In a study first reported more than 25 years ago, the investigators detected electrical currents outside the lens that depended on the activity of lens ion transporters.1 Based on these ionic currents and measures of lens electrical impedance, a model was developed that postulated the existence of fluid flow through the lens fiber cell cytoplasm (hereafter referred to as the fluid circulation model [FCM]).2,3 Although the FCM is more than 20 years old, direct evidence to support it is still lacking. Our analysis suggests that the FCM, as previously described, has conceptual shortcomings and does not appear to be consistent with published data. Contrary to the postulates of the FCM, it is unlikely that fluid circulation through the fiber cell cytoplasm is needed to maintain the metabolism of fiber cells that have degraded their organelles. We suggest that, if water did flow through the cytoplasm of fiber cells from the lens center to its periphery in the manner outlined in the FCM, it would be harmful to lens transparency.
We accept the initial observation that ion currents, generated by active transport, flow around and through the lens. However, we argue that these ion movements do not create a microcirculatory system in which water flows from the lens center to its periphery.
Measuring Fluid Movement in the Lens
Water has been shown to enter across the anterior epithelium and flow out of the lens across the posterior of the fiber mass.4 The authors of this study suggested that water moves through the extracellular space between the fiber cells. When fluorescein was placed on the anterior surface of the rabbit lens in situ, the dye moved through the lens and appeared at the posterior surface 5 to 10 minutes later.5 Candia6 reported in a review article that, by isolating the lens equator from the anterior and posterior surfaces, fluid was observed to move into the lens across the epithelium and out of the equatorial and posterior surfaces. Although these measurements are informative, they do not reveal the pathway taken by water through the lens or whether the water traverses the fiber cell cytoplasm, as predicted by the FCM.
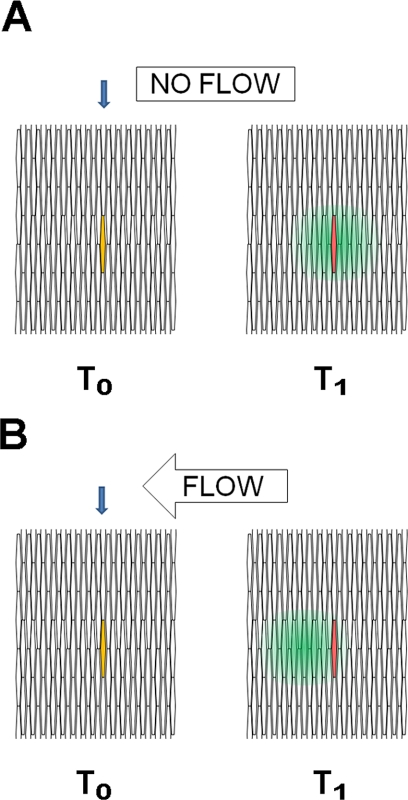
When a fluorescent dye small enough to pass through gap junctions was microinjected into a fiber cell of an intact lens, the dye diffused along the length of the injected cell and radially to fiber cells both deeper and more peripheral in the fiber mass.7 If the injected cell was marked, the peak of fluorescence of the dye remained centered on the injected cell (Bassnett S., personal communication, July 2009).8 The latter observation is not consistent with the FCM, which predicts that the dye would be carried into more peripheral fiber cells by the flow of water toward the lens equator (Fig. 1). One might imagine the fate of dye dropped into a still pool or a slowly flowing river. In both cases, the dye would diffuse radially from its initial position. However, dye placed in the river would move downstream with the flow, whereas dye placed in the pool would spread uniformly from its origin. In the experiments reported to date, the lens behaved like a still pool.
Figure 1.
