Albumin, a major antioxidant in the body, is increased in the retina of monkey eyes with experimental glaucoma and the increase is positively correlated with severity of glaucoma damage. The increased intracellular albumin may contribute to the survival of injured neurons in the glaucomatous retina.
Abstract
Purpose.
To establish the identity of a prominent protein, approximately 70 kDa, that is markedly increased in the retina of monkeys with experimental glaucoma compared with the fellow control retina, the relationship to glaucoma severity, and its localization in the retina.
Methods.
Retinal extracts were subjected to 2-D gel electrophoresis to identify differentially expressed proteins. Purified peptides from the abundant 70 kDa protein were analyzed and identified by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) separation, and collision-induced dissociation sequencing. Protein identity was performed on MASCOT (Matrix Science, Boston, MA) and confirmed by Western blot. The relationship between the increase in this protein and glaucoma severity was investigated by regression analyses. Protein localization in retina was evaluated by immunohistochemistry with confocal imaging.
Results.
The abundant protein was identified as Macaca mulatta serum albumin precursor (67 kDa) from eight non-overlapping proteolytic fragments, and the identity was confirmed by Western blot. The average increase in retinal albumin content was 2.3 fold (P = 0.015). In glaucoma eyes, albumin was localized to some neurons of the inner nuclear layer, in the inner plexiform layer, and along the vitreal surface, but it was only found in blood vessels in control retinas.
Conclusions.
Albumin is the abundant protein found in the glaucomatous monkey retinas. The increased albumin is primarily localized to the inner retina where oxidative damage associated with experimental glaucoma is known to be prominent. Since albumin is a major antioxidant, the increase of albumin in the retinas of eyes with experimental glaucoma may serve to protect the retina against oxidative damage.
Glaucoma is a leading cause of blindness worldwide affecting over 67 million people.1 Primary open-angle glaucoma (POAG) is the most common form of the disease and accounts for 90% of glaucoma patients. This form of the disease is generally insidious in origin, bilateral, and progresses slowly with visual symptoms occurring primarily in late stages of the disease. Normal intraocular pressure (IOP) ranges from 8 to 21 mm Hg, and individuals with IOP higher than 21 mm Hg are considered to be at risk for developing glaucoma. However, some patients with abnormally high IOP never develop visual deficits, while some patients with visual field loss characteristic of glaucoma do not have abnormally high pressures. In some families, the disorder appears to be genetically determined,2 but some of the mutations found associated with POAG show ethnic bias.3–5
Several hypotheses have been proposed for potential mechanisms triggering retinal ganglion cell injury and death in glaucoma. Mechanisms that have been proposed include compromise to blood flow at the optic nerve,6,7 mechanical compression,8 loss of neurotrophic factors and TrkB receptor alterations,9,10 autoimmune mechanisms,11–13 nitric oxide–induced injury to the optic nerve,14–16 and glutamate excitotoxicity.17–19 In addition to these primary mechanisms, a number of studies have provided evidence that oxidative stress and damage contribute to degeneration of retinal ganglion cells in glaucoma.20 Oxidative changes have been reported in the retinas of animals with experimentally induced glaucoma, including a decrease in endogenous antioxidant enzyme activity, an increase in lipid peroxidation21 and protein oxidation,22 and an up-regulation of iron regulatory proteins that have antioxidant properties.23 These oxidative alterations in glaucoma may contribute directly to ganglion cell damage and death, or trigger downstream effectors,20 and can be initiated by many of the mechanisms of glaucomatous neuropathy that have been proposed. Although much is known about the pathologic changes in glaucoma retinas, the precise mechanisms contributing to the injury and death of retinal ganglion cells (RGCs) are not fully understood.
Regardless of the precise mechanisms, gene regulation and expression must be altered in the retinas of glaucomatous eyes compared with normal retinas. Changes may occur at transcriptional, translational, or post-translational levels and can occur as a result of RGC cell death or as a result of protective responses of the retinal cells to the adverse stress. Identification and characterization of proteins that are either up- or downregulated in the glaucomatous eye may provide important insights into the mechanisms contributing to RGC death. In the present investigations of protein levels in the monkey retinas of control eyes and eyes with experimental glaucoma, elevated levels of a protein with a molecular weight of approximately 70 kDa was found in the glaucoma retinas. The protein was isolated by 2-D gel electrophoresis, analyzed by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS), and revealed to be albumin. The prevalence and magnitude of the increase in albumin, after the induction of experimental glaucoma, were determined, as well as the relationship to the severity of glaucoma and the retinal localization in glaucomatous eyes.
