoxic insults. Minimally invasive delivery of the peptide to the retina was explored, and the mechanisms of neuroprotection were elucidated.
Methods.
IQACRG was linked to penetratin (P-IQACRG) to facilitate cellular uptake. RGC labeling by biotinylated-P-IQACRG delivered via intravitreal or subconjunctival injection was demonstrated by avidin-biotin chemistry. The authors used histologic and electrophysiological measures to evaluate the neuroprotective potential of P-IQACRG against RGC death induced by N-methyl-d-aspartate (NMDA) in vitro and in vivo. In addition, they monitored activity of an enzyme that is downstream of caspase-1, matrix metalloproteinase-9 (MMP-9), and protein levels of the caspase-3/7 substrate, myocyte enhancer factor 2C (MEF2C), to determine the effectiveness of IQACRG in blocking excessive caspase activity.
Results.
IQACRG significantly reduced NMDA-induced RGC death in culture and in vivo. Ex vivo electrophysiological recording of the retina on multielectrode arrays demonstrated functional rescue of RGCs by IQACRG. The authors also found that delivery of IQACRG to the retina inhibited NMDA-triggered MMP-9 activity and prevented cleavage of MEF2C protein that would otherwise have been engendered by caspase activation preceding RGC death. Strikingly, subconjunctival injection of P-IQACRG was very effective in preventing NMDA-induced RGC death in vivo.
Conclusions.
These data demonstrate that IQACRG protects RGCs from excitotoxicity in vitro and in vivo. The positive results with subconjunctival administration of P-IQACRG suggest that in the future this treatment may be useful clinically in diseases such as glaucoma and retinal ischemia.
Overactivation of CNS glutamate receptors results in neuronal cell death. This phenomenon was first described in the retina1 and was later named excitotoxicity.2 Among the ionotropic glutamate receptors, the N-methyl-d-aspartate (NMDA) receptor is a predominant contributor to excitotoxicity.3,4 Excitotoxicity is thought to contribute to the pathophysiology of various neurologic disorders, including ophthalmologic diseases affecting the retina and optic nerve.5–13 One such example is glaucoma, in which retinal ganglion cells (RGCs) undergo apoptosis.14 A candidate strategy to protect RGCs is the inhibition of excessive activity of caspases, a family of proteases that execute apoptosis.15
The IQACRG amino acid sequence is conserved in the active site of caspases-1, -2, -3, -6, -7, and -14.16 Synthetic IQACRG peptide is thought to work as an enzymatically inactive caspase mimetic; this peptide binds to caspase substrates as a pseudoenzyme to protect them from proteolysis by caspases.17 For example, IQACRG peptide blocks the cleavage of pro-interleukin (IL)-1β by caspase-1,17 which is essential for the formation of functionally active IL-1β. We and our collaborators have shown that IQACRG protects cultured rat pheochromocytoma-derived PC12 cells17 and primary cerebrocortical neurons from cell death.18 In these studies, IQACRG peptide was linked to penetratin,17,18 the third helix of Drosophila antennapedia, to enhance intracellular delivery of the peptide.19 Here we report the use of the IQACRG peptide, both in vitro and in vivo, as a therapeutic approach to protect RGCs against NMDA excitotoxicity and confirm cellular delivery of the peptide to RGCs in the intact retina.
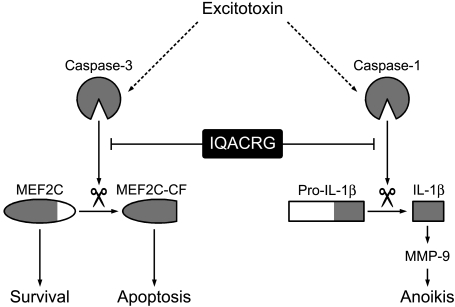
In the present study, we elucidated possible mechanisms of neuroprotective action of IQACRG, showing that the peptide protects caspase substrates from cleavage (illustrated schematically in Fig. 1). For example, in the central nervous system, matrix metalloproteinase (MMP)-9 is an important downstream effecter of IL-1β,20–22 whose activation by caspase-1 is blocked by IQACRG.17 If activated, at least in part by IL-1β, MMP-9 would degrade extracellular matrix, thus contributing to neuronal degeneration by a form of apoptosis known as anoikis.23–26 Here we show an inhibitory effect of IQACRG on MMP-9 activation in the retina. Additionally, the transcription factor myocyte enhancer factor 2C (MEF2C) manifests an antiapoptotic activity in neurons.27–30 After excitotoxic stimulation, however, caspases cleave MEF2C, resulting in the loss of its antiapoptotic activity.30 The present study shows that IQACRG inhibits caspase-mediated proteolysis/inactivation of MEF2C.
Figure 1.
Schema proposing that prevention of caspase cleavage contributes to the neuroprotective effect of IQACRG. Excitotoxins activate caspases in neurons. For example, activated caspase-3 cleaves MEF2C, resulting in loss of its antiapoptotic transcriptional activity and generation of proapoptotic cleaved fragments (MEF2C-CF). Additionally, caspase-1 cleaves pro-IL-1β to functionally active IL-1β. In turn, IL-1β leads to MMP-9 activation. This pathway is thought to contribute to neuronal cell death by a form of apoptosis known as anoikis. IQACRG binds to caspase substrates, thus protecting them from caspase enzymatic activity. Inhibition of caspase cleavage of substrates, such as MEF2C and pro-IL-1β, contributes to neuronal survival.
Finally, we demonstrate that IQACRG protects RGCs when administered by subconjunctival injection (i.e., transscleral delivery). Importantly, this mode of delivery is potentially clinically applicable. Although intravitreal and systemic administration have been widely used by researchers to deliver potent neuroprotective agents to the retina, neither method is optimal for routine clinical practice. Intravitreal injection has risks for infection, damage to the lens, hemorrhage, and retinal detachment; systemic administration can also result in untoward side effects. In contrast, transscleral delivery is a less invasive method of clinical drug administration to the posterior segment of the eye and can be accomplished with minimal uptake by the systemic circulation.31,32 To our knowledge, this is the first successful application of a neuroprotective drug to the retina by the transscleral route.
Materials and Methods
Peptide Synthesis and Coupling with Penetratin
IQACRG and a control scrambled peptide (ICGRQA) were synthesized by our institutional facility (Medicinal Chemistry Facility, Burnham Institute for Medical Research, La Jolla, CA). The peptides were coupled to a 16-amino acid peptide (Activated Penetratin 1; Qbiogene, Irvine, CA), as previously described,17,18 and are designated here as P-IQACRG or scrambled peptide, respectively. For some experiments, we linked IQACRG to a biotinylated 16-amino acid peptide (Biotinylated-Activated Penetratin 1; Qbiogene), designated biotin-P-IQACRG, to track its location by avidin-biotin immunochemistry.
Animals
All experimental procedures using animals were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and our institutional guidelines. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 g each were used for experiments unless otherwise described in the text. Experimental animals were housed in a 12-hour light/12-hour dark cycle with access to food and water ad libitum and were anesthetized with 2% isoflurane, 70% N2O, and 30% O2 during all surgical manipulations.26,33 At the end of the experiments, each animal was euthanatized with an overdose of isoflurane (5%) and was transcardially perfused with phosphate-buffered saline (PBS) to harvest the ocular tissues.
