Adult vascular maintenance and repair are mediated in part by endothelial progenitor cells, recruited by chemokines such as stromal-derived factor (SDF)-1. The authors examined the interaction between growth factors such as IGF and VEGF and the SDF-1 receptor CXCR4, and showed that localized inhibition of CXCR4 may prove beneficial in treating aberrant neovascular disease.
Abstract
Purpose.
Modulators of angiogenesis typically work in an orchestrated manner. The authors examined the interaction between insulinlike growth factor (IGF)-1, vascular endothelial growth factor (VEGF), and stromal derived factor (SDF)-1 in vivo and in vitro using angiogenesis models.
Methods.
The angiogenic effect of SDF-1, alone or in combination with IGF-1 and VEGF, was assessed in human lung microvascular endothelial cells using capillary tube formation and thymidine incorporation. Immunohistochemical analysis for CD31, SDF-1, and CXCR4 was performed on mouse eyes 2 weeks after the initiation of laser rupture of Bruch's membrane, a choroidal neovascularization (CNV) model. CXCR4 antagonist and CXCR4 blocking antibody were tested on inhibition of CNV lesion size in this model. Real-time PCR was used to determine mRNA levels for SDF-1, VEGF, IGF-1, and their cognate receptors in the retinal pigment epithelium/choroid complex of mice that underwent this CNV model.
Results.
IGF-1 and VEGF demonstrated an additive effect on SDF-1–induced in vitro angiogenesis. CXCR4 immunoreactivity was present in both normal and laser-injured mice at the laser burn site and at the ganglion cell layer, the anterior portion of the inner nuclear layer, photoreceptors, and choroidal stroma. SDF-1 was observed in identical locations but was not seen in photoreceptors. mRNA levels for SDF-1, VEGF, and IGF-1 and their receptors were increased after laser injury. CXCR4-neutralizing antibody reduced neovascularization when injected subretinally but not intraperitoneally or intravitreally.
Conclusions.
The potent proangiogenic factors IGF-1 and VEGF both stimulate SDF-1–induced angiogenesis. Local inhibition of CXCR4 is required for an antiangiogenic effect in CNV lesions.
Choroidal neovascularization (CNV), the hallmark of exudative age-related macular degeneration (AMD), is responsible for approximately 90% of cases of severe vision loss from AMD. Vascular endothelial growth factor (VEGF) plays a key role in the regulation of CNV and the accompanying increase in permeability. Current pharmacologic treatments, such as ranibizumab (Lucentis; Genentech, San Francisco, CA) and bevacizumab (Avastin; Genentech), aggressively target VEGF.1,2 However, despite these therapeutic advances, long-term trials using ranibizumab (Lucentis) indicate that a significant population of AMD patients do not respond to VEGF inhibition.1,2 This is not entirely surprising because, in addition to VEGF, other angiogenic and inflammatory mediators are likely to contribute to CNV lesion development. One such mediator, insulinlike growth factor (IGF)-1, produced in neurons and retinal pigment epithelium, has recently been implicated in CNV progression.3 IGF-1 immunoreactivity was abundantly found in human CNV tissue, and the IGF-1 receptor (IGF-1Rc) was highly expressed on retinal pigment epithelial (RPE) cells.3 Moreover, exposure of human RPE cultures to IGF-1 stimulated VEGF secretion.3
Stromal derived factor (SDF)-1 is a newly implicated cytokine in CNV lesion growth4,5 and in the pathogenesis of proliferative diabetic retinopathy.6 Its actions are not limited to the resident vasculature; rather, SDF-1 is a potent stimulator of endothelial precursor cells (EPCs).5 EPCs are bone marrow–derived cells that enhance new vessel growth both by directly incorporating into newly formed vessels and by secreting paracrine factors. CXCR4, the major receptor for SDF-1, is expressed not only on EPCs but also on mature endothelial cells, neural precursors, and smooth muscle progenitors, and it is critical for the migration of these cells to areas of injury and repair.7 Activation of CXCR4 facilitates EPC differentiation to endothelial cells and EPC survival.8
SDF-1, like VEGF, is regulated by hypoxia. Previously, we demonstrated that elevated vitreous SDF-1 levels strongly correlated with vitreous VEGF levels and paralleled the severity of retinopathy.9 When expressed in epiretinal membranes, SDF-1 is associated with VEGFR-2.10 Circulating EPCs are increased in patients with active CNV, suggesting that these cells may be recruited from bone marrow by factors secreted at the sites of active CNV and that they may play a critical role in CNV severity.11 Blocking SDF-1 prevented the recruitment of EPCs to the retina and choroid after injury to these areas and reduced CNV.5
Despite the clear evidence of cooperation between these factors and cytokines for CNV development, no studies have examined the influence of IGF-1 and VEGF on the in vitro angiogenic effect of SDF-1, nor has the effect of CXCR4 inhibition been completely elucidated in CNV lesion formation. We examined the effects of VEGF and IGF-1 on SDF-1–stimulated proliferation and capillary tube formation in vitro and examined the in vivo effect of highly selective CXCR4 antagonist on the neovascular response after laser rupture of Bruch's membrane.