Materials and Methods
Animals
Seven rhesus monkeys (Macaca mulatta) ranging in age from 7 to 11 years of age (six males and one female, ocular hypertensive [OHT]-40) were subjects for the study. The high intraocular pressure (IOP) model of experimental glaucoma was created by laser scarification in the drainage angle to restrict the outflow of aqueous humor from the anterior chamber and, thereby, elevate the IOP.24 Typically, several laser treatments, separated by at least four weeks, were required to create sustained IOP levels and initiate an optic neuropathy. The effects of experimental glaucoma were assessed over periods of 2 to 14 months after elevated IOP by behavioral methods using standard automated perimetry (SAP; Humphrey Field Analyzer; Carl Zeiss Meditec, Inc., Jena, Germany) for monkeys that had been trained to perform a psychophysical detection task.25,26 With well-trained subjects, behavioral SAP produced visual field data for monkeys in the same format as the standard clinical data for humans. The stage of glaucomatous neuropathy was based on the visual fields because of the prior studies that showed a poor relationship between visual field defects and the level or duration of experimental glaucoma,27 but a high correlation between visual field defects and the density of RGCs.28
Male Wistar rats weighing 350 g–450 g were anesthetized with a mixture of ketamine (50 mg/kg), acepromazine (1 mg/kg), and xylazine (5 mg/kg). IOP was elevated by laser photocoagulations (blue-green argon laser; Coherent, Palo Alto, CA) of episcleral veins approximately 50 μm in diameter (1 watt for 0.2 seconds) within 0.5 mm–0.8 mm from the limbus and on the veins around the limbus as described previously.29 IOP was measured (TONO-PEN; Mentor, Norwell, MA30) on rats calmed with acepromazine, 3.0 mg/kg intramuscularly, and proparacaine (0.5%; Opthetic; Allergan, Irvine, CA) applied topically on the eyes to anesthetize the cornea. The average of 15 readings for each eye was taken as the IOP for that eye. Three rats were donated for the study with average control IOP of 15.08 ± 0.0949 (± SD) mm Hg and elevated IOP of 31 ± 2.035 mm Hg for two weeks. All procedures were conducted in accordance with approved procedures by the Institutional Animal Care and Use Committees at The University of Houston, The University of Texas Health Science Center at Houston and Allergan, Inc., and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tissue Preparation
Eyes were removed from deeply anesthetized monkeys and a sample of retina taken by a 5-mm dermal biopsy punch beginning 20° from the fovea and extending peripherally to 40° from the temporal inferior retina in each eye. The retina was detached from the pigment epithelium, placed in small conical tubes, quickly frozen in liquid nitrogen and stored at –80°C. The remaining eyecups were fixed in 4% paraformaldehyde (0.1 M phosphate buffer; pH 7.4).
One-D and Two-D Gel Electrophoresis
To generate cytosolic protein preparations, frozen monkey retinas were ultra-sonicated 10 seconds in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.5% NP-40) with 2% protease inhibitors cocktail (Sigma Aldrich, St. Louis, MO), incubated on ice for 30 minutes, and centrifuged at 14,000 rpm for 15 minutes at 4°C. Protein concentration was determined using a bicinchoninic acid protein assay kit (BCA Protein Assay Kit; Pierce, Rockford, IL) with bovine serum albumin (BSA) as the reference standard. For 1-D gel analysis, 20 μg protein was diluted in an equal volume of 2X Laemmli buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, and 200 mM β-mercaptoethanol), and the samples were boiled for five minutes. After centrifugation, the supernatant was separated on a 15% SDS-PAGE. For 2-D analysis, 20 μg protein was dialyzed against 7 M urea (3.5k MWCO; Pierce Biotechnology, Rockford, IL) for 1.5 hours at room temperature. The retentate was diluted in sample buffer containing 7 M urea, 2 M thiourea, 2% CHAPS, and 65 mM DTT, and 15 μg protein was loaded by passive rehydration onto 7-cm strips (Immobiline DryStrips, pH 3–11NL; GE Healthcare, Piscataway, NJ) overnight at room temperature. For the first dimension separation, isoelectric focusing (IEF) was performed at 15°C using a maximum 50 mA/strip current. The IEF program consisted of: 450 V/hr at 300 V, 325 V/hr ramped to 1000 V, 500 V/hr at 1000 V, 1250 V/hr at 5000V, and 583 V/hr at 7000 V. For the second dimension, IEF strips were equilibrated in buffer containing 50 mM Tris-HCl (pH 6.8), 6 M urea, 30% glycerol, 2% SDS, and 65 mM DTT, followed by the same buffer containing 2.5% iodoacetamide, then separated on a 12% acrylamide gel by SDS-PAGE.31
Total protein was stained (Deep Purple; GE Healthcare) following the manufacturer's suggested protocol, and images of the proteins pattern were scanned (Molecular Imager FX and Quantity One software; Bio-Rad, Hercules, CA). For mass spectrometry identification, gels were restained (Coomassie Colloidal Blue; Invitrogen, Carlsbad, CA) according to the suggested protocol, and the desired spots excised. The gel punches (see Fig. 2) were destained for 15 minutes in 30% ethanol, re-equilibrated with water, and analyzed by LC/MS/MS as described below.
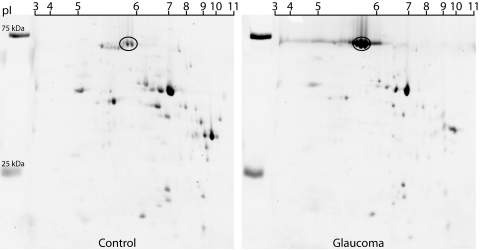
Figure 2.
Separation of the 70 kDa protein by two-dimensional PAGE. Retinal protein extract from monkey OHT 46 was separated by 2-D–SDS-PAGE and stained (Deep Purple; GE Healthcare). The spots corresponding to the abundant 70 kDa protein indicated by the oval were excised, destained, and subjected to proteolytic digestion for determination of amino acid sequences by LC/MS/MS analysis.
