Introduction
Cataract is the leading cause of blindness in the world, accounting for approximately 42% of all blindness.1 Surgical treatment of cataracts imposes a substantial economic burden on health systems. Since cataract is primarily a disease of old age, we are facing a looming cataract epidemic in which the demand for cataract surgery will place greater demands on the resources available for treatment. An alternative approach to surgery is the development of therapies designed to prevent or delay the onset of cataract. It is therefore not surprising that the ultimate goal of many international lens research groups is to determine the causes of lens cataract, with a view toward developing novel anticataract therapies. A major obstacle to achieving this laudable goal is our current understanding of how the normal lens maintains its transparency. It has been proposed that the lens operates an internal microcirculation system that contributes to lens transparency by delivering nutrients to, and removing metabolic wastes from, the deep fiber cells while maintaining steady state lens volume (the lens fluid circulation model [FCM]).2–4 Key features of the model remain to be tested. Such scientific debate is a normal and healthy component of the research discovery process, but the lack of an accepted understanding of lens physiology is compromising progress toward the ultimate goal of developing targeted anticataract therapies.
The purpose of the two perspectives presented in Point/Counterpoint is to formalize this debate. Evidence for and against the FCM will be presented, with the goal of identifying areas of future experimentation that are needed to test its validity. A general overview of the model is provided, followed by a summary of the evidence supporting it by Richard Mathias, Paul Donaldson, and Linda Musil. In the Counterpoint, David Beebe and Roger Truscott present a critique of the model. These articles are followed by brief rebuttals that summarize the critical experiments needed to test the model.
It is important to acknowledge that our understanding of lens physiology has evolved from an initial view of the lens as inert tissue to one that recognizes it as a complex and dynamic organ. This evolution in understanding was initially driven by advances in histologic and electrophysiological recording techniques and then by our ability to determine the molecular identity and cellular localization of key transport proteins associated with the circulation system. Most recently, the ability to combine whole lens electrophysiological recording with transgenic animal models has enabled us to study the physiological roles that specific lens proteins play in the maintenance of lens transparency. It is highly likely that the application of new technologies to the lens will cause us to further modify our current understanding of lens structure and function, a summary of which is provided herein.
The Lens Internal Microcirculation: A Brief Overview
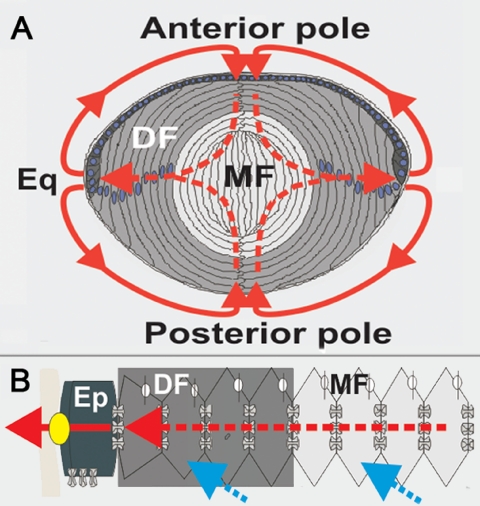
Lens transparency is the direct result of its specialized cellular architecture, which we have proposed is actively maintained by a unique lens physiology. The lens is an avascular tissue surrounded by a tough but porous collagenous capsule (Fig. 1A). Beneath the capsule, a single layer of cuboidal epithelial cells covers the anterior surface. Near the equator, these epithelial cells divide, and the daughter cells elongate and differentiate into the fiber cells that form the bulk of the lens. The fiber cells adopt a flattened hexagonal profile that facilitates their packing into an ordered array in which the spaces between the cells are smaller than the wavelength of light. During differentiation, the fiber cells lose their intracellular organelles and undergo significant changes in the expression of cytoplasmic and membrane proteins. An abundance of soluble cytoplasmic proteins, called crystallins, creates a high index of refraction. The concentration of crystallins is highest in the center of the lens, creating a radial gradient in refractive index that corrects inherent spherical aberration. Lens growth continues throughout the lifetime of an individual, with younger fiber cells being laid down on top of existing fiber cells, resulting in the progressive positioning of older cells deeper in the lens. Each mature fiber cell extends from the anterior to the posterior pole, where it forms a suture with other fiber cells. The lens maintains this precise cellular architecture and prevents light-scattering by controlling the volume of its constituent cells, preventing dilation of the normally narrow extracellular space, and maintaining the solubility of lens crystallins to stop their aggregation.