Methods
Capillary Tube Formation In Vitro
Basement membrane matrix (Matrigel; BD Biosciences, San Jose, CA) was thawed and prepared according to the manufacturer's protocol. Twenty thousand human lung microvascular endothelial cells (HMEC-L) were treated in 1% EBM2 media (Lonza, Walkersville, MD) with SDF-1 (R&D Systems, Minneapolis, MN) at concentrations of 0.1 nM, 1 nM, and 100 nM; VEGF at concentrations of 5 nM, 20 nM, 50 nM, and 100 nM with a modified IGF-1 that does not bind to IGF-binding proteins (long-R-IGF-1; Cell Sciences, Canton, MA) at concentrations of 0.1 nM, 1 nM, 100 nM; a combination of SDF-1 and long-R-IGF-1; or a combination of SDF-1 and VEGF. The basement membrane matrix (Matrigel) was kept at 37°C and 5% CO2, and tube formation was monitored over 10 hours. The cells on basement membrane matrix (Matrigel) were photographed using a Carl Zeiss microscope (Carl Zeiss Inc., Thornwood, NY), and images were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Total tube length in three fields per well were averaged, and three wells were used to produce one value per condition. The entire experiment was performed in triplicate.
Cell Activity Measured as a Function of DNA Synthesis
For thymidine incorporation studies, 30,000 HMEC-L/well were plated in a 24-well plate. Cells were allowed to attach and recover for 24 hours and were then placed in serum-free media and treated with SDF-1 (0.1 nM, 1 nM, 100 nM), long-R-IGF-1 (0.1 nM, 1 nM, 10 nM, 100 nM), VEGF (5 nM, 20 nM, 50 nM, 100 nM), or a combination of SDF and either IGF or VEGF. Full EBM2 media was used as a positive control for the cells. The cells were treated for 24 hours; this was followed by the addition of 40 μL of 0.1 mCi/mL [3H] thymidine (Perkin Elmer, Boston, MA) during the last 2 hours of incubation. The cells were washed twice with 10% trichloroacetic acid (Fisher Scientific, Austin, TX) and lysed with 0.2 N NaOH. One hundred microliters of the cell lysate was counted in a multipurpose scintillation counter (LS 6500; Beckman Coulter, Fullerton, CA).
Animal Studies
All animal procedures used were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Florida Institutional Animal Care and Use Committee. Four groups of adult C57Bl6/J mice (Jackson Laboratories, Bar Harbor, ME) were used. Mice were either uninjected (n = 6) or injected subretinally (n = 18), intravitreally (n = 12), or intraperitoneally (n = 11).
Uninjected Animals
Adult mice were laser-injured in one eye, as previously described,12 and their eyes (n = 6 laser-injured eyes; n = 6 uninjured contralateral eyes) were used for RT-PCR to examine the levels of VEGFR-1, VEGFR-2, VEGF-A, SDF-1, CXCR7, CXCR4, IGF-1, and IGF-1R.
Subretinal Injection
Animals were injected subretinally once with 0.5 μg CXCR4 blocking antibody in 1 μL (n = 6) or 0.5 μg (n = 6; antibody control) denatured/heat-inactivated CXCR4 antibody (R&D Systems) or saline (n = 6) 1 day before laser injury. After 3 weeks, the animals were euthanatized, and the eyes were processed so the vasculature could be visualized, as previously described,12 and CNV lesion volume was measured.
Intravitreal Injection
Another group of mice was injected once intravitreally with 0.5 μg CXCR4 blocking antibody in 1 μL (n = 6) immediately after laser rupture of Bruch's membrane to induce CNV. Saline-injected animals (n = 6) that were laser-injured served as controls. After 3 weeks, the animals were euthanatized, and the eyes were processed to determine CNV lesion volume.
Intraperitoneal Injection
Adult mice were injected with 1 μg CXCR4 antagonist NefM1 intraperitoneally three times a week for 3 weeks (n = 5) after laser injury to induce CNV. Uninjected animals (n = 6) that were laser-injured served as controls.
Immunohistochemistry
Six eyes from three animals in each group were enucleated, fixed in 2% paraformaldehyde in Tris-buffered saline (TBS) at room temperature for 1 hour, and washed in TBS with 5% sucrose three times. Whole eyes were cryopreserved with a series of sucrose solutions, infiltrated with 20% sucrose/OCT in a 2:1 ratio, and frozen as previously reported.13 Serial 8-μm cryosections were used for alkaline phosphatase immunohistochemistry and histology. Alkaline phosphatase (APase) immunohistochemistry was performed according to the method of Bhutto et al.14 using the following primary antibodies overnight at 4°C: rat anti–mouse CD31 (BD PharMingen, San Diego, CA); goat anti–SDF-1 (Santa Cruz, Biotechnology, Santa Cruz, CA) diluted 1:200; and rabbit anti–human CXCR4 (Novus, Littleton, CO). After washing, the sections were incubated with the appropriate biotinylated second-step antibody (Kirkegaard and Perry, Gaithersburg, MD) for 30 minutes at room temperature. Finally, the sections were incubated with streptavidin APase (Kirkegaard and Perry) and then developed with a 5-bromo-4-chloro-3-indoyl phosphate-NBT kit (Vector Laboratories, Inc., Burlingame, CA), yielding a blue reaction product. The sections were fixed and partially bleached using the technique of Bhutto et al.14 so that the reaction product in RPE cells could be evaluated. The first section in the series was stained with hematoxylin and eosin for examining histologic characteristics of the tissue.