Protein Identification
Proteins were identified in the Proteomics Core Facility at the University of Texas Health Science Center at Houston. Analysis was performed using LC/MS/MS (QStar XL; Applied Biosystems, Foster City, CA) equipped with an LC Packings high-performance liquid chromatography (HPLC) for capillary chromatography. Direct analysis of the eluate was made by HPLC coupled to the mass spectrometer by a nanospray ESI (Electrospray Ionization) head. For protein identification, spots of interest were excised from the gel with a clean glass pipette and subjected to in-gel proteolytic digestion with trypsin essentially as described previously.32 After extraction of the peptides, an aliquot was separated by HPLC on a C18 75 μm x 10 cm reversed-phase capillary column (Vydac 218MS3.07510; Grace, Deerfield, IL). The column was developed with a gradient of 2% to 50% acetonitrile in 0.1% formic acid over 30 minutes at a flow rate of 200 nL/min. The nanospray source, fitted with a 30-μm coated tapered fused silica tip (New Objective, Cambridge, MA), was used to elute the peptides directly into the mass spectrometer for tandem mass spectrometry (MS/MS) analysis. The LC/MS/MS instrument (QStar XL; Applied Biosystems) was operated in Information Dependent Acquisition mode using a one second survey scan followed by two consecutive three second product ion scans of 2+, 3+, and 4+ parent ions (m/z 350-1500). Identification was performed using the MASCOT (Matrix Science, Boston, MA) search engine (Matrixscience.com) with an MS and MS/MS mass tolerance of 0.15 Da.
Western Blot Analysis
Fifteen μg of retinal protein extract from the control and the glaucoma retinas were loaded on 8% gels in neighboring lanes for each of seven monkeys, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. The membranes were blocked with 5% nonfat milk in TBST buffer (10 mM Tris, 154 mM NaCl [pH 7.4], 0.05% Tween-20) and incubated overnight at 4°C with a monoclonal anti-albumin antibody (A6684;1:20,000; Sigma Aldrich) in the blocking buffer. Membranes were washed in TBST and exposed to a peroxidase-conjugated goat anti-mouse secondary antibody (Pierce, Rockford, IL) diluted 1:50,000 in blocking buffer for one hour at room temperature. The membranes were washed and immunoreactive bands visualized by pico luminol/enhancer solution (SuperSignal West; Pierce). The blots were stripped and reprobed with monoclonal anti-β-actin antibody (A5441, 1:50,000; Sigma Aldrich) to normalize data for differences in quantity of protein loaded.
Immunohistochemistry
Samples of monkey retina, one millimeter in diameter (contiguous with that for biochemical analysis), were taken from temporal retina between 40° and 45° from the fovea of control and glaucoma eyes and embedded in ultra-low gelling temperature agarose (Sigma-Aldrich). Sections of retina were cut at a thickness of 40 μm on a vibratome (Vibratome 1000 Plus Sectioning System; Technical Products International, Inc., St. Louis, MO), and blocked in a solution containing 3% normal goat serum, 0.3% Triton X-100, and 0.1% sodium azide in PBS for three hours at room temperature. The sections were exposed to the same monoclonal anti-albumin antibody used for the Western blot analysis (1:1000) diluted in 1% normal goat serum, 0.3% Triton X-100, 0.1% sodium azide in PBS for three hours at room temperature. All sections were washed with 1% normal goat serum, 0.3% Triton X-100, and 0.1% sodium azide in PBS for 6 × 30 minutes, and then incubated with Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The nuclei were stained with DAPI (4′-6-diamidino-2-phenylindole), a DNA stain. Images of retina from control and experimental retinas were taken using identical parameters with a confocal microscope (LSM 510; Carl Zeiss Inc., Jena, Germany).
Results
Differential Expression Pattern of Retinal Proteins
The expression pattern of retinal proteins in eyes with experimental glaucoma differed from that of control eyes. Retinal proteins separated by SDS-PAGE and stained with the fluorescent protein dye revealed an apparent increase in protein in a band near 70 kDa, and apparent decreased protein content in several protein bands, in samples from eyes with experimental glaucoma compared to that of control eyes (Fig. 1). The increase in protein abundance near 70 kDa was a robust and consistent finding and was chosen for further investigation to identify proteins(s) whose relative abundance is increased after the induction of experimental glaucoma.
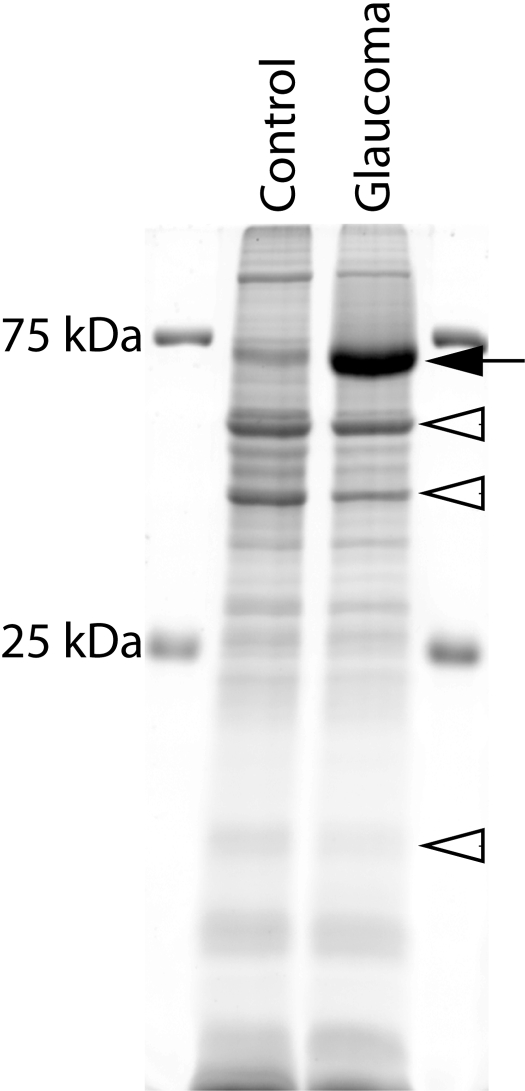
Figure 1.