A cross-sectional view of fiber cells in which a single fiber cell is co-injected with a low-molecular-weight fluorescent dye (green) that can pass through gap junctions and a larger fluorescently labeled molecule (red) that cannot pass through gap junctions. The distribution of fluorescence is shown with increasing time after injection. At the time of injection (T0), the injected cell (blue arrow) appears yellow, because of the co-localization of the green and red dyes. (A) The result if there is no fluid flow through the lens. With increasing time (T1), the low-molecular-weight dye diffuses out on either side of the cell, with the peak of green fluorescence centered over the injected cell (red). (B) The results expected if there were flow through the lens. With increasing time after injection, the peak of green fluorescence of the low-molecular-weight dye would move “downstream,” away from the red fluorescence in the injected cell. The available experimental data support the scheme outlined in (A).
These dual dye-injection studies were performed on lenses isolated from chicken embryos. It is not known when during its development the ion currents are first detectable around the lens. It is possible that no centrifugal flow was observed in these studies because no ion currents were present to drive flow. Thus, one way to explicitly test the predictions of the FCM would be to repeat these studies on adult lenses and monitor the extralenticular ion currents and dye movement at the same time. Such studies would test whether water flows through the cytoplasm of the lens fiber cells, from the center of the lens to its periphery, as predicted by the FCM.
The predictions of the FCM were also not supported by direct measurement of the movement of water molecules in the lens. Nuclear magnetic resonance (NMR) imaging was used to monitor water movement within intact human lenses in real time.9 The pattern of flow predicted by the FCM (i.e., that water would enter initially at the poles and be seen last at the equator) was not observed. Instead, the water moved toward the nucleus uniformly from the lens surface and at a rate consistent with passive diffusion, with no evidence of flow. In a subsequent investigation, diffusion tensor NMR microimaging was used to study the movement of water within the fiber cell cytoplasm.10 In this study, the water diffused freely within the lens fiber cell, but diffusion was constrained significantly by cell membranes. Of note, quiver plots, which reveal the “preferred” direction of diffusion, demonstrated that access to the center of the lens was largely via movement of water along the equatorial plane, not from the poles, as predicted by the FCM.
Potential Problems with the FCM
The FCM assumes that ions (Na+ and Cl−) and small metabolites such as glucose diffuse from the fluids surrounding the lens into the extracellular space surrounding mature fiber cells, deep in the lens. The gradients that result from the movement of ions across fiber cell membranes are postulated to provide the motive force for fluid flow.3 Glucose is assumed to provide a substrate for metabolism in the lens core. However, studies by proponents of the FCM showed that a barrier to extracellular diffusion, located at the interface between the differentiating and mature fiber cells, inhibits small molecules such as glucose from diffusing into the extracellular space of the lens core.11 This obstruction was not discussed in papers describing the FCM. Its existence seems contrary to the model.
Lens fiber cell membranes have a high concentration of gap junctions and aquaporin water channels. The FCM suggests that water channels contribute to the flow of water through the lens, with ions and some of the water passing through gap junctions.3 However, unidirectional movement of water through water channels would create osmotic differences across the lateral membranes of the fiber cells. As water flows through these channels, intracellular solutes would be left behind. The resulting gradients would create an osmotic force that would oppose the flow of water at the surface of every fiber cell (Fig. 2). This effect would preclude unidirectional flow through aquaporin channels as a means of moving the water from the center to the periphery of the lens. Osmotic resistance would also be generated at gap junctions, since unidirectional flow of water and ions through the gap junctional channels would leave behind proteins and other solutes too large to pass through them. Although the osmotic braking force generated at gap junctions would be less than at water channels, it would still be additive for every fiber cell along the radius of the lens. Neither of these impediments to unidirectional flow was discussed in the published version of the FCM.3
Figure 2.
The consequence of unidirectional flow through aquaporin water channels. Water molecules (●) pass through the channels, leaving ions (○) behind. This movement creates lower water concentration on the upstream side of the membrane, generating osmotic pressure opposite the direction of flow. Without a means of selectively moving ions across lens membranes, unidirectional flow through aquaporin channels is not possible.
Mature Fiber Cells Have No Need for Metabolic Activity, and Published Studies Show that Little Metabolism Occurs There
The FCM assumes that centrifugal fluid flow is essential for providing nutrients such as glucose to the fiber cells that lack organelles and for removing the waste products of metabolism (lactate, in the case of anaerobic glycolysis). Therefore, a basic assumption of the FCM is that mature fiber cells have active metabolism.