Volumetric Analysis of CNV Lesions
Using laser scanning confocal microscopy, images were taken throughout the entire thickness of the lesion. Five-micrometer thickness z-sections were stacked and analyzed using ImageJ software for each lesion. Threshold pixel intensity was set for one sample, and this setting was retained for all analyses (i.e., density slicing). The points on each image that exceeded the threshold were then totaled and multiplied by the slice thickness to determine the total vascular lesion volume. This was repeated for each of the lesions in each animal, and from this a mean lesion volume was determined for each animal. These lesion volumes were used to calculate the average and SEM for each injection condition.
Real-Time PCR
Eyes underwent laser-induced rupture of Bruch's membrane or were used as controls. After the eyes were dissected, the neural retinas were removed. mRNA from three posterior cups or neural retina was harvested (Total Aurum RNA kit; Bio-Rad, Hercules, CA) according to the manufacturer's protocol; the pooled samples were run in triplicate. Fifteen microliters of the mRNA were reverse transcribed using buffer (Iscript; Bio-Rad) and transcriptase and then were incubated at 42°C for 30 minutes and denatured at 85°C for 5 minutes. FAM-labeled primers for TATA binding protein, SDF, CXCR4, VEGFA, CXCR7, KDR, Flt1, IGF-1, and IGF-1R were purchased from Applied Biosystems (ABI; Foster City, CA). Universal master mix (TaqMan; ABI) was used according to the manufacturer's protocol to run the PCR reactions on a PCR machine (7500 Fast Real Time; ABI).
Isolation of EPCs
Circulating EPCs (CD34+ cells) were isolated from healthy volunteers at Shands Teaching Hospital at the University of Florida in accordance with an approved protocol from the Institutional Review Board. Sixty microliters of blood per volunteer was collected aseptically into heparinized glass vacuum tubes, reacted with magnetic bead-conjugated anti–CD34 antibody, and separated according to the manufacturer's directions (Stem Cell Technologies, Vancouver, BC, Canada). Enriched cells were then washed three times with PBS.
Characterization of VEGF Receptor Expression in CD34+ EPCs
Protein expression of VEGF receptors (VEGFR-1 and VEGFR-2) in CD34+ EPC was evaluated using flow cytometry analysis. CD34+ cells were exposed to 0.1 nM or 100 nM SDF-1 for 0 minute or 15 minutes or for 1 hour, 4 hours, or 6 hours in 5% CO2 at 37°C. After treatment, the cells were permeabilized (Cytofix/Cytoperm Kit; BD Biosciences, San Jose, CA). The cells were blocked with 10% normal human serum (Jackson Immuno Research Laboratories, West Grove, PA) in PBS. Ten micrograms of anti–VEGFR-1 (Santa Cruz Biotechnology) or 10 μg anti–VEGFR-2 antibody (NeoMarkers, Fremont, CA) was added to the cells and then incubated for 30 minutes on ice. The cells were washed with PBS and incubated with 23 μg FITC-conjugated goat anti–mouse antibody (Jackson ImmunoResearch Laboratories) in the dark for 30 minutes on ice. Cells were then washed and analyzed by flow cytometry. The isotype control for both VEGFR-1 and VEGFR-2 antibodies was anti–GFP (Molecular Probes, Carlsbad, CA) antibody (15 μg). Apoptotic dead cells were removed before analysis by 7-aminoactinomycin D (Sigma-Aldrich, St. Louis, MO) positive selection. Data were acquired with a flow cytometer (FACSCalibur; BD Biosciences) and were analyzed (BD Cell Quest; BD Biosciences).
Statistical Analysis
Mean tube length on basement membrane matrix (Matrigel) for each combination of SDF-1 with IGF-1 or VEGF was compared with the tube length achieved by SDF-1 alone using Student's t-test assuming unequal variance. Thymidine incorporation was analyzed by multivariate ANOVA. In the intravitreous injections, CNV lesion volumes resulting from individual treatments within injection space were compared with each other by Student's t-test assuming unequal variance. For mRNA analysis, individual mRNA levels were compared with those of untreated eyes by Student's t-test assuming unequal variance. For surface marker expression after SDF-1 challenge, the percentage of cells positive for the marker examined was compared with untreated cells using Student's t-test assuming unequal variance.
Results
In Vitro Tube Formation Using SDF-1 and IGF-1
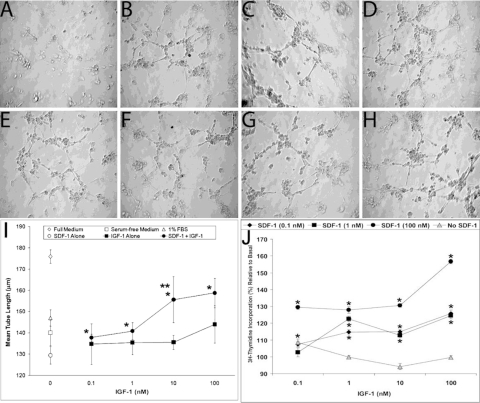
We performed the basement membrane matrix (Matrigel) tube formation assay with SDF-1 and IGF-1. Using the concentrations of SDF-1 (0.1 nM and 100 nM) that we previously found to be most potent in EPC and HREC migration assay,15 we examined the effect of combining IGF-1 with SDF-1 on tube formation (Figs. 1B–D, 1F–H) in serum-free medium. Cells cultured in serum-free media alone were used as the negative control (Fig. 1A), and 1% FBS served as the positive control (Fig. 1E). Numerous tubes formed in all experimental conditions examined except serum-free medium alone. Mean tube length was calculated and demonstrated that IGF-1 had minimal effect except at the highest concentration of IGF-1 used (100 nM). However, the combination of even the lowest concentration of SDF-1 (0.1 nM) with IGF-1 resulted in a marked increase in tube length (Fig. 1I).