Retinal Ganglion Cell Cultures
RGC cultures were prepared from postnatal day 7 rat pup retinas, as previously described.34 Briefly, after enzymatic dissociation, RGCs were collected by two-step immunopanning. The first step was performed with mouse anti–macrophage monoclonal antibody (MAC1) to exclude macrophages, and the second was performed with mouse anti–Thy 1.1 monoclonal antibody (clone 2G12) to purify RGCs. RGCs were plated in medium (Neurobasal; Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen), l-glutamine, brain-derived neurotrophic factor, ciliary neurotrophic factor, and forskolin.
Three days after plating, cultures were pretreated with P-IQACRG (200 nM), scrambled peptide, or a broad-spectrum caspase inhibitor (zVAD-fmk; Biomol, Plymouth Meeting, PA; 50 μM).17,18 Control cultures did not receive pretreatment. Three hours after pretreatment, cultures were exposed to NMDA (200 μM) and glycine (5 μM) for 20 minutes in Earle's balanced salt solution (EBSS).18 For sham preparation, cultures were stimulated with vehicle (glycine alone, which is not neurotoxic in its own right). Cultures were then rinsed with fresh EBSS and incubated for an additional 18 hours. RGCs were fixed, permeabilized, and stained with propidium iodide to count total and pyknotic nuclei, as previously described.18
Intravitreal Injections
In a series of experiments, drugs were injected with a 33-gauge needle inserted into the vitreous through the superotemporal sclera under general (isoflurane) and topical anesthesia (one drop of lidocaine applied to the eye).35 Animals with lens damage or vitreal hemorrhage were euthanatized and excluded from analyses. Five paradigms of intravitreal injection were conducted:
First, biotin-P-IQACRG (150 pmol) in 3.5 μL PBS was injected into the vitreous to study cellular uptake of the peptide in the retina. Equimolar amounts of P-IQACRG (without biotin) and the biotin molecule itself (Sigma-Aldrich, St. Louis, MO) served as controls.
Second, to study the potential neuroprotective effect of P-IQACRG after excitotoxic insult, NMDA (15 nmol) plus glycine (10 nmol) was injected with or without peptide (P-IQACRG or scrambled peptide, 150 pmol). Control animals received vehicle alone (glycine).
Third, to study the therapeutic potential of transscleral application of the neuroprotective caspase substrate-binding peptide, we injected NMDA and glycine intravitreally to induce an insult, preceded at various intervals by subconjunctival injection of P-IQACRG or controls.
Fourth, to study the possible effect of P-IQACRG on MMP activity, we injected intravitreal NMDA plus glycine in conjunction with either P-IQACRG or scrambled peptide.
Fifth, to study the possible effect of P-IQACRG on MEF2C protein levels, we injected intravitreal NMDA plus glycine in conjunction with either P-IQACRG, scrambled peptide, or zVAD-fmk.36
Subconjunctival Injection
Initially, a prostaglandin F2 analogue, latanoprost (Sigma-Aldrich; 0.005% in 6 μL saline),37 was administrated topically to eyes once a day for 12 days to increase scleral permeability.38 Saline alone was instilled as a sham treatment. Next, to investigate peptide delivery, we injected biotin-P-IQACRG (3 nmol in 8 μL PBS) into the superotemporal quadrant of the posterior subconjunctival space using a 33-gauge needle.
The possible neuroprotective effect of the caspase substrate-binding peptide was evaluated in rats that received latanoprost instillation by subsequent injection of P-IQACRG or scrambled peptide as a control. We calculated the amount to be injected based on in vitro neuroprotective efficacy. Injections consisted of 15 nmol peptide in 37.5 μL PBS administered into the posterior subconjunctival space in three quadrants. Subconjunctival injections were made twice daily (for a total daily dose of 30 nmol) starting 1 day before intravitreal NMDA injection. The last (fourth) subconjunctival injection was made immediately after the intravitreal injection of NMDA. We use the term “subconjunctival injection” instead of “sub-Tenon injection” because the Tenon tissue is indistinguishable from the conjunctiva in rats during this type of procedure.
Avidin Precipitation and Blot Analysis
Ocular tissues were collected 8 and 24 hours after intravitreal and subconjunctival injection of biotin-P-IQACRG, respectively. Vitreous and retina were homogenized in an immunoprecipitation buffer (150 mM NaCl, 50 mM Tris-HCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, pH 7.4) containing a protease inhibitor cocktail (Complete Mini, EDTA-free; Roche, Basel, Switzerland) and were clarified by centrifugation. Retinal lysates (600 μg protein) were processed for avidin precipitation and blot analysis. The entire vitreous from each eye was used to maximize the protein concentration. After preabsorption with Sepharose beads to eliminate nonspecific binding, samples were incubated with avidin beads (Immobilized Neutravidin Protein; Pierce, Rockford, IL). After several rinses, the avidin beads were boiled in sample buffer (tricine SDS; Invitrogen) with a sample reducing agent (NuPAGE; Invitrogen). Samples were subjected to SDS-PAGE on 10% to 20% tricine gel (Invitrogen) and transferred to a small (0.2-μm)-pore polyvinylidene difluoride membrane (Immobilon-PSQ Membrane; Millipore, Billerica, MA). After fixation with 4% glutaraldehyde, the membranes were blocked with bovine serum albumin and incubated with extravidin-peroxidase (Sigma-Aldrich; 1:3000 dilution).39 Peroxidase activity was visualized on x-ray film with enhanced chemiluminescence (ECL Plus Kit; GE Healthcare Life Sciences, Piscataway, NJ).
Immunohistochemistry and TUNEL Staining
Tissue preparation, cryosectioning, immunohistochemistry, and apoptotic assays were performed as previously described.35 To visualize biotin-P-IQACRG, sections were reacted with fluorescein-conjugated streptavidin (Vector Laboratories, Burlingame, CA; 1:300 dilution). Mouse anti–microtubule associated protein-2 (MAP2) monoclonal antibody (Sigma-Aldrich; 1:1000 dilution) was used with Alexa Fluor 555 goat anti–mouse IgG secondary antibody (Invitrogen; 1:500 dilution) to label cell bodies and neurites of RGCs and amacrine cells in our preparations.40 To localize MEF2C protein in the retina, specimens were incubated with rabbit anti–MEF2C polyclonal antibody (Cell Signaling, Beverly, MA; 1:100 dilution) and Alexa Fluor 488 donkey anti–rabbit IgG secondary antibody (Invitrogen; 1:500 dilution). The specificity of MEF2C antibody staining was confirmed by lack of immunofluorescence in retinas harvested from MEF2C knockout mice.41 We also stained retinas with mouse anti–NMDA receptor subunit 1 (NR1) monoclonal antibody (Upstate, Lake Placid, NY; 1:100 dilution). A transcription factor Brn-3b served as an RGC marker in the retina,42 as detected by goat anti–Brn-3b polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution) in combination with Alexa Fluor 555 donkey anti–goat IgG secondary antibody (Invitrogen, 1:500 dilution). Cell nuclei were counterstained with Hoechst 33342 (Invitrogen; 200 nM). Images were captured with a deconvolution fluorescence microscope (Axiovert 100M; Zeiss, Jena, Germany) with analysis software (Slidebook 4.0; Intelligent Imaging Innovations, Denver, CO). Illumination and exposure times were kept constant for precise comparison between images. To detect apoptotic nuclei, the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) method was carried out using a kit (In Situ Cell Death Detection Kit, TMR Red; Roche). Numbers of total and TUNEL-positive nuclei in the ganglion cell layer (GCL) were counted per length of section, as previously described.43
Retrograde Labeling and Quantification of Surviving RGCs
RGCs were retrogradely labeled by injection of 5% aminostilbamidine (Fluoro-Gold; Invitrogen) into the superior colliculi 4 days before intravitreal injection of NMDA.26,33,44 Twenty-four hours after NMDA injection, retinas were fixed and prepared as flat-mounts for observation. Aminostilbamidine-labeled RGCs with diameters of at least 8 μm were counted in 12 areas (at one-sixth, one-half, and five-sixths of the radius in each retinal quadrant) for a total area of 2.25 mm2 per eye, as previously described.26,33,44,45 Values represent RGC density (number of cells/mm2).