Figure 1.
Lens structure and function. (A) Differentiating (DF) and mature (MF) fiber cells with measured external (solid arrows) and hypothesized internal (dotted arrows) current flows. (B) Equatorial (Eq) cross section showing ion uptake from extracellular space (blue) and cell-to-cell efflux (red) via gap junctions and Na pump (yellow). Ep, epithelial cells.
Although the energy necessary to drive these processes in the differentiating fiber cells of the outer cortex can be provided by aerobic metabolism, the remaining bulk of lens fiber cells lack mitochondria and therefore must use anaerobic glycolysis to satisfy their energy requirements. Because of its size, the lens cannot rely on passive diffusion alone to transport nutrients to deeper lying cells or to transport waste products back to the surface (discussed later). Furthermore, most fiber cells lack the usual potassium channels and Na/K pumps necessary to generate the negative membrane potential needed to control their steady state cell volume. Faced with these metabolic and physiological constraints, the lens requires a specialized transport system to deliver nutrients, remove waste products, and impose the negative membrane potential necessary to the maintain steady state volume of the fiber cells.
A common feature of all vertebrate lenses studied to date is the existence of a standing flow of ionic current that is directed inward at the poles and outward at the equator (Fig. 1A). Using a combination of electrical impedance measurements and theoretical modeling, Mathias et al.4 have proposed that these currents measured at the lens surface represent the external portion of a circulating ionic current that drives a unique internal microcirculatory system that maintains fiber cell homeostasis and therefore lens transparency. Briefly, the working model is that the current, which is carried primarily by Na+, enters at all locations around the lens along the extracellular clefts between fiber cells. It eventually crosses the fiber cell membranes, then flows from cell to cell toward the lens surface via an intracellular pathway mediated by gap junction channels. Because the gap junction coupling conductance in the outer shell of differentiating fibers (Fig. 1A) is concentrated at the equator,5,6 the intracellular current is directed to the equatorial epithelial cells where the highest densities of Na/K pumps are located to actively transport Na+ out of the lens.7–9 Thus at the equator, the intracellular current that is leaving the lens is highly concentrated, causing the net current to be outward. At the poles, there is very little intracellular current. The net current is therefore predominantly inward, along the extracellular spaces (Fig. 1B).
The driving force for these fluxes is hypothesized to be the difference in the electromotive potential of surface cells and inner fiber cells. Data from ion substitution experiments performed on whole lenses4 and more recently on isolated fiber cells,10 suggest that the surface cells, including epithelial cells and newly differentiating fiber cells, contain Na/K pumps and K+ channels, which together generate a negative electromotive potential. Fiber cells deeper in the lens lack functional Na/K pumps and K+ channels, and their permeability is dominated by nonselective cation and Cl− conductances, with molecular identities that remain to be determined. In these inner cells, a negative membrane potential is maintained by virtue of their connection to surface cells via gap junctions. This electrical connection, together with the different membrane properties of the surface and inner cells causes the standing current to flow. In this model, the circulating current creates a net flux of solute that in turn generates fluid flow. The extracellular flow of water convects nutrients toward the deeper lying fiber cells, whereas the intracellular flow removes wastes and creates a well stirred intracellular compartment. Furthermore, the active removal of Na+ at the lens equatorial surface serves to maintain a favorable transmembrane Na+ gradient that is used by secondary active transporters expressed by mature fiber cells to accumulate nutrients delivered to the lens core by the circulation system. Thus, transport by surface cells is not only responsible for the delivery of nutrients to inner fiber cells and their subsequent uptake, but also imposes the negative membrane potential necessary to maintain the steady state volume of the fiber cells.