Figure 1.
Basement membrane matrix assay showing tube formation and branch points with different concentrations of SDF-1 and IGF-1. (A) Cells cultured in serum-free media with no growth factor as a negative control. (B–D) Representative images of cells cultured with 0.1 nM SDF-1, 0.1 nM IGF-1, and a combination of the two, respectively. (E) Cells cultured with 1% FBS as a positive control. (F–H) Representative images of cells cultured with 1 nM SDF-1, 1 nM IGF-1, and a combination of the two, respectively. (B–H, I) The high number of tubes and branch points formed shows the enhanced tube formation when cells are exposed to IGF-1 in combination with SDF-1 compared with IGF-1 alone. *P < 0.05 vs. SDF-1 alone. **P < 0.05 vs. IGF-1 alone. (J) Thymidine incorporation with different combinations of SDF-1 and IGF-1. Thymidine incorporation increases with increasing SDF-1 concentration. This effect is enhanced with increasing concentrations of IGF-1. All data are corrected to the level of incorporation of thymidine in serum-free medium in the absence of either SDF-1 or IGF-1. *P < 0.05 for all values on graph compared with serum-free medium alone.
As assessed by thymidine incorporation, IGF-1 did not stimulate DNA synthesis at any of the doses tested. This is not unexpected considering that IGF-1 is a progression factor but does not stimulate mitosis. However, when increasing concentrations of SDF-1 were added to IGF-1–stimulated cultures (Fig. 1J), thymidine incorporation increased in a dose-dependent manner, demonstrating the greatest effect, as expected, at the highest concentration of SDF-1 (100 nM) and IGF-1 (100 nM) tested (P < 0.01 for all concentrations above basal; Fig. 1J).
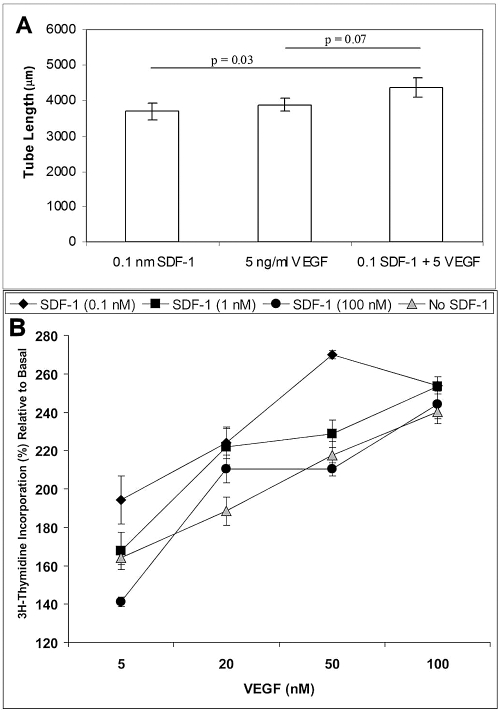
We next examined the effect of the combination of low doses of SDF-1 (0.1 nM) and VEGF (5 nM) on tube formation (Fig. 2A). Together these two factors significantly increased tube length in the basement membrane matrix (Matrigel) assay compared with SDF-1 alone (P = 0.03). VEGF induced a dose-dependent increase in thymidine incorporation. Interestingly, in marked contrast to the results with IGF-1, we observed that the lowest concentration of SDF-1 resulted in the greatest stimulatory effect on VEGF-induced thymidine incorporation (P < 0.05; Fig. 2B). This was not entirely unexpected because this lowest dose of SDF-1 (0.1 nM) has a profound effect on cell migration.15 Exposure of the endothelial cells to the intermediate concentration (1 nM) of SDF-1 and increasing VEGF resulted in a dose-dependent increase in thymidine incorporation (Fig. 2B). Exposure of the cells to the highest concentration of SDF-1 (100 nM) and increasing VEGF had minimal effect compared with VEGF alone (Fig. 2B).
Figure 2.
Synergistic effect on tube length. (A) Combination of SDF-1 and VEGF significantly increased tube length in a basement membrane matrix assay compared with use of SDF-1 alone (P = 0.03). The effect of the combination approached, but did not achieve, statistical significance when compared with VEGF alone (P = 0.07). Bars represent SE. (B) Thymidine incorporation with different combinations of SDF-1 and VEGF. Note that the thymidine incorporation increases with increasing VEGF; the effect is greatest, however, with the lowest concentration of SDF-1. P < 0.05 for all values on graph compared with SFM alone.