Multiple Electrode Array Recordings
Twenty-four hours after treatment, we enucleated the eyes and prepared flat retinas by relaxing incisions under dim red light in Ames' medium (Sigma-Aldrich). Residual vitreous, after mechanical removal, was enzymatically digested with 0.5 mg/mL hyaluronidase (Sigma-Aldrich) for 5 minutes at 37°C.46 Retinas were centered with the vitreal surface facing the electrodes of a multiple electrode array (MEA; model 500/30iR-Ti; Multi Channel Systems, Reutlingen, Germany). After allowing the preparation to equilibrate, spontaneous and light-evoked activity were recorded in Ames' medium after the addition of agents to block synaptic inhibition, the GABAA receptor blocker, picrotoxin (100 μM), and the glycine receptor antagonist, strychnine (10 μM). Light-evoked responses were induced by 10 exposures lasting 1 second each to diffuse full-field white light.47 Data were acquired with software (MC Rack; Multi Channel Systems) and high-pass filtered (>200 Hz). RGC action potentials were detected as spikes if they crossed a threshold of 5 SD over background noise. Spike rates for each electrode were analyzed (NeuroExplorer; Nex Technologies, Dallas, TX).
Gelatin Zymography
To determine the enzymatic activity of MMP-2 and -9 (gelatinase-A and -B, respectively), we used gelatin zymography.23,25,26 Six hours after intravitreal injection of NMDA, retinas were lysed in homogenizing buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5 mM CaCl2, 0.02% NaN3, 0.05% Brij 35, 1% Triton X-100, and protease inhibitor cocktail). After centrifugation, supernatants (containing 600 μg protein) were incubated with gelatin-conjugated beads (Gelatin Sepharose 4B; GE Healthcare Life Sciences) for affinity precipitation. Eluates mixed with SDS sample buffer were separated by SDS-PAGE on 10% gelatin zymogram gels (Invitrogen). Gels were incubated at 37°C for 3 days for in-gel digestion and then were stained (Coomassie Brilliant Blue R-250; Pierce). Destaining excess dye enabled areas of gelatinase activity to appear as clear bands. A human fibrosarcoma cell line, HT-1080 (American Type Culture Collection, Manassas, VA), and a rat mammary carcinoma cell line, BC148 (a kind gift from J. Guy Lyons, Sydney Cancer Centre, Royal Prince Alfred Hospital, Camperdown, Australia), were used for positive controls. The intensity of bands corresponding to MMP-2 and -9 was quantified by densitometry using NIH Image version 1.60 (National Institutes of Health, Bethesda, MD).
Western Blot Analysis
Retinas were harvested for homogenization 5 hours after intravitreal NMDA injection (45 nmol). After clarification by centrifugation, lysates (containing 40 μg protein) were subjected to Western blot analysis. Rabbit anti–MEF2C polyclonal antibody (1:1000 dilution) and peroxidase-conjugated donkey anti–rabbit IgG antibody (GE Healthcare Life Sciences; 1:5000 dilution) were used as primary and secondary antibodies, respectively. Peroxidase activity was visualized by chemiluminescence. To confirm equal loading, membranes were reprobed with mouse anti–actin monoclonal antibody (clone C4; Chemicon, Temecula, CA; 1:5000 dilution) followed by incubation with peroxidase-conjugated sheep anti–mouse IgG antibody (GE Healthcare Life Sciences; 1:5000 dilution).
Statistical Analyses
All data are expressed as mean ± SEM. Statistical significance between groups was analyzed by either Student's t-test (for single comparisons) or one-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) for multiple comparisons. P < 0.05 was taken to be significant.
Results
P-IQACRG Protects Cultured Rat RGCs from NMDA-Induced Excitotoxicity
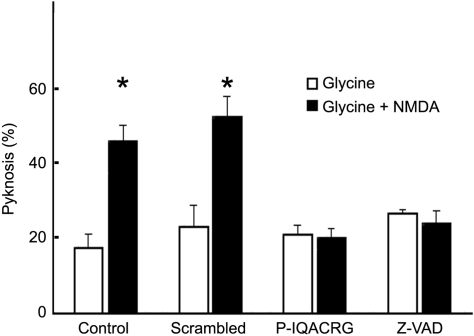
We first tested whether P-IQACRG could inhibit NMDA-mediated excitotoxicity in purified RGC cultures (Fig. 2). In control cultures (no pretreatment), nearly half the RGCs manifested pyknotic nuclei 18 hours after brief exposure to NMDA. Preincubation of the cultures with P-IQACRG, as with the classical caspase inhibitor zVAD, inhibited NMDA-induced RGC death by more than twofold.
Figure 2.
Protection of RGCs by caspase substrate-binding peptide IQACRG and canonical caspase inhibitor z-VAD. RGC cultures were pretreated for 3 hours with control (scrambled) peptide, P-IQACRG (200 nM) or the broad-spectrum caspase inhibitor zVAD-fmk (50 μM) before 20-minute exposure to NMDA (200 μM) plus glycine (5 μM). Control cultures did not receive these pretreatments. Eighteen hours later, RGC cultures were fixed and stained with propidium iodide to count total and pyknotic nuclei. The basal level of pyknosis was determined in cultures exposed to vehicle (glycine) alone. Data are mean ± SEM (n = 5 each group). *P < 0.05 by ANOVA.
Retinal Uptake of Intravitreally Injected Biotin-P-IQACRG
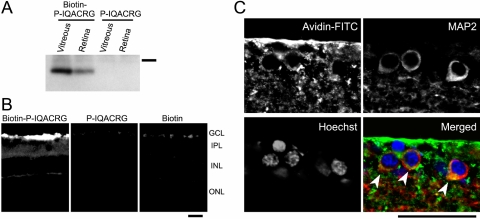
We analyzed tissue lysates by avidin-biotin chemistry 8 hours after intravitreal injection of biotin-P-IQACRG to investigate the extent of uptake of the peptide into the retina. Peroxidase-conjugated avidin detected a band in the vitreous and retina of eyes injected with biotin-P-IQACRG (Fig. 3A). Importantly, the band was of appropriate size for biotin conjugated to the peptide, indicating that free biotin in the absence of peptide was not present in the retinal tissue. In contrast, no detectable signal was present in eyes injected with P-IQACRG without biotin, which served as a negative control.
Figure 3.