We know of no metabolic pathway that has been demonstrated in mature nuclear fiber cells (Fig. 3). Typical cells expend most of their metabolic energy maintaining their transmembrane potential and synthesizing proteins. Neither of these activities occurs in mature fiber cells. Even if some low level of metabolic activity were found in the cells, substrates for these reactions could diffuse through gap junctions from metabolically active superficial cells, which have organelles.
Figure 3.
The zone of active metabolism in the adult human lens. The dark band at the top represents the lens epithelium. The gray oval at the periphery of the fiber mass includes all the lens fiber cells with nuclei and other membrane-bound organelles. The epithelium and the outer fiber cells are responsible for nearly all the metabolism in the lens. The available data suggest that the lens center has little or no metabolic activity. Based on experimental data, the filled arrows indicate the major route of entry of metabolites at the germinative zone, adjacent to the lens equator.12 The open arrows depict the direction of diffusion of critical metabolites such as glutathione, from the metabolically active superficial fiber cells where they are produced, to the deeper fiber cells.
Consistent with this view, results of studies suggest that little enzyme activity survives in fiber cells after they have finished elongating and degraded their organelles. In all species examined, enzyme activity is concentrated in the outer cortical zone of the lens. In the young rat, the cortex contains 75% of the total phospholipase A2 activity.13 Incorporation of tritiated water into cholesterol and fatty acids, as well as leucine into aquaporin-0, occurred in the outer 10% of the lens, with peak incorporation in the outer 3% to 6%, corresponding to the fiber cells that contain organelles.14 Similarly, immunochemically detectable transglutaminase was localized to epithelial cells and a thin zone of the peripheral cortex in human lenses.15
Glucose transporters are readily detectable in the membranes of mature fiber cells,16,17 which raises the question of whether these transporters are functional and whether glycolysis persists deep in the lens. Glycolysis requires the concerted activity of 10 enzymes. Loss of the activity of any one would block the pathway. No glucose-6-phosphate dehydrogenase (G6PD) activity was detected in the center of lenses in rats older than 6 months, but inactive G6PD molecules were detected.18,19 The presence of inactivated enzymes in the nuclei of lenses may well be a general phenomenon, as the presence of superoxide dismutase, aldolase, and glyceraldehyde-3-phosphate dehydrogenase has also been documented.18–21 Active lactate dehydrogenase (LDH), the enzyme that converts pyruvate to lactate in the final step of anaerobic glycolysis, was readily detected by histochemical assay in the nucleated superficial fiber cells of bovine and human lenses, but not in the fiber cells that were 50 to 150 μm beneath the capsule and lacked nuclei.22 The sharp boundary between cells with LDH activity and those lacking it suggests that the activity of the enzyme was lost during or soon after fiber cell denucleation. In the absence of mitochondria, LDH activity is necessary to produce the NAD+ that is essential for upstream steps in glycolysis. Without the NAD+ produced by LDH, glycolysis would come to an abrupt halt. Given these observations and the fact that macromolecules present in the nuclei of adult human lenses have been “cooking” at body temperature for decades with no means to replace them, it is unlikely that glycolysis or any other major metabolic pathway functions in human nuclear fiber cells (Fig. 3). With little metabolic activity in the nucleus, there is no requirement for fluid flow to deliver substrates and remove the products of metabolism. If the FCM is necessary for metabolism in the lens nucleus, metabolic activity should be demonstrable there.
Is There a Need for Coupling between the Cortex and Nucleus?
It is apparent from the previous section that the lens nucleus, particularly in adult humans, is quiescent, with little or no enzymatic activity. A corollary is that, for nearly all purposes, there is no need for appreciable communication between the metabolically active cortex and the lens nucleus. There is, however, at least one exception.