In Vivo Assessment of SDF-1/CXCR-4 Axis in CNV Lesion Formation
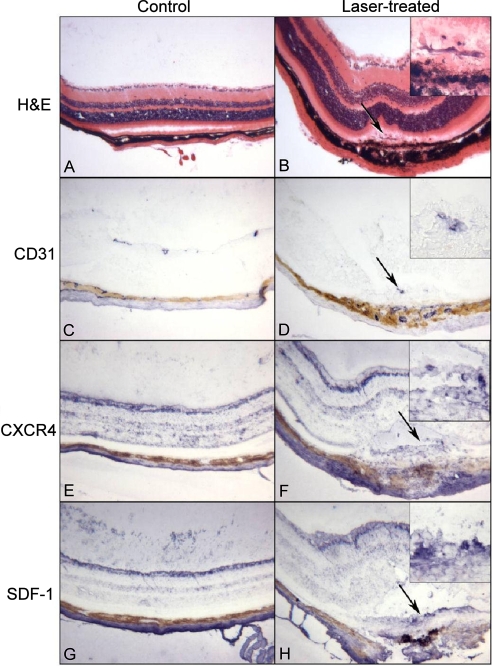
In animals in which Bruch's membrane was ruptured to induce CNV, laser burn sites were visible with hematoxylin and eosin staining (Figs. 3B vs. 3A). Disorganized hypertrophic RPE cells were present, and pigmented cells were evident anterior to the RPE monolayer (Fig. 3B). With anti-CD31 (a marker for endothelial cells), it was apparent that the CNV within laser burns and some of the cells anterior to the RPE monolayer were endothelial cells (Figs. 3D vs. 3C). CXCR4 immunoreactivity was present both in normal mice and in laser-treated mice in the ganglion cell layer, the anterior portion of the inner nuclear layer, photoreceptors, and choroidal stroma. CXCR-4 was also present in some cells anterior to the retinal pigment epithelium in the laser burn site (Figs. 3E, 3F). SDF-1 was present in the inner retina and the inner portion of the inner nuclear layer and the choroidal stroma in normal and laser-treated mice. It was also present at the laser burn site in cells anterior to the remaining RPE monolayer (Figs. 3G, 3H).
Figure 3.
Comparison of normal retina and choroid (A, C, E, G) and sites of laser burns (B, D, F, H) using histology (A, B) and alkaline phosphatase immunohistochemistry (C–H). (B, D, F, H) Arrow represents the same site. The normal laminar architecture of the retina and choroid (A) is disrupted by the laser injury (B). In the laser burn, cells (arrow) are present anterior to the RPE monolayer, which appears hypertrophic. At higher magnification (B, inset) some of those cells appear spindle-shaped, and the retinal pigment epithelium is disorganized. CD31 is present in most blood vessels in normal retina and choroid (C) and in cells in the site of the laser burn (D, arrow). At high magnification (D, inset), the CD31+ cells appear to be a site of CNV. CXCR4 is present at the same sites in normal retina and choroid (E), as in normal areas in the laser-treated mice (F). However, CXCR4 also labels the cells in the laser burn that are anterior to the RPE monolayer (F, inset) and around the area with apparent CNV (D; arrow and inset in F). SDF-1 is present in the innermost retina, a portion of the inner nuclear later in normal retina (G), and normal areas of the laser-injured eyes (H). SDF-1 is also prominent in the cells in the laser burn that appear to be organizing to form a scar (arrow) where CNV is present (D, arrow). (A, B) Hematoxylin and eosin staining. (C–H) APase reaction product and partial bleaching of the tissue.
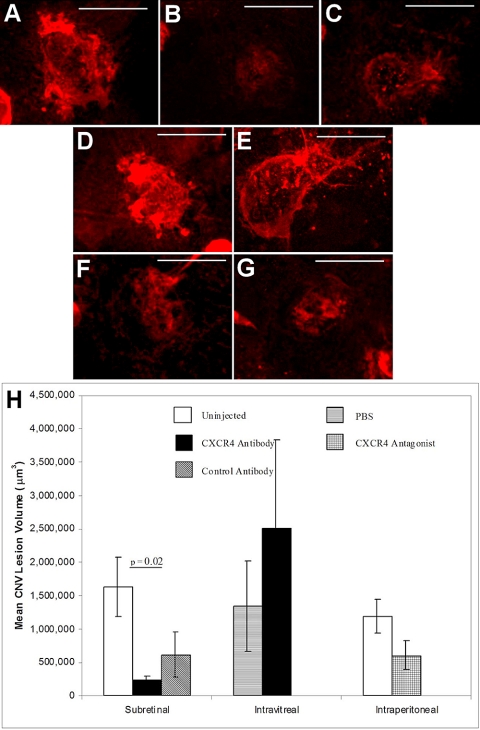
CNV Lesion Size in Response to CXCR4 Inhibition
In the group of animals undergoing subretinal injection with CXCR4 antibody (Fig. 4B), a significant decrease in CNV lesion volume was observed compared with uninjected controls (Figs. 4A, 4H; P = 0.02). In marked contrast, intravitreal injection of CXCR4 antibody (Fig. 4E) did not achieve similar results (Fig. 4H). Intraperitoneal injections of the CXCR4 antagonist NefM1 at the concentration shown to effectively reduce tumor growth (Fig. 4G) did not significantly reduce the CNV lesion volume compared with uninjected control eyes (Fig. 4F).
Figure 4.
Quantification of CNV lesion volume in the posterior cups. (A–G) Representative immunofluorescence micrographs of CNV lesions on posterior cups removed from eyes after injection by administration through a subretinal (A, uninjected control; B, CXCR4 antibody; C, control antibody), intravitreal (D, PBS; E, CXCR4 antibody), or intraperitoneal (F, uninjected control; G, CXCR4 antagonist) route. Posterior cups were stained with rhodamine-agglutinin, imaged, and quantified to show the vascular volume. Scale bars, 100 μm. (H) Plot of the quantitative measures of CNV lesion volumes for the conditions tested. For intravitreal and intraperitoneal injections, there was a trend toward a difference when CXCR4 antibody or antagonist was injected compared with the controls. With a subretinal injection, however, the CXCR4 antibody injections decreased the CNV volume significantly compared with uninjected eyes (P = 0.02).