Modeling Lens Function Predicts the Existence of a Circulating Fluid Flux
Although we acknowledge that the circulation model is not universally accepted, it is important to distinguish between the circulating ionic currents that have been experimentally measured7,11,12 at the surface in lenses from different species and our model, in which a circulating current carried primarily by Na+ generates a circulation of fluid inside the lens.4 The data on the existence of the circulating ionic currents are firm and well supported by data on the distinct spatial localizations of gap junction conductance,5 Na/K pump currents,8 and the preferential influx of Na+ at the anterior and posterior poles.7,11,12 In contrast, circulating fluid flows in the lens have been more difficult to measure directly and are at present only predicted to occur from indirect measurements and models of the measured electrical properties of the lens.
The initial model of the electrical properties of the lens predicted the distribution of induced voltages when a current was injected into a central cell of a spherical syncytial tissue.13 The model was based on the structure of the lens and was used to determine the membrane conductances of fiber and surface cells and to determine the effective resistances of intracellular and extracellular pathways.4 Our impedance data suggest that most of the Na+ leak conductance of the lens is associated with fiber cell membranes,14 whereas others have localized Na,K-ATPase activity to the epithelium at the lens surface.4 This spatial segregation of Na+ influx (inner cells) from Na+ efflux (surface cells) suggests that there is a circulation of Na+. However, in our initial attempts to use the model to calculate the magnitude of this circulating Na+ current, we naively neglected water flow. In the absence of water flow, the model predicted that voltage and ion gradients would develop in the intracellular and extracellular spaces that actually opposed the circulation. This result was contrary to experimental measurement of both surface current flows12 and Na,K-ATPase activity, which suggests that a large Na+ leak into the lens must exist to account for the large pump current. Even more bothersome was the prediction that large transmembrane osmotic gradients would develop, making it impossible to neglect water flow unless membrane water permeability was zero. Because it is clearly not zero,15 it is now apparent that water flow cannot be neglected in modeling lens current flows.
The subsequent inclusion of water flow16 dramatically altered the model and produced a series of specific predictions about the distributions of intracellular ions, voltages, and hydrostatic pressures that could be experimentally tested. Predictions of an intracellular voltage gradient from peripheral to central fiber cells of ∼10 mV have now been confirmed by microelectrode measurements in lenses from several species.16–18 Similarly, a prediction of a surface-to-central-fiber-cell gradient in the intracellular Na+ concentration of ∼10 mM was recently confirmed by measurements of the intracellular Na+ concentrations in different regions of the mouse lens.19 Earlier models also did not include the increase in gap junction coupling from the poles to the equator that was measured experimentally.5 Higher coupling in peripheral equatorial fiber cells is proposed to guide intracellular outwardly directed current flow to the equator where Na,K-ATPase activity is concentrated.8,9 Inclusion of this axial variation in gap junctional coupling into the model accurately predicted surface current inflow and outflow of a magnitude similar to those recorded by vibrating probe measurements.11,12 Last, the model predicted that there would be a large intracellular hydrostatic pressure gradient (several hundred millimeters of mercury) to drive the intracellular flow of fluid from the central cells to the surface cells. We have recently measured this intracellular hydrostatic pressure in mouse lenses (Mathias RT, et al. IOVS 2010;51:ARVO E-Abstract 3459) and found that it varied from ∼300 mm Hg in the central cells to 0 mm Hg in the surface cells.
The Lens Circulation System: Unresolved Questions
Although these many successes of the model calculations have served to reinforce our conviction that water fluxes convect nutrients into the lens faster than can be achieved by passive diffusion, it is fair to say that for many seeing is believing, and water flow in the lens has yet to be measured experimentally. Furthermore, some have questioned the need for a specialized transport system to enhance the delivery of nutrients to the “metabolically inert” fiber cells of the lens core. Finally, the model is evolving as new data are accumulated. These issues are now discussed, to identify areas for future experimentation.
Visualizing Circulating Ion and Fluid Fluxes within the Lens.