Identification of a 70 kDa protein overexpressed in the retina of monkeys with experimental glaucoma. Total protein dye (Deep Purple; GE Healthcare)-stained gel of retinal proteins from the control (C) and glaucoma (G) eye of the same monkey (OHT 46; inferior 40°–45°) separated by SDS-PAGE. Although equal amounts of proteins (20 μg) were loaded in each lane, the protein content near 70 kDa was obviously greater in the glaucoma retina (lane 2) than in the control retina. This same pattern of labeling is seen in six of the monkeys studied. Only five to six prominent protein bands were detected since the amount of protein loaded in each well is 1.5–6 times lower than that in most studies. Filled arrow: increased 70 kDa protein band; open arrowheads: decreased proteins.
Identity of the Abundant Protein Near 70 kDa
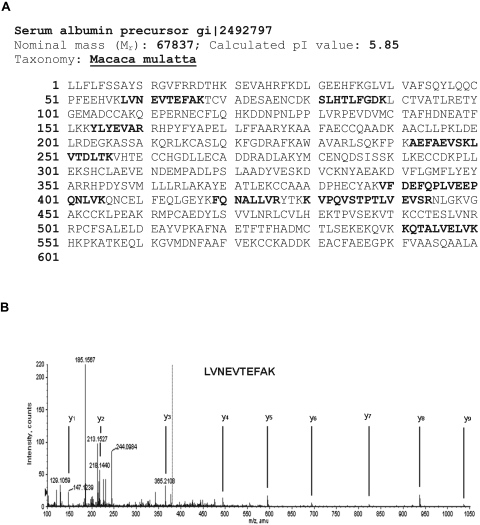
The protein band near 70 kDa may contain several proteins, therefore further protein separation by 2-D gel was conducted and spots containing the abundant protein near 70 kDa were removed for analysis by mass spectrometry (Fig. 2). In-gel proteolytic digestion of the excised spots with trypsin recovered eight peptides. MS/MS collision-induced dissociation was used to establish the amino acid sequences of eight peptides generated from the trypsin proteolytic digestion. MASCOT search results using the peptide masses and amino acid sequences positively identified the protein as serum albumin (predicted MW-67,837 Da, pl-5.85). As shown in Figure 3A, eight non-overlapping peptides (shown in bold) gave over 15% sequence coverage, and the predicted molecular weight and isoelectric point is in agreement with the spot migrations (Fig. 2). Identity or extensive homology is indicated for peptides with ion scores > 42 (P < 0.05). Ions scores for the eight tryptic peptides identifying albumin ranged from 46 to 65. The MS/MS spectrum for a representative peptide is shown in Figure 3B.
Figure 3.
Identification of the 70 kDa protein as albumin by MS/MS analysis and MASCOT search. (A) The amino acid sequence of serum albumin (Macaca mulatta) is shown at the top with bold regions indicating the eight tryptic peptides identified by LC/MS/MS analysis. The eight peptides are a part of the amino acid sequence of serum albumin. (B) An example spectrum of the peptide LVNEVTEFAK is shown. The spectrum shown in this example was obtained by LC/MS/MS analysis of the doubly charged [M + 2H+] ion.
To confirm the protein identity as albumin, Western blot analysis was conducted on equal amounts of retinal protein extracts from control and glaucomatous retinas. A prominent single band for albumin was detected in all lanes near 70 kDa, when the membrane was probed with an antibody directed against human albumin (antibody specificity determined before use). This positive labeling with the antibody to human albumin confirmed the identity of the abundant protein as albumin (Fig. 4A).
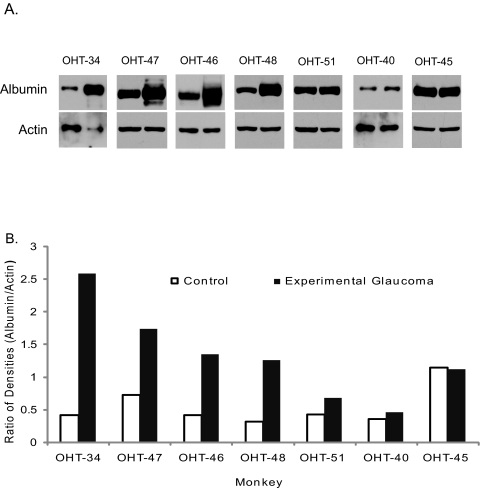
Figure 4.
Confirmation that albumin is elevated in the retina of monkeys with experimental glaucoma by Western Blot analysis. Equal amounts of retinal protein extracts from control and glaucoma monkey eyes were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with an antibody to human serum albumin. Each pair of blots contains sample from the control (first lane) and glaucoma eye (second lane) of the same monkey for each of seven monkeys. (A) Labeling for albumin is apparently greater in the retina of the glaucoma eye compared with the companion control eye. (B) Quantitative analysis of the relative change of albumin in the retina of glaucoma eyes to that of the fellow control eye for each monkey, after normalizing to β-actin, show increased amounts of albumin in all but one of the glaucoma eyes compared with control eyes.