SDF-1, IGF-1, and VEGF and Their Cognate Receptors after Laser Rupture
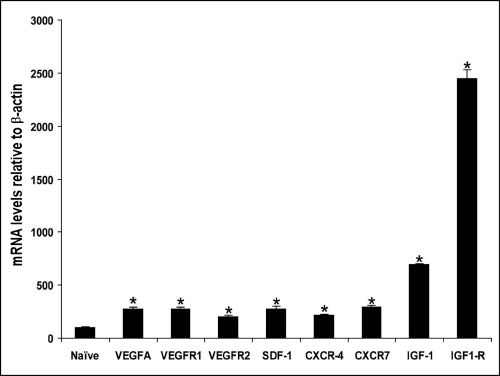
Laser rupture of Bruch's membrane resulted in a marked increased expression of mRNA for VEGFR-1, VEGFR-2, VEGF-A, SDF-1, CXCR4, CXCR-7, the alternate SDF-1 receptor, IGF-1, and IGF-1R compared with uninjured eyes (Fig. 5; P < 0.001). IGF-1 and IGF1R mRNA levels were especially striking, demonstrating almost 7-fold and 25-fold increases over normal, respectively.
Figure 5.
Analysis of mRNA levels in the posterior cups. Neural retinas were removed from posterior cups; the retinal pigment epithelium/choroid complex was pooled to analyze the levels of VEGF-A, VEGFR-1, VEGFR-2, SDF-1, CXCR4, CXCR7, IGF-1, and IGF-1R in response to laser injury. Note that in all cases mRNA levels are significantly higher in the laser-injured eye than in the uninjured naive eye (P < 0.001).
VEGF Receptor Surface Expression in Response to SDF-1
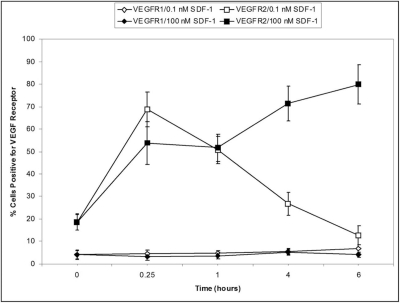
Because activation of VEGF receptors has been implicated in EPC differentiation to endothelial cells, we next examined the effect of SDF-1 (0.1 and 100 nM) on VEGFR-1 and VEGFR-2 expression in human CD34+ EPCs using FACS. SDF-1 exposure increased VEGFR-2, but not VEGFR-1, expression in CD34+ cells. Furthermore, the lowest concentration of SDF-1 resulted in a rapid increase in expression of VEGFR-2 that dropped with time, whereas at the higher SDF-1 concentration (100 nM), VEGFR-2 expression was maintained (Fig. 6).
Figure 6.
Percentage of cells expressing VEGFR-1 or VEGFR-2 in CD34+ EPCs after SDF-1 exposure. CD34+ EPCs were exposed to 0.1 nM SDF-1, 100 nM SDF-1 or medium alone (control) for 15 minutes, 1 hour, 4 hours, and 6 hours. Cells were stained with anti-VEGFR-1 or anti-VEGFR-2 and analyzed by flow cytometry. 0.1 nM SDF-1 increased the expression of VEGFR-2 at 15 minutes (□). 100 nM SDF-1 sustained the increase in VEGFR-2 expression (■).
Discussion
The role of SDF-1 and CXCR4 in ocular health and pathology is just now being elucidated given that many ocular cells are sources of SDF-1 in the eye.16 SDF-1 and CXCR4 are known to have dynamic and complementary expression patterns, with both proteins playing important roles in vascularization.17 Mice lacking CXCR4 or SDF-1 die in utero, with disruption in the development of vasculature networks in any organs.18
Typically, CXC chemokines lacking ELR motifs such as SDF-1 are antiangiogenic; interestingly, however, SDF-119 stimulates endothelial cell growth.15 Darash-Yahana et al.20 demonstrated that tumor-associated blood vessels express SDF-1. Subcutaneous xenografts of PC3 cells that overexpressed CXCR4 in mice showed significantly greater blood vessel density, functionality, and invasiveness of tumors into the surrounding tissues. Neutralizing SDF-1/CXCR4 interactions with antibodies to CXCR4 inhibited CXCR4-dependent tumor growth and vascularization.21 Vessel density and functionality, as well as metastasis to the regional lymph nodes and lungs, were significantly increased in these tumors overexpressing CXCR4.20 Similar effects of CXCR4 overexpression on tumor growth were also noted in breast cancer lines.22
Although in previous studies we focused on directly blocking SDF-1 and showed a reduction in CNV lesion size,5 in this study we used two approaches for reducing CXCR4 activation, a CXCR4 blocking antibody and a CXCR4 modulator, NefM1, that downregulates CXCR4 expression. NefM1 has been used to reduce tumor size in a rodent colon cancer model and has been shown to inhibit endothelial cell proliferation in vitro.23
In this report, we show that the inhibition of CXCR4 using a blocking antibody reduced CNV lesion size when administered as a subretinal injection but that intravitreal administration of CXCR4 antibody did not. Moreover, when NefM1 was given by way of the intraperitoneal route, it did not reduce CNV lesion size. Lima e Salva et al.4 used pharmacologic antagonists of CXCR4 (AMD 8664, TC14012, and compound 3) and demonstrated reduced CNV lesion size in the identical model system we describe here. They did not test systemic delivery but used periocular administration, which provides high ocular bioavailability, and observed CNV inhibition to a degree similar to what we observed with subretinal administration. Their results, as well as ours, suggest that the route of administration is paramount to using CXCR-4 antagonist as a therapeutic strategy for CNV.4
The concentration of NefM1 we used was previously found to be effective for the inhibition of tumor growth23; however, it had minimal effect on CNV lesion size. Interestingly, the effect of knocking down CXCR4 expression in tumor models has not always been consistent, generating conflicting data regarding the role of SDF-1 signaling on tumor cells and associated angiogenesis,24,25 and further speaks to the complexity of the system. The benefit of targeted ocular delivery rather than systemic delivery is nevertheless clearly shown in our study of CNV.