Although fluid flow patterns such as those represented in Figure 1 are difficult to measure within the lens, findings in some recent studies are consistent with their existence. Fischbarg et al.20 were able to detect translens (anterior to posterior) fluid movement when the lens was placed in a Ussing chamber that forced the pattern of current flow to be translens (anterior to posterior). Although interpreted differently by Fischbarg et al., this observation is consistent with our prediction that fluid follows the path of Na+ flux such that when the Na+ flux is forced to be translens, the pattern of fluid flow should follow. In this regard, magnetic resonance imaging (MRI) techniques offer the potential to noninvasively probe the structure and function of the lens. We have obtained preliminary data from MRI-based diffusion tensor imaging that show that the technique can be used to map the directionality of water movement in the bovine lens.21 As would be predicted, the direction of water movement appears to follow the directionality of the Na+ flux. Future work is needed to determine whether it can be altered after perturbations designed to disrupt the Na+ flux that drives the circulation system.
Nutrient Delivery to Mature Fiber Cells.
Because the lens is a large (with respect to diffusion distances), avascular organ,23,24 it is our basic contention that some form of delivery system other than passive diffusion is necessary to support ionic and metabolic homeostasis of central fiber cells. This requirement is based on Einstein's law of diffusion, which simply states that the average time for diffusion to occur is proportional to the distance squared. This law means that diffusion is quite rapid over short distances, but is extremely slow over the longer distances found in organs. For example, glucose diffuses 10 μm deep into a lens in ∼1 second but requires 11 days to diffuse 1 cm. In comparison, a convective fluid flow with a velocity of 1 μm/s2, the rate calculated for fluid entry into the lens,4 moves glucose up to 1 cm in less than 3 hours. Obviously, convection becomes essential when diffusion distances are significantly larger than cellular dimensions, the lenses of all mammalian species are sufficiently large that the diffusion of metabolites such as glucose to the central fiber cells would take many hours at least, whether it be via the extracellular space or an intracellular route mediated by gap junction channels.
Therefore, if the physical constraints imposed on a simple diffusion-based delivery system are accepted, then the issue shifts to whether the metabolic requirements of mature fiber cells are sufficiently high to warrant a faster delivery system. The metabolic requirements of the lens core are obviously much lower than those of the cortex, where the high levels of protein and lipid synthesis associated with fiber cell differentiation and elongation are fuelled by oxidative metabolism. In the core, the major known metabolic requirement is to maintain the reduced levels of glutathione (GSH) to prevent protein cross-linking, crystallin aggregation, and light-scattering.24 The replenishment of GSH from glutathione disulfide (GSSG) is mediated by the enzyme glutathione reductase and requires NADPH as a reducing equivalent, which is in turn produced via the hexokinase shunt pathway.25 Hence, it is apparent that the core of the lens needs energy, but it is not clear whether the necessary reducing environment in the core of the lens is maintained by the local metabolism of glucose to maintain NADPH levels, or the importation of reducing equivalents from the cortex. We do know that low levels of glutathione reductase activity persist in the lens nucleus, albeit at lower levels than in the cortex,26 and that mature fiber cells express transporters that are potentially capable of mediating the uptake of nutrients convected to them via the circulation system.27 Furthermore, in preliminary experiments, we have shown that the Na+-dependent glucose transporter SGLT2 is expressed in the nucleus and can accumulate SGLT-specific glucose analogues.28 Thus, the production of reducing equivalents via the anaerobic metabolism of glucose convected into the nucleus by the circulation and the expression of glutathione reductase in the nucleus suggest that the recycling of GSH can occur locally.
In summary, the lens is a complex and dynamic tissue that requires an understanding of how regional differences in lens biochemistry, physiology, and cell biology contribute to the maintenance of lens transparency. It is our contention that the lens internal circulation system is central to this integrative lens biology that controls lens transparency. However, the circulation system remains a model that must be rigorously tested and refined if we are to accumulate the necessary functional insights to combat the looming cataract epidemic.
Acknowledgments
The authors thank Jim Hall for invaluable discussions and a critical review of the manuscript.
Footnotes
Supported by the New Zealand Health Research Council (PJD), the Marsden Fund (PJD), and National Institute of Health Grants EY014622 (LM) and EY06391 (RTM).