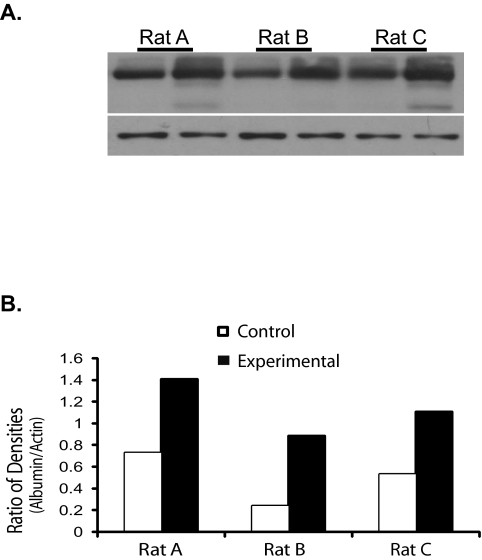
To quantitatively analyze the increase in albumin in the glaucomatous retina, the mean ratio in immunolabeling density for albumin between the two eyes of each monkey was compared, after normalizing the density of the albumin band to that of β-actin in the same lane. To control for possible variation between monkeys, the non-lasered eye (control) was compared with the laser-treated glaucomatous eye of the same monkey. The paired t-test was performed to see if the difference in albumin expression between the control and experimental retinas is significant. This analysis revealed that the average increase of albumin was 2.3-fold in the glaucoma retinas (Fig. 4B; log-transformed paired t-test; P = 0.0150; SE of ratio = 1.28) versus the matched control retinas. These results clearly indicate that an increase in albumin may be a common feature of glaucoma in monkey. We then extended our study to include another popular glaucoma animal model and to test whether elevated albumin can be observed in the glaucomatous eyes in rats. Three rats with ocular hypertension for two weeks were donated for the study. Intraocular pressure was elevated by laser coagulation and the IOP monitored as described previously.33 The average IOP of control eyes was 15.08 ± 0.0949 (± SD) mm Hg, and the average IOP in fellow ocular hypertensive eyes was 31 ± 2.035 mm Hg. A similar increase (2.4-fold; log-transformed paired t-test; P = 0.0483; SE of ratio = 1.23) in albumin content was found in the three rats with experimental glaucoma (Fig. 5). Retinal ganglion cell loss at two weeks is 26% ± 7%.33,34 Data from the seven monkeys and three rats with experimental glaucoma strongly suggest that elevation of albumin in retina may be a general response that is caused by the death of retinal ganglion cells initiated by high intraocular pressure.
Figure 5.
Increased albumin content is observed in the retina of OHT rat eyes. (A) Equal amounts of retinal protein extracts from control (lanes 1, 3, and 5) and from OHT (lanes 2, 4, and 6) eyes were probed with an antibody directed against rat albumin (rabbit anti-rat albumin; RAL-25A, 1:5000; Immunology Consultants Laboratory Inc., Newberg, OR). Albumin content appears greater in the retinas from OHT eyes compared with their fellow control eyes. (B) Quantitative analysis of the relative albumin content (normalized to β-actin) between the eyes of the three rats clearly shows more albumin in the retina of OHT eyes.
Relationship between Albumin Level and Severity of Glaucoma
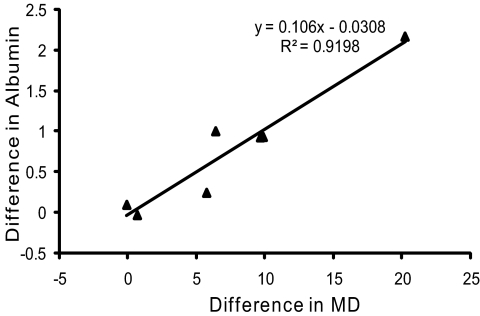
The obvious variation in the level of albumin among the seven experimental monkeys (0.5–2.7-fold increase; Fig. 4) indicated a possible correlation between the magnitude of albumin elevation and the severity of glaucoma damage or duration of glaucoma. Comparison of elevated albumin to duration of glaucoma showed no clear correlation (Table 1). However, examination of the data in Table 1 suggested a positive correlation with glaucoma severity. The perimetric mean deviation (MD), which is an average of visual sensitivity loss across test locations, provides a global measure of visual deficits that is commonly used as an indicator of the severity of glaucoma (Table 1). Regression analysis was performed to determine whether a correlation is found between albumin abundance and glaucoma severity or duration of glaucoma. The difference in mean deviation between the control and glaucoma eye for each monkey was plotted against the difference in density of normalized albumin labeling between the two eyes of each monkey (Fig. 6). The linear regression analysis indicates that albumin density increases 0.106 (± 0.2014) units above the control eye (P = 0.0006) for each unit of increase in mean deviation (R2 = 0.9198). However, no correlation was found between duration of glaucoma and the abundance of albumin by linear regression analysis (R2 = 0.0067). The lack of correlation between duration and retinal pathology in glaucomatous monkey retinas is consistent with our previous findings.27 These analyses further confirmed a positive association between the MD and increased albumin content and, even with the small sample, strongly imply that albumin content in the retina increases with the severity of glaucomatous damage and not with duration.
Table 1.
Monkey Subjects: Mean Deviation and Duration
| Monkey | Mean Deviation (dB) |
Duration (mo) | |
|---|---|---|---|
| Control | Glaucoma | ||
| OHT-34 | 0.00 | −20.22 | 2 |
| OHT-47 | 2.45 | −8.85 | 11 |
| OHT-46 | 0.24 | −9.91 | 2 |
| OHT-48 | 0.19 | −10.06 | 14 |
| OHT-51 | 2.82 | −8.56 | 9 |
| OHT-40 | 2.48 | −2.39 | 2 |
| OHT-45 | 2.62 | −3.30 | 5 |
Figure 6.
Increase in albumin is positively correlated to the severity of visual field defect. Scatterplot of the differences in immunolabeling densities of albumin and the differences in the mean deviation (indicator of severity) reveals a positive association (R2 = 0.9198) between the severity of visual field defect and increased albumin immunolabeling density.