Glutathione (GSH) is essential for maintenance of lens transparency.23–25 Like the enzymes just described, the enzymes and cofactors necessary for the reduction of GSH (glutathione reductase [GR], NADPH, and enzymes of the hexose monophosphate shunt) are located in the lens periphery. The lens cortex contains more than 20 mM GSH.26,27 GR, the enzyme that is necessary for the reduction of oxidized glutathione, has been measured in the cortex and nucleus. Precise dissection methods have revealed a steep gradient of GR, with highest specific activity in the outermost cortical fibers, decreasing to no detectable activity in the inner parts of older lenses.28 GSH is 80% to 90% lower in the lens nucleus than in the cortex,29 and age exacerbates this difference (for example, see Ref. 30). Treatment of rabbit lenses with hyperbaric oxygen (HBO) for 4 hours decreased GSH levels by less than 10% in the superficial cortex, but by 70% in the nucleus.31 In guinea pigs given prolonged HBO treatments, the level of GSH in the nucleus became nearly 10 times lower than that in the cortex, and GSH- and cysteine-protein–mixed disulfides in the nucleus increased dramatically.25,30 It has been suggested that the decline in GSH concentration that occurs in the nucleus of the human lens with increasing age is caused by a barrier to diffusion from the periphery to the center of the lens.32 The decline in GSH and the resulting increase in GSSG leads to oxidation of cysteine and methionine residues in proteins, a hallmark of age-related nuclear cataracts.33
When monkey lenses were incubated with 35S cysteine for various times and the movement of label within the lens followed by autoradiography, the amino acid entered primarily at the equator (Fig. 3).12 The movement of cysteine within the lens was then followed over time. The major pathway appeared to involve diffusion along the length of the fiber cells and orthogonal movement across the fibers in the equatorial plane, in accordance with the distribution of connexons (Fig. 3). These experiments showed that metabolites are transported into the cytoplasm of lens cells close to the equator and then move inward toward the nucleus along the equatorial plane. This direction is opposite that of the water and solute flow predicted by the FCM.
The experiments cited herein have shown that the capacity to reduce GSH is minimal in the lens nucleus. GSH is synthesized and reduced in the cortex, and reduced glutathione diffuses from the periphery of the lens to its center to maintain a reducing environment there. Therefore, to maintain lens transparency it is necessary to preserve a pathway for diffusion between the cortex and nucleus.
Fluid Flow from Center to Periphery Would Harm the Lens
The proponents of the FCM estimate that the magnitude of water flow and diffusion in the lens are similar.3 They also point out that, because flow is directional and diffusion is random, flow would be more efficient at moving metabolites and waste products through the lens. However, if water flowed from the center to the periphery of the lens through gap junction channels, it would counter the diffusion of GSH through these same channels in the opposite direction (Fig. 4). Therefore, directional flow from the lens center to its periphery would effectively restrict reduced glutathione from reaching the lens nucleus. As described earlier, maintaining sufficient reduced glutathione in the nucleus is essential in the maintenance of transparency. Therefore, the mechanism of the FCM, as it is presently described, would be harmful to the lens.
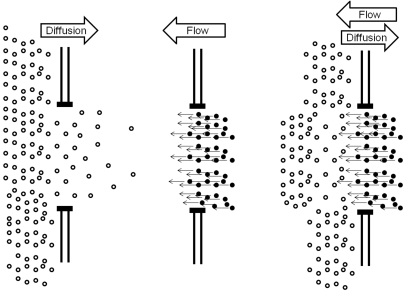
Figure 4.
The diffusion of a metabolite such as glutathione and the flow of water in the opposite direction through a gap junction channel. Left: metabolite molecules moving through a gap junction by diffusion down a concentration gradient. Each molecule moves randomly. Net movement is due to the concentration difference across the junction. Middle: the flow of water through a gap junction, as proposed by the FCM. The net movement of all water molecules is in the same direction, resulting in bulk flow of solvent. Right: these movements are combined. It is evident that, if the magnitude of flow and diffusion were similar, the vectorial flow of water would oppose the movement of the metabolite, retarding or preventing its movement across the gap junction.