Retinal uptake of biotin-labeled P-IQACRG. Ocular tissues were harvested 8 hours after intravitreal injection of 150 pmol biotin-P-IQACRG, P-IQACRG (without biotin label, which served as a negative control), or biotin alone. (A) Avidin-affinity precipitates of the vitreous and retina were separated on SDS-PAGE, and blots were incubated with peroxidase-conjugated avidin. After biotin-P-IQACRG injection, blots displayed a band corresponding to the biotinylated molecule (n = 5 experiments; bar on right indicates 3.5 kDa). (B) Localization of P-IQACRG in the retina. In animals receiving biotin-P-IQACRG, fluorescein-streptavidin histochemistry revealed the presence of biotinylated peptide in the inner retina, with the strongest signal in the ganglion cell layer (left). In eyes injected with P-IQACRG alone, we observed little if any signal (center). Eyes injected with biotin alone displayed restricted distribution of avidin fluorescence around the inner limiting membrane (right). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer. (C) When avidin histology (green) was combined with MAP2 immunostaining for neurons (red), deconvolution microscopy revealed MAP2-positive neurons in the GCL that contained the biotinylated peptide in the cytosol (yellow, marked by arrowheads). Cell nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 50 μm; n ≥ 4 experiments for each.
To further confirm the uptake of biotin-P-IQACRG by retinal cells in vivo, we examined the retina by immunohistochemistry. We observed fluorescein-conjugated streptavidin signal in the inner retina of eyes that had been intravitreally injected with biotin-P-IQACRG (Fig. 3B). Although staining for endogenous biotin has been reported in Müller cells in fish and amphibian,49 we did not detect such signals in the mammalian retina under our conditions. In eyes injected with the biotin molecule alone, distribution of avidin fluorescence was limited to the vitreal surface of the retina. When observed at a higher magnification, individual MAP2-positive neurons in the GCL displayed biotin signal in their cytosol (Fig. 3C, arrowheads). These results indicate that penetratin was effective in transporting the IQACRG peptide into retinal cells in the GCL.
Inhibition of NMDA-Induced Apoptosis in the GCL by P-IQACRG
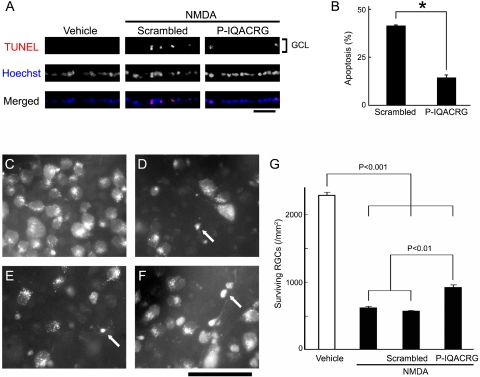
We and others had previously shown that intravitreal injection of relatively low doses of NMDA induces RGC death with predominantly apoptotic features.33,50 To study the potential effect of P-IQACRG in vivo in the retina, rats were intravitreally injected with NMDA/glycine along with the neuroprotective peptide. Although there were virtually no TUNEL-positive nuclei in the retinas of animals injected with vehicle (glycine alone), injection of NMDA induced apoptosis in cells of the GCL within 8 hours (Fig. 4A). Coinjection of P-IQACRG significantly reduced the percentage of TUNEL-positive cells in the GCL (14.4% ± 1.4% apoptotic cells) compared with scrambled peptide (41.3% ± 0.5%; P < 0.001; Fig. 4B).
Figure 4.
P-IQACRG protects RGCs from apoptosis after intravitreal NMDA injection. (A) NMDA (15 nmol) and glycine (10 nmol) were injected intravitreally along with 150 pmol P-IQACRG or scrambled (control) peptide. Eight hours later, retinal sections were subjected to TUNEL staining (red in merged image) to assess apoptosis and were counterstained with Hoechst 33342 to label cell nuclei (blue); colabeling appears violet. Injection of NMDA led to apoptosis in the GCL, whereas vehicle (glycine alone) did not. Administration of P-IQACRG but not the scrambled peptide protected the GCL from apoptosis. Scale bar, 50 μm. (B) Quantitative assessment of number of TUNEL-positive nuclei in the GCL revealed that P-IQACRG inhibited apoptosis induced by NMDA (*P < 0.001, Student's t-test). Data are mean ± SEM (n = 5–6 for each group). (C–F) Representative photomicrographs of aminostilbamidine-labeled RGCs in retinal flat-mounts 24 hours after intravitreal injection of vehicle (glycine alone; n = 4; C), NMDA plus glycine (n = 5; D), NMDA plus glycine treated with scrambled peptide (n = 9; E), or NMDA plus glycine treated with P-IQACRG (n = 10; F). NMDA injection resulted in fewer RGCs and pyknotic-appearing somas in many of the remaining cells (arrows). Scale bar, 50 μm. (G) Surviving RGCs were counted in retinal flat-mounts and were expressed as RGC density per square millimeter. Each bar represents mean ± SEM. Statistical significance was assessed with a one-way ANOVA followed by Fisher's PLSD multiple comparisons test.
Intravitreal Injection of P-IQACRG Protects RGCs from NMDA-Induced Excitotoxicity
Next, we wanted to ensure that the cells protected by P-IQACRG were indeed RGCs rather than displaced amacrine or other cell types that are present in rodent GCL. For this purpose, we identified RGCs by retrograde-aminostilbamidine labeling. We scored the number of surviving RGCs in retinal flat-mounts 24 hours after NMDA injection. In eyes injected with vehicle (glycine alone), aminostilbamidine-labeled RGCs manifested normal morphology (Fig. 4C). The density of RGCs in this control group was 2280.0 ± 42.2/mm2. Intravitreal injection of NMDA resulted in a significant decrease in the number of surviving RGCs (to 626.4 ± 13.0 /mm2; P < 0.001; Fig. 4D). Dying cells manifested pyknotic features (arrows). Scrambled peptide did not protect RGCs (562.1 ± 10.7/mm2; Fig. 4E). P-IQACRG treatment, however, significantly increased the number of surviving RGCs after NMDA injection (to 911.7 ± 36.3/mm2; P < 0.01; Figs. 4F, G).
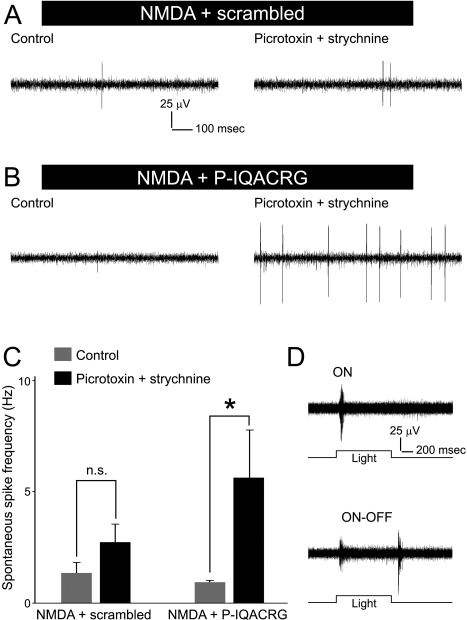
P-IQACRG Preserves Electrophysiological Activity of RGCs
Next, we analyzed the effect of treatment with P-IQACRG on RGC function using MEA electrophysiological techniques. We found that RGCs surviving NMDA-induced excitotoxicity exhibited occasional bursts of activity during baseline recording (controls in Figs. 5A, B). The addition of picrotoxin and strychnine to repress the inhibitory input to RGCs unmasked additional spontaneous spiking, especially in P-IQACRG-treated retinas (Figs. 5A–C; P < 0.05). This observation suggests that there might have been an increase in the number of potentially active RGCs in P-IQACRG-treated retinas over control-treated retinas. Additionally, we were able to record light-evoked responses in P-IQACRG-treated retinas exposed to NMDA/glycine (Fig. 5D), showing that the surviving RGCs that received the neuroprotective peptide appeared to be light responsive and, therefore, functional.