Localization of Albumin in Retina
Immunohistochemistry was used to identify the retinal localization of increased albumin. Sections of monkey retina, from the inferior temporal retina between 40° and 45° from the fovea of control and glaucoma eyes, were immunolabeled with the monoclonal anti-albumin antibody, stained with DAPI and examined by confocal microscopy. In control retinas, albumin was localized predominately to blood vessels (Fig. 7A). No labeling for albumin was detected in retinal neurons or glia. In contrast, the glaucomatous retinas (Fig. 7B) contained a high level of albumin immunoreactivity in some neurons of the inner nuclear and the inner plexiform layers, and along the vitreal surface.
Figure 7.
Abnormal localization of albumin in the retina of monkeys with experimental glaucoma. Albumin in the retina of Monkey OHT 46 was localized by immunohistochemical detection. Samples, one millimeter in diameter, were taken from the inferior temporal retina between 40° and 45° from the fovea of control and glaucoma eyes. (A) Albumin immunoreactivity (green) is found in blood vessels of the inner retina of control retinas (arrowheads). (B) Glaucoma retina shows albumin (green) in the inner retina in some cell bodies of the inner nuclear layer (INL; arrows), in the inner plexiform layer (IPL), and along the inner limiting membrane (ILM). (C) Higher magnification of control retina shows 3 to 4 ganglion cell nuclei in the ganglion cell layer (GCL). (D) A similar area in glaucomatous retina shows no remaining ganglion cell nuclei. Nuclei (red) stained with DAPI (4′-6-diamidino-2-phenylindole), which binds to DNA. ONL, outer nuclear layer. Scale bars, 20 μm.
Discussion
Using two independent methods, proteomic analysis and Western blot, we have demonstrated that albumin is an abundant protein in the retinas of glaucomatous monkey eyes. The average albumin content is increased approximately 2.3-fold in the glaucomatous retinas when compared to retinas of fellow-eye controls. Further, a similar increase in albumin content is found in the three retinas of glaucomatous rat eyes. We demonstrated by immunohistochemistry that in addition to the typical location of albumin in blood vessels, albumin is also found at abnormally high levels outside of blood vessels in glaucomatous retinas. Albumin is found localized to some neuronal cell bodies of the inner nuclear layer and within cell processes, as indicated by positive immunolabeling for albumin in the inner plexiform layer in glaucomatous eyes. These results suggest that elevation of albumin may be a general consequence in the pathophysiology of glaucoma.
Some of the intravascular albumin is redistributed into the anterior and posterior chambers and the vitreous of the eye via the ciliary body and iris root.35 In extraocular tissues, albumin has many functions which include maintenance of osmotic pressure, transport of hormones, fatty acids, bilirubin, binding to toxins and metal ions rendering them inactive, along with antioxidant properties.36 In vitreous, albumin is the dominant protein, and although its functions in the eye are not yet fully characterized, it likely serves many of the same functions as it does in other body tissues.
There is evidence indicating that oxidative injury contributes to retinal damage in glaucoma.37 In rats with experimental glaucoma, oxidized proteins have been detected in the retina by Western blot analysis and are localized predominately to the inner retina.22 Evidence of oxidative damage also has been shown in retinas of human patients with glaucoma.37 The present investigations have demonstrated that albumin levels are increased in the retina of monkeys with experimental glaucoma, and it appears to be localized to the same retinal regions reported to exhibit oxidative damage in eyes of rats and humans with glaucoma.20,37 The intracellular increase of albumin in the retinas of monkey eyes with experimental glaucoma also may occur in response to increased oxidative injury.
Several studies have supported a role for albumin in neuronal protection and recovery from oxidative insults.38 After transient ischemia in rats, several studies showed that intravenous serum albumin treatment reduced histologic damage, enhanced behavioral recovery,39 and improved vascular dynamics.40 Based on the positive findings in the rat model of stroke, clinical trials were initiated and are currently in Phase III to evaluate the efficacy of albumin as part of the treatment strategy for stroke patients.41 Albumin treatments also enhance functional recovery of rats with experimental spinal cord injury.42 The neuroprotective effects of albumin may be exerted directly on neurons in the models of stroke and spinal cord injury since in vitro studies show that the excitotoxic effects of glutamate on spinal cord neurons is reduced when treated with albumin,42 and it protects cortical neurons,43 as well as non-neuronal cells such as pancreatic β cells from hydrogen peroxide-induced cell death.44 In our laboratory, preliminary studies show that albumin protects RGC-5 (immortalized retinal ganglion cells) against cell death induced by oxidative insult (RR, LCD, XCZ, unpublished observation, 2008). Together, these studies indicate that albumin contributes to the protection of cells experiencing oxidative injury. These studies suggest that the presence of extravascular albumin in the inner retina of eyes with glaucoma may assist in protecting retinal neurons against oxidative damage.
Extravasated albumin can be translocated intracellularly. For example, albumin is taken up by cell bodies and dendrites of neurons in the brain after transient ischemia.45,46 Lens epithelial cells also take up albumin after in vivo injection into the anterior chamber of rat eyes and it plays a role in the transport of long chain fatty acids into the lens epithelium47,48 for subsequent incorporation into numerous lipids. It is possible that albumin, along with fatty acids, is translocated into cells experiencing oxidative injury in glaucoma and that the amount of albumin taken up is relative to the degree of injury.