Conclusions
Based on the arguments herein, we suggest that the theory behind the FCM is unlikely to be correct and that such movement is not needed by the lens. The model also appears to be incompatible with maintenance of the reducing environment that is necessary for the transparency of the lens nucleus. Before the FCM can be accepted, it must be demonstrated, not just hypothesized, that an internal circulatory system moves fluid through the cytoplasm of adult lens fiber cells. Its proponents should also show that a major metabolic pathway—for example, glycolysis—functions in mature fiber cells.
Footnotes
Supported by National Institutes of Health Grants EY013570 (RJWT), EY04853 (DCB), EY015863 (DCB); Australian National Health and Medical Research Council (NHMRC) Grants 457085 (RJWT) and 512334 (RJWT); and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences (DCB).
Disclosure: D.C. Beebe, None; R.J.W. Truscott, None
References
- 1.Robinson KR, Patterson JW. Localization of steady currents in the lens. Curr Eye Res 1982;2:843–847 [DOI] [PubMed] [Google Scholar]
- 2.Mathias RT, Rae JL. Transport properties of the lens. Am J Physiol 1985;249:c181–c190 [DOI] [PubMed] [Google Scholar]
- 3.Mathias R, Kistler J, Donaldson P. The lens circulation. J Membr Biol 2007;216:1–16 [DOI] [PubMed] [Google Scholar]
- 4.Fischbarg J, Diecke FP, Kuang K, et al. Transport of fluid by lens epithelium. Am J Physiol Cell Physiol 1999;276:C548–C557 [DOI] [PubMed] [Google Scholar]
- 5.Stepanova LV, Marchenko IY, Sychev GM. Direction of fluid transport in the lens. Bull Exp Biol Med 2005;139:50–51 [DOI] [PubMed] [Google Scholar]
- 6.Candia OA. Electrolyte and fluid transport across corneal, conjunctival and lens epithelia. Exp Eye Res 2004;78:527–535 [DOI] [PubMed] [Google Scholar]
- 7.Rae JL. The movement of procion dye in the crystalline lens. Invest Ophthalmol 1974;13:147–150 [PubMed] [Google Scholar]
- 8.Shestopalov VI, Bassnett S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J Cell Sci 2000;113:1913–1921 [DOI] [PubMed] [Google Scholar]
- 9.Moffat BA, Landman KA, Truscott RJW, Sweeney MHJ, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res 1999;69:663–669 [DOI] [PubMed] [Google Scholar]
- 10.Moffat BA, Pope JM. Anisotropic water transport in the human eye lens studied by diffusion tensor NMR micro-imaging. Exp Eye Res 2002;74:677–687 [DOI] [PubMed] [Google Scholar]
- 11.Donaldson PJ, Grey AC, Merriman-Smith BR, et al. Functional imaging: new views on lens structure and function. Clin Exp Pharmacol Physiol 2004;31:890–895 [DOI] [PubMed] [Google Scholar]
- 12.Sweeney MHJ, Garland DL, Truscott RJW. Movement of cysteine in intact monkey lenses: the major site of entry is the germinative region. Exp Eye Res 2003;77:245–251 [DOI] [PubMed] [Google Scholar]
- 13.Cenedella RJ. Regional distribution of lipids and phospholipase A2 activity in normal and cataractous rat lens. Curr Eye Res 1985;4:113–120 [DOI] [PubMed] [Google Scholar]
- 14.Cenedella RJ, Shi H. Spatial distribution of 3-hydroxy-3-methylglutaryl coenzyme A reductase messenger RNA in the ocular lens: relationship to cholesterologenesis. J Lipid Res 1994;35:2232–2240 [PubMed] [Google Scholar]
- 15.Hidasi V, Adany R, Muszbek L. Localization of transglutaminase in human lenses. J Histochem Cytochem 1995;43:1173–1177 [DOI] [PubMed] [Google Scholar]