Figure 5.
Electrophysiological activity of surviving RGCs. (A, B) Retinal flat-mounts were prepared for electrophysiological analysis 24 hours after intravitreal injection of NMDA (15 nmol) plus glycine (10 nmol) with either scrambled peptide or P-IQACRG (150 pmol). Ex vivo retinal recordings were performed on MEAs initially in normal extracellular solution (control, left) and then after the addition of picrotoxin (100 μM) and strychnine (10 μM) to suppress inhibitory input (right). RGCs exhibited spontaneous spiking activity, which increased in the presence of picrotoxin and strychnine, particularly in retinas treated with P-IQACRG. (C) Spontaneous spike frequency obtained from multiple electrodes was averaged for each retina and compared (n = 6 retinas for each treatment). P-IQACRG-treated retinas exhibited a statistically significant increase in spike frequency in the presence of picrotoxin and strychnine (*P < 0.05; n.s., not significant). (D) Retinal responses to full-field illumination (1 second in duration). Surviving RGCs in P-IQACRG-treated retinas displayed light-evoked responses. These traces represent recordings in medium lacking picrotoxin and strychnine and illustrate an ON response (top) and an ON-OFF response (bottom).
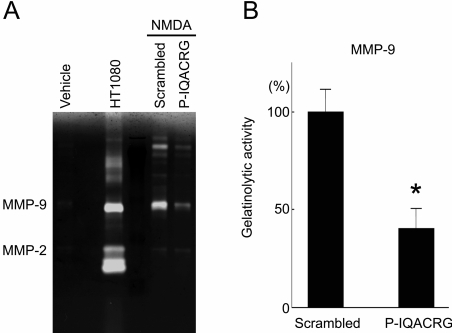
Inhibition of NMDA-Triggered MMP-9 Activity by P-IQACRG
We next further investigated the potential mechanism and targets of P-IQACRG in its neuroprotective action in the retina. Previous studies had shown that intravitreal injection of NMDA induces MMP-9 activity in the retina26 and that the inhibition of MMP-9 activity prevents, at least in part, RGC death caused by excitotoxic insult.24,26 Similarly, in the present study, we detected MMP-9 activity in retinas after intravitreal NMDA injection (Fig. 6A). Concurrent treatment with P-IQACRG inhibited NMDA-induced MMP-9 activity in the retina (P < 0.01 compared with scrambled peptide; Fig. 6B). In contrast, MMP-2 activity in the retina remained relatively minimal.
Figure 6.
Analysis of retinal MMP-9 gelatinase activity after excitotoxic insult. Rats received intravitreal injection of vehicle (10 nmol glycine alone) or NMDA (20 nmol) plus glycine along with either scrambled peptide or P-IQACRG (150 pmol). Six hours after injection, retinas were harvested for gelatin zymography to assess MMP activity. (A) MMP-2 and -9 in tissue homogenates were affinity-precipitated with gelatin beads and separated by SDS-PAGE on polymerized gelatin. Gelatin digestion indicated MMP activity and was visualized as a clear band on dye-stained gels. Conditioned medium from the fibrosarcoma cell line HT1080, which is known to contain gelatinase activity, served as a positive control. (B) Quantitative comparison of gelatin zymography by densitometric analysis (mean value of eyes treated with scrambled peptide set at 100%). Statistical analyses revealed that P-IQACRG inhibited NMDA-induced MMP-9 activity (*P < 0.01 by t-test). Values are mean ± SEM (n = 5–6 for each group).
Protection of Retinal MEF2C from Caspase Cleavage by P-IQACRG
Next, we studied the effect of P-IQACRG on activity of the transcription factor MEF2C because we had previously shown that this antiapoptotic transcription factor could be cleaved by caspase-3 and -7 in neurons.30 Once cleaved, MEF2C loses its antiapoptotic activity and displays dominant-negative properties, resulting in increased apoptosis.30 In the present study, we hypothesized that IQACRG, as a caspase substrate-binding peptide, would protect MEF2C from caspase cleavage.
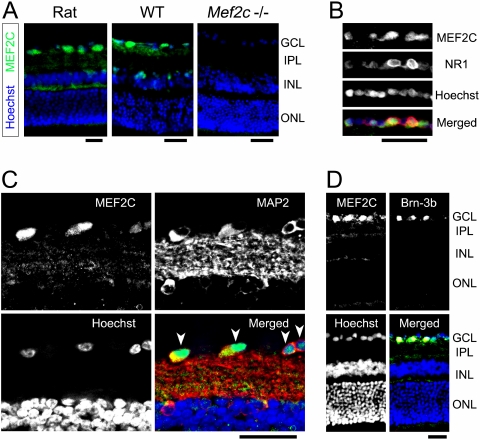
As a first step, we analyzed the localization of MEF2C in the retina. Anti–MEF2C antibody decorated cells most strongly in the GCL of the rat retina and, to some degree, in the plexiform layers (Fig. 7A, left). The staining pattern for MEF2C protein was similar in wild-type mouse retina but with less prominent plexiform labeling (Fig. 7A, center). As expected, retinas of conditional Mef2c knockout mice41 were devoid of MEF2C immunofluorescence (Fig. 7A, right), ensuring the specificity of the antibody. Additionally, activation of NMDA receptors on cerebrocortical neurons is known to activate caspase cleavage of MEF2C.30 To assess NMDA receptor localization in conjunction with MEF2C, we stained for the NR1 subunit, which is present in all functional NMDA receptors. Interestingly, transcription of this NMDA receptor subunit is regulated by MEF2C.51 We found that NR1-expressing cells in the GCL displayed prominent MEF2C immunoreactivity (Fig. 7B). At higher magnification, the MAP2-positive neurons in the GCL clearly exhibit MEF2C immunoreactivity, with the strongest signal in their nuclei (Fig. 7C, arrowheads). Moreover, immunostaining for the RGC-specific marker Brn-3b42 revealed that MEF2C immunoreactivity was especially strong in RGCs (Fig. 7D). Hence, we found that in the retina MEF2C predominantly localizes to RGCs.
Figure 7.
Localization of retinal MEF2C. Retinal sections were stained immunohistochemically for the transcription factor MEF2C. (A) Immunostaining for MEF2C (green) revealed prominent expression in the GCL of the normal adult rat retina (left). Wild-type mouse retina exhibited similar MEF2C protein localization (center). In contrast, Mef2C conditional knockout mice exhibited no MEF2 immunofluorescence, as expected (right). Cell nuclei were counterstained with Hoechst 33342 (blue). (B) Retinal sections were double-immunolabeled with MEF2C and NMDA receptor subunit 1 (NR1) (green and red, respectively, in merged image). GCL labeling revealed MEF2C protein localization in cells expressing NMDA receptors. (C) Double immunostaining of MEF2C and MAP2 (green and red, respectively, in merged image) clearly displayed individual neurons in the GCL expressing MEF2C (arrowheads). Note that cell nuclei in the GCL show robust MEF2C-immunoreactivity. (D) Double immunostaining for MEF2C and the RGC marker Brn-3b confirmed that MEF2C was expressed in RGCs. Colocalization of MEF2C and Brn-3b (green and red, respectively, in merged image) was evidenced by yellow staining (n ≥ 3 animals for each). Scale bars, 50 μm. IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer.