The increased abundance of albumin in the retina of glaucomatous eyes is likely derived from one or more of these sources: (1) retinal vasculature, (2) vitreous, or (3) de novo synthesis within retina. Normally, as with the brain, there is a blood–retina barrier that is established by the retinal pigment epithelium and the tight junctions formed between the endothelial cells lining the lumen of retinal vessels. Typically, albumin and other proteins or hormones destined for the extravascular space must be transported across the endothelium. Enhanced transport, or a breakdown in the blood–retina barrier, would result in an increase in extravascular albumin. An increase in vascular permeability has been reported to occur in the optic nerve head of patients with open-angle glaucoma.49 Several other studies have reported evidence of vascular anomalies leading to reduced vascular perfusion of the retina and the optic nerve in patients with glaucoma, which can lead to hypoxia. Hypoxia in human glaucoma is supported by the presence of increased immunoreactivity for hypoxia-inducible factor α (HIF-1α) in the inner retina, blood vessels, and optic nerve compared with age-matched controls.50 Hypoxia can lead to disruption of the endothelial barrier function in neural vasculature.51 The presence of increased albumin in the retinas of the monkeys and rats with experimental glaucoma may be the result of a breakdown of the blood–retinal barrier; however, entry of albumin from the vitreous or de novo synthesis in response to oxidative stress similar to that reported in an in vitro model of cornea wound healing52 cannot be eliminated. Additional studies will be necessary to establish the source of the increased intracellular albumin in the retinas of monkeys with experimental glaucoma.
In conclusion, this study shows that albumin is increased in the retinas of monkeys with experimental glaucoma. The increase in albumin is localized predominately to the same region of inner retina and to neurons that typically exhibit increased oxidative damage in glaucoma. Our findings also show that the amount of retinal albumin is positively correlated with severity of glaucoma damage. Because albumin is a major antioxidant in the body, the increased intracellular albumin in the glaucomatous retina may contribute to the antioxidant activity of injured neurons. The protective effect against oxidants provided by albumin maybe insufficient to save the RGCs, but sufficient for protecting cells in the inner nuclear layer.
Footnotes
Supported by National Eye Institute Grants EY10608 and EY001139; and Research to Prevent Blindness.
Disclosure: L. Carter-Dawson, None; Y. Zhang, None; R.S. Harwerth, None; R. Rojas, None; P. Dash, None; X.C. Zhao, None; E. WoldeMusssie, Allergen, Inc. (F); G. Ruiz, Allergen, Inc. (F); A. Chuang, None; W.P. Dubinsky, None; J.B. Redell, None
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challa P. Glaucoma genetics. Int Opthalmol Clin 2008; 48: 73–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia LY, Tam PO, Chiang SW, et al. Multiple gene polymorphisms analysis revealed a different profile of genetic polymorphisms of primary open-angle glaucoma in northern Chinese. Mol Vis 2009; 15: 89–98 [PMC free article] [PubMed] [Google Scholar]
- 4.Caixeta-Umbelino C, de Vasconcellos JP, Costa VP, et al. Lack of association between optineurin gene variants T34T, E50K, M98K, 691_692insAG and R545Q and primary open angle glaucoma in Brazilian patients. Ophthalmic Genet 2009; 30: 13–18 [DOI] [PubMed] [Google Scholar]
- 5.Campos-Mollo E, Sánchez-Sánchez F, López-Garrido MP, López-Sánchez E, López-Martínez F, Escribano J. MYOC gene mutations in Spanish patients with autosomal dominant primary open-angle glaucoma: a founder effect in southeast Spain. Mol Vis 2007; 13: 1666–1673 [PubMed] [Google Scholar]
- 6.Hayreh SS. Factors influencing blood flow in the optic nerve head. J Glaucoma 1997; 6: 412–425 [PubMed] [Google Scholar]
- 7.Kerr J, Nelson P, O'Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol 1998; 126: 42–51 [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 1983; 95: 673–691 [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci 2000; 41: 3460–3466 [PubMed] [Google Scholar]
- 10.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci 2000; 41: 764–774 [PubMed] [Google Scholar]
- 11.Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Invest Ophthalmol Vis Sci 1995; 36: 1968–1975 [PubMed] [Google Scholar]
- 12.Wax MB, Tezel G, Edward PD. Clinical and ocular histopathological findings in a patient with normal-pressure glaucoma. Arch Ophthalmol 1998; 116: 993–1001 [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2/IL-2 levels in patients with glaucoma. Am J Ophthalmol 2001; 131: 421–426 [DOI] [PubMed] [Google Scholar]
- 14.Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A 1999; 96: 9944–9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shareef S, Sawada A, Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci 1999; 40: 2884–2891 [PubMed] [Google Scholar]
- 16.Liu B, Neufeld AH. Nitric oxide synthase-2 in human optic nerve head astrocytes induced by elevated pressure in vitro. Arch Ophthalmol 2001; 119: 240–245 [PubMed] [Google Scholar]
- 17.Sullivan RK, Woldemussie E, Macnab L, Ruiz G, Pow DV. Evoked expression of the glutamate transporter GLT-1c in retinal ganglion cells in human glaucoma and in a rat model. Invest Ophthalmol 2006; 47: 3853–3859 [DOI] [PubMed] [Google Scholar]
- 18.Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol 1999; 43(suppl 1): S142–150 [DOI] [PubMed] [Google Scholar]
- 19.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res 2008; 173: 495–510 [DOI] [PubMed] [Google Scholar]