- 16.Lucas V, Zigler J. Transmembrane glucose carriers in the monkey lens: quantitation and regional distribution as determined by cytochalasin B binding. Invest Ophthalmol Vis Sci 1987;28:1404–1412 [PubMed] [Google Scholar]
- 17.Merriman-Smith R, Donaldson P, Kistler J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest Ophthalmol Vis Sci 1999;40:3224–3230 [PubMed] [Google Scholar]
- 18.Dovrat A, Scharf J, Eisenbach L, Gershon D. G6PD molecules devoid of catalytic activity are present in the nucleus of the rat lens. Exp Eye Res 1986;42:489–496 [DOI] [PubMed] [Google Scholar]
- 19.Dovrat A, Gershon D. Rat lens superoxide dismutase and glucose-6-phosphate dehydrogenase: studies on the catalytic activity and the fate of enzyme antigen as a function of age. Exp Eye Res 1981;33:651–661 [DOI] [PubMed] [Google Scholar]
- 20.Dovrat A, Gershon D. Studies on the fate of aldolase molecules in the aging rat lens. Biochim Biophys Acta 1983;757:164–167 [DOI] [PubMed] [Google Scholar]
- 21.Dovrat A, Scharf J, Gershon D. Glyceraldehyde 3-phosphate dehydrogenase activity in rat and human lenses and the fate of enzyme molecules in the aging lens. Mech Ageing Dev 1984;28:187–191 [DOI] [PubMed] [Google Scholar]
- 22.Pau H, Hartwig H-G, Fassbender R. Topographical distribution of lactate dehydrogenase activity in human clear eye lenses and in lenses with different types of senile cataract: a histochemical investigation. Graefes Arch Clin Exp Ophthalmol 1997;235:611–617 [DOI] [PubMed] [Google Scholar]
- 23.Calvin H, Medvedovsky C, David J, et al. Rapid deterioration of lens fibers in GSH-depleted mouse pups. Invest Ophthalmol Vis Sci 1991;32:1916–1924 [PubMed] [Google Scholar]
- 24.Calvin HI, Zhu G, Wu J, Banerjee U, Fu JS. Progression of mouse buthionine sulfoximine cataracts in vitro is inhibited by thiols or ascorbate. Exp Eye Res 1997;65:341–347 [DOI] [PubMed] [Google Scholar]
- 25.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther 2000;16:121–135 [DOI] [PubMed] [Google Scholar]
- 26.Reddy VN. Metabolism of glutathione in the lens. Exp Eye Res 1971;11:310–328 [DOI] [PubMed] [Google Scholar]
- 27.Reddy V. Glutathione and its function in the lens: an overview. Exp Eye Res 1990;50:771–778 [DOI] [PubMed] [Google Scholar]
- 28.Zhang WZ, Augusteyn RC. Ageing of glutathione reductase in the lens. Exp Eye Res 1994;59:91–95 [DOI] [PubMed] [Google Scholar]
- 29.Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res 1995;60:219–235 [DOI] [PubMed] [Google Scholar]
- 30.Padgaonkar VA, Lin LR, Leverenz VR, Rinke A, Reddy VN, Giblin FJ. Hyperbaric oxygen in vivo accelerates the loss of cytoskeletal proteins and MIP26 in guinea pig lens nucleus. Exp Eye Res 1999;68:493–504 [DOI] [PubMed] [Google Scholar]
- 31.Giblin FJ, Schrimscher L, Chakrapani B, Reddy VN. Exposure of rabbit lens to hyperbaric oxygen in vitro: regional effects on GSH level. Invest Ophthalmol Vis Sci 1988;29:1312–1319 [PubMed] [Google Scholar]
- 32.Bova LM, Sweeney MH, Jamie JF, Truscott RJ. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci 2001;42:200–205 [PubMed] [Google Scholar]
- 33.Truscott RJW. Age-related nuclear cataract: oxidation is the key. Exp Eye Res 2004;80:709–725 [DOI] [PubMed] [Google Scholar]