A possible explanation for the NefM1 result is that NefM1 may not reach sufficiently high levels in the target tissue. Overall, our data suggest that not only is ocular delivery critical, local delivery (subretinal rather than intravitreal) may be imperative. Subretinal blockade may be needed because antibodies and drugs that block CXCR-4 may bind in the retina before they reach the subretinal space. The bioavailability of the agent used is also essential because small molecule antagonists such as used by Lima e Silva et al.4 may be more effective than the blocking antibodies we used. Our results support those of previous reports from Lima e Silva and confirm that the blockade of CXCR4 activation results in decreases in CNV lesion size.4
In certain cell types, NefM1 induces internalization of CXCR4; thus, NefM1may activate signaling mechanisms to cause proliferation, thus limiting its clinical usefulness as an antiangiogenic.23,26,27 Internalization of a receptor from ligand binding typically leads to receptor degradation. However, Earp et al.28 showed that binding of EGF to the receptor leads to receptor degradation, but this was counterbalanced by a 3.5-fold increase in receptor synthesis; however, this effect may be cell-type specific. Although we do not know whether this type of compensation applies to NefM1 peptide and CXCR4 specifically in our system, we may consider these possibilities to explain the disparate effect observed with this agent in ocular tissue versus tumor.
More recently, a second receptor for SDF-1, CXCR7, has been discovered.29 It is present on hematopoietic stem cells and human CD34+ EPCs (Afzal A, Grant MB, unpublished results, 2007). It is also important for signaling to more committed progenitors and mature blood cells, such as T-lymphocytes and monocytes. We also observed that after laser injury, mRNA for CXCR7 is increased, suggesting that simply blocking CXCR4 may not be sufficient to achieve optimal SDF-1 blockade but that both receptors may have to be blocked to reach optimal therapeutic reduction of CNV.
SDF-1, CXCR-4, and CXCR-7 expression are increased in CNV lesions, making this system a therapeutic target. Our in vitro studies suggest that an anti–SDF-1 approach may be used in conjunction with anti-VEGF or anti–IGF-1 strategies to improve outcomes. Our studies show that the lowest concentration of SDF-1 tested enhanced the proliferative response of endothelial cells to VEGF, and this lowest concentration of SDF-1 was actually more potent than the highest concentration in stimulating VEGF-induced proliferation. This is not entirely uncommon in that cytokines such as SDF-1 typically have a “peak” effect; thus, concentrations lower or higher than this ideal level typically result in a reduced physiological response.
The repertoire of receptor expression in EPCs and endothelial cells no doubt dictates the angiogenic outcome, with the G protein–coupled receptor CXCR4 possibly regulating the tyrosine kinase receptors IGF-1R and VEGFR. Given the potential diversity of receptors constitutively expressed by EPC and endothelial cells, such interactions may be expected to alter signal coupling.
SDF-1 does not work in isolation, but its actions are orchestrated by other growth factors such as VEGF and IGF-1. Three forms of cross-talk between G protein–coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) have been demonstrated in different cellular systems. First, RTKs can be transactivated by GPCRs.30 Second, GPCRs can be transactivated by RTKs. For example, it has been shown that IGF-1 stimulated phosphorylation of the chemokine receptor CCR5 in MCF-7 cells and that chemotaxis induced by IGF-1 was inhibited by a neutralizing antibody to the ligand CCL5. Transactivation of CCR5 by IGF-1 was. Therefore, indirect, requiring the activity of CCL5 with its receptor.31 Third, bidirectional transactivation between the two receptor systems has also been observed. For example, the TKR platelet-derived growth factor receptor is phosphorylated by sphingosine-1-phosphate (S1P), leading to the activation of downstream effectors, including Shc, and the p85 regulatory subunit of the class IA PI3K.32 PDGF has been shown to transactivate the S1P receptor, a GPCR.33 Studies by Akekawatchai et al.34 showed coprecipitation of IGF-1R, CXCR4, and the G protein subunits Giα2 and Gβ, indicating a constitutive physical association between these molecules in breast cancer cells. CXCR4/IGF-1R receptor integration may play an important role in cancer metastasis and in development because both IGF-1/IGF-1R and SDF-1/CXCR4 are essential.35,36
Taken together, our results provide new information regarding the SDF-1/CXCR4/CXCR-7 axis in CD34+ EPCs, endothelial cells, and CNV lesions. Our findings add a new dimension to the understanding of chemokine-mediated neovascularization and support a new era of potential therapeutic interventions targeting this chemokine. Our work emphasizes the requirement of local delivery for targeting CXCR-4 and supports that combination therapy with anti-VEGF or anti–IGF-1 agents and simultaneous blockade of CXCR-7 may represent the optimal therapeutic strategy for CNV.