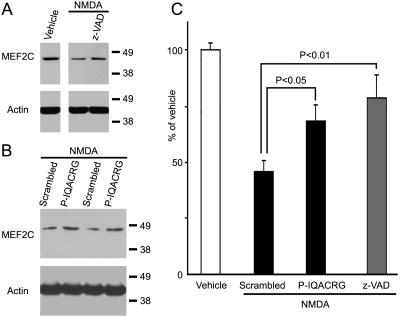
P-IQACRG Inhibits NMDA-Induced Cleavage of MEF2C by Caspases
We tested the effect of P-IQACRG on MEF2C protein levels in the retina by Western blot analysis. Intravitreal injection of NMDA reduced intact MEF2C protein levels in the retina 5 hours later (Fig. 8A), consistent with our prior report that NMDA induces caspase-3 and -7 activation with consequent cleavage of MEF2C.30 Concurrent intravitreal injection of the broad-spectrum caspase inhibitor zVAD-fmk significantly attenuated the NMDA-triggered reduction in MEF2C protein (Figs. 8A, C), suggesting that the reduction in MEF2C protein levels occurred because of caspase activity. Intravitreal injection of P-IQACRG also significantly inhibited the NMDA-induced reduction in MEF2C protein levels (Figs. 8B, C). Actin was used as a loading control. These data are consistent with the notion that IQACRG inhibited NMDA-triggered caspase proteolysis of MEF2C.
Figure 8.
Analysis of levels of the caspase-3 substrate MEF2C after P-IQACRG administration. Rats received intravitreal injection of NMDA (45 nmol) plus glycine (10 nmol) along with the broad-spectrum caspase inhibitor zVAD-fmk (10 nmol), scrambled peptide, or P-IQACRG (150 pmol each). Vehicle injection (glycine alone) served as a control. Five hours after injection, retinas were harvested, and protein samples were subjected to Western blot analysis. (A) NMDA injection attenuated MEF2C protein levels in the retina. Simultaneous injection of zVAD blocked the NMDA-induced reduction in MEF2C. Equal loading was confirmed by the level of actin. (B) The decrease in MEF2C immunoreactivity after NMDA injection was largely prevented by P-IQACRG compared with scrambled peptide. (C) Densitometric analysis of MEF2C immunoreactivity allowed quantitative comparison of Western blot analysis with the mean value of vehicle-injected eyes set at 100%. Values are mean ± SEM (n = 4–7 for each group). Statistical significance was determined by one-way ANOVA followed by Fisher's PLSD multiple comparisons test.
Subconjunctival Injection of P-IQACRG Protects RGCs from NMDA-Induced Excitotoxicity
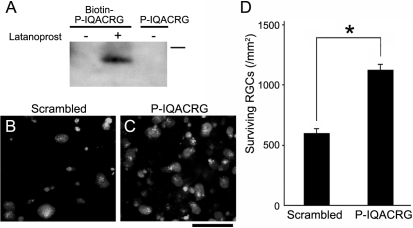
To develop a more clinically relevant treatment, we next administered P-IQACRG subconjunctivally. In this regard, we initially studied whether biotin-labeled P-IQACRG could reach the retina after posterior subconjunctival injection. After a single subconjunctival injection of biotin-P-IQACRG, we could not detect label in the retina (Fig. 9A, left lane). Previously, it was reported that the prostaglandin latanoprost could facilitate the penetration of peptides through the sclera.38,52 Therefore, we instilled latanoprost onto the sclera before the subconjunctival administration of biotin-P-IQACRG and then monitored biotin-P-IQACRG in the retina. Under these conditions, we observed the presence of biotin-P-IQACRG in the retina, as revealed by avidin-biotin immunochemistry (Fig. 9A, center lane; Supplementary Fig. S1, http://www.iovs.org/cgi/content/full/51/2/1198/DC1).
Figure 9.
Subconjunctival treatment with P-IQACRG protects RGCs from NMDA insult. (A) Rats received subconjunctival injection of biotin labeled P-IQACRG (3 nmol) after 12 days of daily instillation of saline or latanoprost. Twenty-four hours after subconjunctival injection of the peptides, retinal lysates were analyzed by avidin-biotin chemistry. Only animals receiving biotin-P-IQACRG plus latanoprost displayed a band representing the biotin-labeled peptide (n = 5 experiments; bar represents 3.5 kDa). (B, C) Rats received daily latanoprost instillation followed by subconjunctival injection of either scrambled peptide or P-IQACRG (30 nmol × 2 days). Before the last subconjunctival administration, NMDA (15 nmol) was delivered intravitreally. Twenty-four hours later, surviving RGCs were assessed by the presence of retrograde label (aminostilbamidine). RGCs were more densely distributed in eyes treated with P-IQACRG. Scale bar, 50 μm. (D) Quantification of surviving RGCs in retinal flat-mounts of animals treated with subconjunctival P-IQACRG or scrambled peptide after NMDA intravitreal injection. Subconjunctival injection of P-IQACRG protected RGCs from NMDA excitotoxicity. Values are mean ± SEM (*P < 0.001 by t-test; n = 9 for each group).
We next tested whether subconjunctival administration of P-IQACRG after latanoprost instillation could provide neuroprotection for RGCs. To obtain comparable or slightly higher levels of peptide in the retina after subconjunctival administration compared with those achieved by intravitreal injection of 150 pmol P-IQACRG, we calculated that a daily dose of 30 nmol of P-IQACRG would be necessary based on calculations from preliminary experiments using densitometry of avidin-biotin immunoblots of retina (Supplementary Fig. S2, http://www.iovs.org/cgi/content/full/51/2/1198/DC1). To monitor neuroprotection by P-IQACRG, 24 hours after intravitreal injection of NMDA, aminostilbamidine-labeled RGCs were counted in retinal flat-mounts. Eyes treated subconjunctivally with P-IQACRG exhibited an increase in surviving RGCs (1125.7 ± 40.8/mm2) compared with scrambled peptide (600.9 ± 29.6/mm2; P < 0.001; Student's t-test; Figs. 9B–D). Thus, subconjunctivally injected P-IQACRG may offer neuroprotection for RGCs.
Discussion
Excitotoxicity is thought to contribute, at least in part, to the pathophysiology of diseases affecting the retina and optic nerve, including diabetic retinopathy, retinal artery occlusion, optic neuropathy, and possibly glaucoma.7–13 Intravitreal injection of NMDA represents a relatively acute animal model for these retinal disorders that can be used to elucidate mechanisms of neuronal cell death and to evaluate potential neuroprotective agents. With this in vivo model, the present study demonstrated that the caspase substrate-binding peptide IQACRG protected RGCs from NMDA-induced excitotoxic damage by histologic criteria. Additionally, we developed a new functional assay for RGC activity after excitotoxic insult using electrophysiological recordings with an MEA system. This functional assay demonstrated that IQACRG protected spontaneous electrical activity and the light response of RGCs after NMDA insult. Although in our paradigm it is possible that the IQACRG peptide merely delayed RGC dysfunction or death, we would argue that a delay in damage might still prove beneficial in the management of a chronic progressive disease such as glaucoma.