Disclosure: P.J. Donaldson, None; L. Musil, None; R.T. Mathias, None
References
- 1.Brian G, Taylor H. Cataract blindness: challenges for the 21st century. Bull World Health Organ 2001;79(3):249–256 [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci 2001;16:118–123 [DOI] [PubMed] [Google Scholar]
- 3.Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol 2007;216:1–16 [DOI] [PubMed] [Google Scholar]
- 4.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev 1997;77:21–50 [DOI] [PubMed] [Google Scholar]
- 5.Baldo GJ, Mathias RT. Spatial variations in membrane properties in the intact rat lens. Biophys J 1992;63:518–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le AC, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol 2001;154:197–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candia OA, Zamudio AC. Regional distribution of the Na(+) and K(+) currents around the crystalline lens of rabbit. Am J Physiol Cell Physiol 2002;282:C252–C262 [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J Membr Biol 2000;178:89–101 [DOI] [PubMed] [Google Scholar]
- 9.Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na, K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci 2003;44:4395–4399 [DOI] [PubMed] [Google Scholar]
- 10.Webb KF, Donaldson PJ. Differentiation-dependent changes in the membrane properties of fiber cells isolated from the rat lens. Am J Physiol Cell Physiol 2008;294:C1133–C1145 [DOI] [PubMed] [Google Scholar]
- 11.Parmelee JT. Measurement of steady currents around the frog lens. Exp Eye Res 1986;42:433–441 [DOI] [PubMed] [Google Scholar]
- 12.Robinson KR, Patterson JW. Localization of steady currents in the lens. Curr Eye Res 1982;2:843–847 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg RS, Barcilon V, Mathias RT. Electrical properties of spherical syncytia. Biophys J 1979;25:151–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathias RT, Rae JL, Ebihara L, McCarthy RT. The localization of transport properties in the frog lens. Biophys J 1985;48:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol 1999;170:191–203 [DOI] [PubMed] [Google Scholar]
- 16.Mathias RT, Rae JL. Steady state voltages in the frog lens. Curr Eye Res 1985;4:421–430 [DOI] [PubMed] [Google Scholar]
- 17.Baldo GJ, Gong X, Martinez-Wittinghan FJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol 2001;118:447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses lacking alpha3 connexin. Proc Natl Acad Sci U S A 1998;95:15303–15308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Gao J, Sun X, et al. The effects of GPX-1 knockout on membrane transport and intracellular homeostasis in the lens. J Membr Biol 2009;227:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischbarg J, Diecke FP, Kuang K, et al. Transport of fluid by lens epithelium. Am J Physiol Cell Physiol 1999;276:C548–C557 [DOI] [PubMed] [Google Scholar]
- 21.Vaghefi E, Pontre B, Donaldson PJ, Hunter PJ, Jacobs MD. Visualization of transverse diffusion paths across fiber cells of the ocular lens by small animal MRI. Physiol Meas 2009;30:1061–1073 [DOI] [PubMed] [Google Scholar]
- 22.Fischbarg J, Diecke FPJ, Kuang K, et al. Transport of fluid by lens epithelium. Am J Physiol Cell Physiol 1999;276:C548–C557 [DOI] [PubMed] [Google Scholar]
- 23.Harris JE, Hauschildt JD, Nordquist LT. Transport of glucose across the lens surfaces. Am J Ophthalmol 1955;39:161–169 [DOI] [PubMed] [Google Scholar]
- 24.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther 2000;16:121 [DOI] [PubMed] [Google Scholar]
- 25.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res 2006;31:1–11 [DOI] [PubMed] [Google Scholar]
- 26.Zhang WZ, Augusteyn RC. Ageing of glutathione reductase in the lens. Exp Eye Res 1994;59:91–95 [DOI] [PubMed] [Google Scholar]
- 27.Donaldson PJ, Lim J. Membrane transporters: new roles in lens cataract. In: Rizzo JF, Tombran-Tink J, Barnstable CJ. eds. Ocular Transporters in Ophthalmic Diseases and Drug Delivery Totowa, NJ: Humana Press Inc.; 2008:83–104 [Google Scholar]
- 28.Merriman-Smith BR, Grey AC, Varadaraj R, Kistler J, Mathias RT, Donaldson PJ. The functional implications of the differentiation dependent expression of glucose transporters in the rat lens. Exp Eye Res 2004;79(suppl):105 15183105 [Google Scholar]