Acknowledgments
The authors thank Scott McLeod for his photographic and artistic contribution to Figure 1.
Footnotes
Supported by National Institutes of Health Grants EY012601 (MBG), EY007739 (MBG), EY01765 (Wilmer), and EY016151 (GAL). GAL is the G. Edward and G. Britton Durell Professor of Ophthalmology.
Disclosure: N. Sengupta, None; A. Afzal, None; S. Caballero, None; K.-H. Chang, None; L.C. Shaw, None; J.-J. Pang, None; V.C. Bond, None; I. Bhutto, None; T. Baba, None; G.A. Lutty, None; M.B. Grant, None
References
- 1.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–870 [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am 2006;19:361–372 [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal R, Strauss O. Investigations of RPE cells of choroidal neovascular membranes from patients with age-related macular degeneration. Adv Exp Med Biol 2003;533:107–113 [PubMed] [Google Scholar]
- 4.Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J 2007;21:3219–3230 [DOI] [PubMed] [Google Scholar]
- 5.Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci 2005;46:343–348 [DOI] [PubMed] [Google Scholar]
- 6.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 2005;115:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006;24:1254–1264 [DOI] [PubMed] [Google Scholar]
- 8.Smadja DM, Bieche I, Uzan G, et al. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol 2005;25:2321–2327 [DOI] [PubMed] [Google Scholar]
- 9.Brooks HL, Jr, Caballero S, Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol 2004;122:1801–1807 [DOI] [PubMed] [Google Scholar]
- 10.Guerin E, Sheridan C, Assheton D, et al. SDF1-alpha is associated with VEGFR-2 in human choroidal neovascularisation. Microvasc Res 2008;75:302–307 [DOI] [PubMed] [Google Scholar]
- 11.Yodoi Y, Sasahara M, Kameda T, Yoshimura N, Otani A. Circulating hematopoietic stem cells in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2007;48:5464–5472 [DOI] [PubMed] [Google Scholar]
- 12.Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:4908–4913 [DOI] [PubMed] [Google Scholar]
- 13.Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci 1993;34:477–487 [PubMed] [Google Scholar]
- 14.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:1544–1552 [DOI] [PubMed] [Google Scholar]
- 15.Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 2006;55:102–109 [PubMed] [Google Scholar]
- 16.Bhutto IA, McLeod DS, Merges C, Hasegawa T, Lutty GA. Localisation of SDF-1 and its receptor CXCR4 in retina and choroid of aged human eyes and in eyes with age related macular degeneration. Br J Ophthalmol 2006;90:906–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci 2008;49:2178–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998;393:591–594 [DOI] [PubMed] [Google Scholar]
- 19.Razmkhah M, Talei AR, Doroudchi M, Khalili-Azad T, Ghaderi A. Stromal cell-derived factor-1 (SDF-1) alleles and susceptibility to breast carcinoma. Cancer Lett 2005;225:261–266 [DOI] [PubMed] [Google Scholar]
- 20.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J 2004;18:1240–1242 [DOI] [PubMed] [Google Scholar]
- 21.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res 2000;6:3530–3535 [PubMed] [Google Scholar]
- 22.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–56 [DOI] [PubMed] [Google Scholar]
- 23.Bumpers HL, Huang MB, Powell M, et al. Effects of HIV-1 Nef, a cytotoxic viral protein, on the growth of primary colorectal cancer. Cancer Biol Ther 2005;4:65–69 [DOI] [PubMed] [Google Scholar]
- 24.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004;64:8604–8612 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal 2005;17:1578–1592 [DOI] [PubMed] [Google Scholar]
- 26.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol 2004;78:11084–11096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol 2004;78:3099–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earp HS, Austin KS, Blaisdell J, et al. Epidermal growth factor (EGF) stimulates EGF receptor synthesis. J Biol Chem 1986;261:4777–4780 [PubMed] [Google Scholar]
- 29.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 2006;203:2201–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi T, Cui CH, Kanda A, Kayaba H, Ohta K, Chihara J. Activation of epidermal growth factor receptor via CCR3 in bronchial epithelial cells. Biochem Biophys Res Commun 2004;320:292–296 [DOI] [PubMed] [Google Scholar]
- 31.Mira E, Lacalle RA, Gonzalez MA, et al. A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep 2001;2:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimoto T, Lungu AO, Berk BC. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res 2004;94:1050–1058 [DOI] [PubMed] [Google Scholar]
- 33.Waters C, Sambi B, Kong KC, et al. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem 2003;278:6282–6290 [DOI] [PubMed] [Google Scholar]
- 34.Akekawatchai C, Holland JD, Kochetkova M, Wallace JC, McColl SR. Transactivation of CXCR4 by the insulin-like growth factor-1 receptor (IGF-1R) in human MDA-MB-231 breast cancer epithelial cells. J Biol Chem 2005;280:39701–39708 [DOI] [PubMed] [Google Scholar]
- 35.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 1998;95:9448–9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molyneaux KA, Zinszner H, Kunwar PS, et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003;130:4279–4286 [DOI] [PubMed] [Google Scholar]