- 20.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 2006; 25: 490–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med 2004; 37: 803–812 [DOI] [PubMed] [Google Scholar]
- 22.Tezel G, Yang G, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci 2005; 46: 3177–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farkas RH, Qian J, Goldberg JL, Quigley HA, Zack DJ. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci 2004; 45: 1410–1417 [DOI] [PubMed] [Google Scholar]
- 24.Quigley HA, Holman RM. Laser energy levels for trabecular meshwork damage in the primate eye. Invest Ophthalmol Vis Sci 1987; 24: 1305–1307 [PubMed] [Google Scholar]
- 25.Harwerth RS, Smith EL, III, DeSantis L. Behavioral perimetry in monkeys. Invest Ophthalmol Vis Sci 1993; 34: 31–40 [PubMed] [Google Scholar]
- 26.Harwerth RS. Glaucoma Models: Primate. In: Pang IH, Clark A. eds. Animal Models for Retinal Disease Totowa, NJ: Humana Press; 2009. In press [Google Scholar]
- 27.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci 2004; 45: 3152–3160 [DOI] [PubMed] [Google Scholar]
- 28.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Crawford ML. Scaling the structure-function relationship for clinical perimetry. Acta Ophthalmol Scand 2005; 83: 448–455 [DOI] [PubMed] [Google Scholar]
- 29.Hare W, WoldeMussie E, Lai R, et al. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv Ophthalmol 2001; 45(suppl 3): S284–289 [DOI] [PubMed] [Google Scholar]
- 30.Moore CG, Epley D, Milne ST, Morrison JC. Long-term non-invasive measurement of intraocular pressure in the rat eye. Curr Eye Res 1995; 8: 711–717 [DOI] [PubMed] [Google Scholar]
- 31.Redell JB, Zhao J, Dash PK. Acutely increased cyclophilin a expression after brain injury: a role in blood-brain barrier function and tissue preservation. J Neurosci Res 2007; 85: 1980–1988 [DOI] [PubMed] [Google Scholar]
- 32.Simpson RJ. Proteins and Proteomics: A Laboratory Manual Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003: 400–401 [Google Scholar]
- 33.WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci 2001; 42: 2849–2855 [PubMed] [Google Scholar]
- 34.Woldemussie E, Wijono M, Ruiz G. Müller cell response to laser-induced increase in intraocular pressure in rats. Glia 2004; 47: 109–119 [DOI] [PubMed] [Google Scholar]
- 35.Mestriner AC, Haddad A. Serum albumin enters the posterior chamber of the eye permeating the blood-aqueous barrier. Graefes Arch Clin Exp Ophthalmol 1994; 232: 242–251 [DOI] [PubMed] [Google Scholar]
- 36.Peters T., Jr All about Albumin: Biochemistry, Genetics, and Medical Applications San Diego, CA: Academic Press; 1996: 432 [Google Scholar]
- 37.Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci 2007; 48: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatokun AA, Stone TW, Smith RA. Oxidative stress in neurodegeneration and available means of protection. Front Biosci 2008; 13: 3288–3311 [DOI] [PubMed] [Google Scholar]
- 39.Belayev L, Saul I, Busto R, et al. Albumin treatment reduces neurological deficit and protects blood-brain barrier integrity after acute intracortical hematoma in the rat. Stroke 2005; 36: 326–331 [DOI] [PubMed] [Google Scholar]
- 40.Nimmagadda A, Park HP, Prado R, Ginsberg MD. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke 2008; 9: 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill MD, Moy CS, Palesch YY, et al. The albumin in acute stroke trial (ALIAS); design and metholodgy. Int J Stroke 2007; 2: 214–219 [DOI] [PubMed] [Google Scholar]
- 42.Cain LD, Nie L, Hughes MG, et al. Serum albumin improves recovery from spinal cord injury. J Neurosci Res 2007; 85: 1558–1567 [DOI] [PubMed] [Google Scholar]
- 43.Gum ET, Swanson RA, Alano C, et al. Human serum albumin and its N-terminal tetrapeptide (DAHK) oxidant-induced neuronal death. Stroke 2004; 35: 590–595 [DOI] [PubMed] [Google Scholar]
- 44.Kiaer C, Thams P. Serum albumin protects from cytokine-induced pancreatic b cell death by a phosphoinositide 3-kinase-dependent mechanism. Endocrine 2009; 35: 325–332 [DOI] [PubMed] [Google Scholar]
- 45.Maeda M, Akai F, Nishida S, Yanagihara T. Intracerebral distribution of albumin after transient cerebral ischemia: light and electron microscopic immunocytochemical investigation. Acta Neuropathol 1992; 84: 59–66 [DOI] [PubMed] [Google Scholar]
- 46.Løberg EM, Karlsson BR, Torvik A. Neuronal uptake of plasma proteins after transient cerebral ischemia/hypoxia. Immunohistochemical studies on experimental animals and human brains. APMIS 1993; 101: 777–783 [DOI] [PubMed] [Google Scholar]
- 47.Sabah JR, Davidson H, McConkey EN, Takemoto LJ. In vivo passage of albumin from the aqueous humor into the lens. Mol Vis 2004; 10: 254–259 [PubMed] [Google Scholar]
- 48.Sabah JR, McConkey E, Welti R, Albin K, Takemoto LJ. Role of albumin as a fatty acid carrier for biosynthesis of lens lipids. Exp Eye Res 2005; 80: 31–36 [DOI] [PubMed] [Google Scholar]
- 49.Arend O, Remky A, Plange N, Kaup M, Schwartz B. Fluorescein leakage of the optic disc in glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2005; 243: 659–664 [DOI] [PubMed] [Google Scholar]
- 50.Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 2004; 122: 1348–1356 [DOI] [PubMed] [Google Scholar]
- 51.Koto T, Takubo K, Ishida S, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol 2007; 170: 1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mushtaq S, Naqvi ZA, Siddiqui AA, Palmberg C, Shafqat J, Ahmed N. Changes in albumin precursor and heat shock protein 70 expression and their potential role in response to corneal epithelial wound repair. Proteomics 2007; 7: 463–468 [DOI] [PubMed] [Google Scholar]