Previous data had suggested that IQACRG prevents the processing of IL-1β through the inhibition of caspase-1 activity, though other caspase enzymes may be inhibited as well.17 We thus hypothesized that a contributing mechanism to neuroprotection by IQACRG might involve the suppression of neuroinflammatory pathways downstream of IL-1β. A growing body of evidence suggests that caspase-1 and IL-1β participate in neuronal damage, including excitotoxic damage.21,53–55 For example, intravitreal injection of NMDA increased IL-1β levels in the retina before RGC death.56,57 Additionally, caspase-1 inhibition58 or IL-1β siRNA57 abrogated NMDA-induced RGC death. However, none of these studies were able to distinguish between the precursor/proform of IL-1β and the mature/active form. This uncertainty occurred because of the lack of specific antibodies to distinguish between the two forms of IL-1β. Similarly, this technical difficulty prevented us from identifying an inhibitory effect of IQACRG on caspase-1 by directly monitoring IL-1β processing in the retina. Alternatively, therefore, we analyzed MMP-9 activity as a readout of IL-1β signaling because MMP-9 is activated downstream of IL-1β activation in neurons.20–22 Inhibition of MMP-9 activity is known to promote neuronal survival under pathologic conditions.23–26 Along these lines, we had previously shown that exposure to NMDA triggers MMP-9 activity in the retina, specifically in RGCs, contributing to subsequent RGC death.26 In the present study, we demonstrate that IQACRG inhibits NMDA-induced MMP-9 activation. Taken in conjunction with previous observations,17,20–26 these results are consistent with the notion that IQACRG protects neurons by preventing caspase-1 cleavage of IL-1β to trigger neurotoxic MMP-9 activity.
Additionally, we investigated another caspase-mediated cascade potentially influenced by IQACRG. Previous work had shown that the transcription factor MEF2C activates an antiapoptotic pathway in neurons at a transcriptional level.28–30 However, we found that caspase cleavage of MEF2C abolishes this transcriptional activity, thereby promoting apoptosis.30 In the present study, we found a reduction in full-length MEF2C protein levels in the retina after intravitreal injection of NMDA, consistent with the caspase-dependent cleavage of MEF2C.30 Administration of IQACRG prevented this decrease in MEF2C levels. Thus, neuroprotection by IQACRG may be mediated, at least in part, through the inhibition of MEF2C degradation. Additional caspase-mediated apoptotic pathways may be affected by IQACRG,59 but the fact that two such pathways, related to pro–IL-1β and MEF2C, have been implicated here implies that caspase inhibition is indeed a contributory factor to the neuroprotective properties of IQACRG in the retina (Fig. 1). Importantly, we postulate that the reversible mode of caspase inhibition by IQACRG17 would produce fewer clinical side effects than irreversible caspase pseudosubstrates, such as zVAD, which are known to produce problems when tested in humans.
Concerning the administration of IQACRG to the eye, transscleral drug delivery (e.g., subconjunctival or sub-Tenon injection) is a clinically tolerated and less invasive method of drug delivery to the posterior segment of the eye than intravitreal injection.31,32 For example, subconjunctivally administered drugs have been shown to inhibit pathologic angiogenesis in the retina.60–62 Heretofore, however, no neuroprotective drug has been reported to be delivered by this method. Because both human and rodent sclera are permeable to molecules as large as 70 kDa,63,64 smaller molecules could theoretically diffuse across the sclera. Nonetheless, other studies have shown that cell-penetrating peptides, such as TAT, derived from the human immunodeficiency virus, fail to reach the retina when injected into the subconjunctival space.65 Hence, we sought a different method for delivering P-IQACRG to the retina. Thus, in this study, we took advantage of a recently discovered method of allowing small peptides to cross the sclera by transiently permeabilizing the tissue with prostaglandin derivatives. In our case, prostaglandin F2 analogue (latanoprost), which is clinically used in glaucoma treatment to lower intraocular pressure, was used to enhance scleral permeability.38,52 Instillation of latanoprost facilitated the delivery of subconjunctivally injected P-IQACRG to the retina, as expected, and this combination therapy protected RGCs. Our data thus suggest that latanoprost instillation can be an effective strategy for the delivery of neuroprotective agents through the sclera to the diabetic, ischemic, or glaucomatous retina. Further studies in more relevant disease models should allow us to determine whether P-IQACRG has future clinical applications in these blinding diseases.
Supplementary Material
Acknowledgments
The authors thank Ziwei Huang and I. M. Krishna Kumar for peptide synthesis; Robert N. Weinreb, James D. Lindsey, Shu-ichi Okamoto, Tomohiro Nakamura, Jianguo Fang, Zezong Gu, Makoto Aihara, and Toru Nakazawa for helpful discussions; Jiankun Cui and Ken Mizuno for technical suggestions about surgical procedures; Hao Li, Hiroshi Nakamura, and Jun Ueda for their expertise in histology; Rameez Zaidi for his help with MEA data analysis; and Holly Dorlon, Dawn Dunsmore, and Bobbie Jo Larraga for manuscript preparation.
Footnotes
Supported in part by National Institutes of Health Grants R01 EY05477, R01 EY09024, and P01 HD29587 (SAL) and Blueprint Core Grant P30 NS057096 (La Jolla Interdisciplinary Neuroscience Center); the Astellas Foundation for Research on Metabolic Disorders; and the Japanese Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (MS).
Disclosure: M. Seki, None; W. Soussou, None; S. Manabe, None; S.A. Lipton, P
References
- 1.Lucas DR, Newhouse JP. The toxic effect of sodium l-glutamate on the inner layers of the retina. AMA Arch Ophthalmol 1957; 58: 193–201 [DOI] [PubMed] [Google Scholar]
- 2.Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature 1970; 227: 609–611 [DOI] [PubMed] [Google Scholar]
- 3.Hahn JS, Aizenman E, Lipton SA. Central mammalian neurons normally resistant to glutamate toxicity are made sensitive by elevated extracellular Ca2+: toxicity is blocked by the N-methyl-d-aspartate antagonist MK-801. Proc Natl Acad Sci USA 1988; 85: 6556–6560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sucher NJ, Aizenman E, Lipton SA. N-methyl-d-aspartate antagonists prevent kainate neurotoxicity in rat retinal ganglion cells in vitro. J Neurosci 1991; 11: 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988; 1: 623–634 [DOI] [PubMed] [Google Scholar]
- 6.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994; 330: 613–622 [DOI] [PubMed] [Google Scholar]
- 7.Ambati J, Chalam KV, Chawla DK, et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol 1997; 115: 1161–1166 [DOI] [PubMed] [Google Scholar]
- 8.Lieth E, Barber AJ, Xu B, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 1998; 47: 815–820 [DOI] [PubMed] [Google Scholar]
- 9.Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol 1998; 116: 906–910 [DOI] [PubMed] [Google Scholar]
- 10.Dreyer EB, Lipton SA. New perspectives on glaucoma. JAMA 1999; 281: 306–308 [DOI] [PubMed] [Google Scholar]
- 11.Lipton SA. Retinal ganglion cells, glaucoma and neuroprotection. Prog Brain Res 2001; 131: 712–718 [PubMed] [Google Scholar]
- 12.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol 2003; 48(suppl 1): S38–S46 [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi Y, Yagihashi T, Kezuka J, Muramatsu D, Usui M, Iwasaki T. Glutamate levels in aqueous humor of patients with retinal artery occlusion. Retina 2006; 26: 432–436 [DOI] [PubMed] [Google Scholar]
- 14.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res 1999; 18: 39–57 [DOI] [PubMed] [Google Scholar]
- 15.Naskar R, Vorwerk CK, Dreyer EB. Saving the nerve from glaucoma: memantine to caspases. Semin Ophthalmol 1999; 14: 152–158 [DOI] [PubMed] [Google Scholar]
- 16.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995; 376: 37–43 [DOI] [PubMed] [Google Scholar]
- 17.Troy CM, Stefanis L, Prochiantz A, Greene LA, Shelanski ML. The contrasting roles of ICE family proteases and interleukin-1beta in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc Natl Acad Sci USA 1996; 93: 5635–5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenneti L, D'Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-d-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem 1998; 71: 946–959 [DOI] [PubMed] [Google Scholar]
- 19.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol 1998; 8: 84–87 [PubMed] [Google Scholar]
- 20.Vecil GG, Larsen PH, Corley SM, et al. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res 2000; 61: 212–224 [DOI] [PubMed] [Google Scholar]
- 21.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol 2005; 5: 629–640 [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Xu Z-Z, Wang X, et al. Distinct roles of matrix metalloproteases in the early and late-phase development of neuropathic pain. Nat Med 2008; 14: 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Z, Kaul M, Yan B, et al. S-Nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 2002; 297: 1186–1190 [DOI] [PubMed] [Google Scholar]
- 24.Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem 2002; 277: 47461–47468 [DOI] [PubMed] [Google Scholar]
- 25.Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 2005; 25: 6401–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manabe S, Gu Z, Lipton S. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci 2005; 46: 4747–4753 [DOI] [PubMed] [Google Scholar]
- 27.Leifer D, Krainc D, Yu Y, et al. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci USA 1993; 90: 1546–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 1999; 286: 785–790 [DOI] [PubMed] [Google Scholar]
- 29.Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA 2000; 97: 7561–7566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto S, Li Z, Ju C, et al. Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc Natl Acad Sci USA 2002; 99: 3974–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci 2000; 41: 961–964 [PubMed] [Google Scholar]
- 32.Ambati J, Adamis AP. Transscleral drug delivery to the retina and choroid. Prog Retin Eye Res 2002; 21: 145–151 [DOI] [PubMed] [Google Scholar]
- 33.Manabe S, Lipton S. Divergent NMDA signals leading to proapoptotic and antiapoptotic pathways in the rat retina. Invest Ophthalmol Vis Sci 2003; 44: 385–392 [DOI] [PubMed] [Google Scholar]
- 34.Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci 1998; 39: 972–981 [PubMed] [Google Scholar]
- 35.Seki M, Tanaka T, Nawa H, et al. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 2004; 53: 2412–2419 [DOI] [PubMed] [Google Scholar]
- 36.Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci 1998; 18: 4656–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aihara M, Lindsey JD, Weinreb RN. Reduction of intraocular pressure in mouse eyes treated with latanoprost. Invest Ophthalmol Vis Sci 2002; 43: 146–150 [PubMed] [Google Scholar]
- 38.Weinreb RN. Enhancement of scleral macromolecular permeability with prostaglandins. Trans Am Ophthalmol Soc 2001; 99: 319–343 [PMC free article] [PubMed] [Google Scholar]
- 39.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem 1996; 271: 18188–18193 [DOI] [PubMed] [Google Scholar]
- 40.Seki M, Nawa H, Fukuchi T, Abe H, Takei N. BDNF is upregulated by postnatal development and visual experience: quantitative and immunohistochemical analyses of BDNF in the rat retina. Invest Ophthalmol Vis Sci 2003; 44: 3211–3218 [DOI] [PubMed] [Google Scholar]
- 41.Li H, Radford JC, Ragusa MJ, et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci USA 2008; 105: 9397–9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron 1993; 11: 689–701 [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, Behrens A, Le-Niculescu H, et al. Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Invest Ophthalmol Vis Sci 2002; 43: 1631–1635 [PubMed] [Google Scholar]
- 44.Kikuchi M, Tenneti L, Lipton SA. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J Neurosci 2000; 20: 5037–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danias J, Shen F, Goldblum D, et al. Cytoarchitecture of the retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci 2002; 43: 587–594 [PubMed] [Google Scholar]
- 46.Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 2006; 50: 923–935 [DOI] [PubMed] [Google Scholar]
- 47.Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron 2001; 32: 439–449 [DOI] [PubMed] [Google Scholar]
- 48.Lyons JG, Siew K, O'Grady RL. Cellular interactions determining the production of collagenase by a rat mammary carcinoma cell line. Int J Cancer 1989; 43: 119–125 [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharjee J, Nunes Cardozo B, Kamphuis W, Kamermans M, Vrensen GF. Pseudo-immunolabelling with the avidin-biotin-peroxidase complex (ABC) due to the presence of endogenous biotin in retinal Muller cells of goldfish and salamander. J Neurosci Methods 1997; 77: 75–82 [DOI] [PubMed] [Google Scholar]
- 50.Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-d-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci 1999; 40: 2391–2397 [PubMed] [Google Scholar]
- 51.Krainc D, Bai G, Okamoto S, et al. Synergistic activation of the N-methyl-D-aspartate receptor subunit 1 promoter by myocyte enhancer factor 2C and Sp1. J Biol Chem 1998; 273: 26218–26224 [DOI] [PubMed] [Google Scholar]
- 52.Aihara M, Lindsey JD, Weinreb RN. Enhanced FGF-2 movement through human sclera after exposure to latanoprost. Invest Ophthalmol Vis Sci 2001; 42: 2554–2559 [PubMed] [Google Scholar]
- 53.Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull 1992; 29: 243–246 [DOI] [PubMed] [Google Scholar]
- 54.Hagan P, Poole S, Bristow AF, Tilders F, Silverstein FS. Intracerebral NMDA injection stimulates production of interleukin-1 beta in perinatal rat brain. J Neurochem 1996; 67: 2215–2218 [DOI] [PubMed] [Google Scholar]
- 55.Hara H, Friedlander RM, Gagliardini V, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA 1997; 94: 2007–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kido N, Inatani M, Honjo M, et al. Dual effects of interleukin-1beta on N-methyl-d-aspartate-induced retinal neuronal death in rat eyes. Brain Res 2001; 910: 153–162 [DOI] [PubMed] [Google Scholar]
- 57.Kitaoka Y, Munemasa Y, Nakazawa T, Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res 2007; 1142: 247–255 [DOI] [PubMed] [Google Scholar]
- 58.Kwong JM, Lam TT. N-methyl-d-aspartate (NMDA) induced apoptosis in adult rabbit retinas. Exp Eye Res 2000; 71: 437–444 [DOI] [PubMed] [Google Scholar]
- 59.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ 2007; 14: 66–72 [DOI] [PubMed] [Google Scholar]
- 60.Escalona-Benz E, Jockovich ME, Murray TG, et al. Combretastatin A-4 prodrug in the treatment of a murine model of retinoblastoma. Invest Ophthalmol Vis Sci 2005; 46: 8–11 [DOI] [PubMed] [Google Scholar]
- 61.Economopoulou M, Bdeir K, Cines DB, et al. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood 2005; 106: 3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruysberg LP, Franklin AJ, Sanders J, et al. Effective transscleral delivery of two retinal anti-angiogenic molecules: carboxyamido-triazole (CAI) and 2-methoxyestradiol (2ME2). Retina 2005; 25: 1022–1031 [DOI] [PubMed] [Google Scholar]
- 63.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability: effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci 1995; 36: 1893–1903 [PubMed] [Google Scholar]
- 64.Kim TW, Lindsey JD, Aihara M, Anthony TL, Weinreb RN. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci 2002; 43: 1809–1816 [PubMed] [Google Scholar]
- 65.Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci 2006; 47: 2589–2595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.