Based on published data, this ecological correlation study showed evidence to support the hypothesis that variation in the risk allele frequency of the Y402H polymorphism across ethnicities explains variation in prevalence of late AMD when data on people of African ancestry are excluded.
Abstract
Purpose.
To investigate whether variation in the distribution of the risk allele frequency of the Y402H single-nucleotide polymorphism (SNP) across various ethnicities and geographic regions reflects differences in the prevalence of late age-related macular degeneration (AMD) in those ethnicities.
Methods.
Published data were obtained via a systematic search. Study samples were grouped into clusters by ethnicity and geographic location and the Spearman correlation coefficient of the prevalence of late AMD and risk allele frequencies was calculated across clusters.
Results.
Across all ethnicities, AMD prevalence was seen to increase with age. Populations of European descent had both higher risk allele frequencies and prevalence of late AMD than did Japanese, Chinese, and Hispanic descendants. Results for African descendants were anomalous: although allele frequency was similar to that in European populations, the age-specific prevalence of late AMD was considerably lower. The correlation coefficient for the association between allele frequency and AMD prevalence was 0.40 (95% confidence interval [CI] = −0.36 to 0.84, P = 0.28) in all populations combined and 0.71 (95% CI = 0.02–0.94, P = 0.04) when people of African descent were excluded.
Conclusions.
Evidence was found at the population level to support a positive association between the Y204H risk allele and the prevalence of AMD after exclusion of studies undertaken on persons of African ancestry. Data in African, Middle Eastern, and South American populations are needed to provide a better understanding of the association of late AMD genetic risk across ethnicities.
Age-related macular degeneration (AMD) is the leading cause of blindness in older people in Western populations. Several lifestyle and environmental risk factors have been identified for AMD,1 and more recently, genetic variants with strong effects have also been identified.2 The genetic variants have mainly been studied in people of European origin. In this study, we focused on the single-nucleotide polymorphism (SNP) Y402H in the complement factor H (CFH) gene, which is characterized by a substitution of histidine for tyrosine at codon 402 on the long arm of chromosome 1, region 31 (rs1061170). This SNP is particularly striking because of the strength of its association with late AMD. Odds ratios (ORs) >5 were found for those homozygous for the Y402H risk allele, making this genetic association one of the strongest for a complex disorder yet to be reported.3
Although most of the population-based studies reporting the prevalence of late AMD have been undertaken in people of European origin in Western settings, studies are now emerging from other geographic areas. These, along with studies in different ethnic subgroups within Western populations (principally the United States) suggest that there is considerable variation in the distribution of risk alleles of the Y402H SNP4–8 and that these variations may in part explain the differences in the prevalence of late AMD in the respective population groups. To our knowledge, no studies have been conducted to examine whether differences in the frequency of this variant between populations or ethnic groups can explain differences in the prevalence of late AMD. We therefore conducted an ecological correlation of the prevalence of late AMD with the frequencies of the Y402H risk allele across ethnic groups and geographic regions. We considered only late AMD because of the differences in classification and grading of early AMD across studies.
Methods
We systematically searched the literature for studies of the prevalence of late AMD and studies that included representative data on the frequency of Y402H genotypes or alleles in the general population.
AMD Prevalence
We searched PubMed for relevant articles using the search terms “prevalence” in combination with “age-related macular degeneration” or “AMD,” limiting our search to English language papers. The articles that we deemed eligible were those that clearly confirmed the use of robust systems of fundus grading to determine the diagnosis of AMD9,10 and reported prevalence of late AMD (either neovascular, geographic atrophy, or a combination of both), by age groups and ethnicity of the sample. The search identified 22 eligible articles, some of which reported prevalence in samples from two or more ethnic groups, resulting in a total of 29 unique samples. The studies reported AMD prevalence using different age ranges starting from as low as 35 to above 90.
CFH Y402H Polymorphism
We searched PubMed for relevant articles by using the search terms “age-related macular degeneration” and “gene” or “complement factor H” as well as “complement factor H” on its own. We searched for articles on association studies of AMD as well as non-AMD diseases with the Y402H polymorphism. Among these, we included those that reported SNP allele or genotype frequencies of non-AMD cases. Studies were included irrespective of whether Hardy-Weinberg equilibrium (HWE) was observed for the Y402H genotypes in non-AMD cases, since this is not necessarily reflective of poor study design, but may instead be reflective of copy number variation within the complement factor genetic region.11 Furthermore, in studies in which HWE was observed, the association between the Y402H risk allele and AMD has been shown to be the same as in studies in which HWE was not observed (see, for example, Thakkinstian et al.12). Our search resulted in 50 eligible articles, some of which included two or more samples or samples from more than one ethnic group, hence 67 unique samples. Tables 1 and 2 give general characteristics of the prevalence and Y402H studies that were selected in our search.
Table 1.
General Characteristics of AMD Prevalence Studies Selected
| Study | Year | Study Name | Country | Ethnicity | % Male | Mean Age (SD) | Sample Size (n) | Grading System |
|---|---|---|---|---|---|---|---|---|
| Andersen et al.20 | 2008 | Inuit | Greenland | Inuit | 660 | ICSARM | ||
| Augood et al.21 | 2006 | Eureye | 7 European countries* | European | 4753 | ICSARM | ||
| Chen et al.22 | 2008 | Shihpai | Taiwan | Chinese | 62.3 | 71.8 (4.8) | 1058 | WARMGS |
| Friedman et al.23 | 1999 | BES | Unites States | African | 1836 | |||
| Unites States | European | 2475 | ICSARM | |||||
| Gupta et al.24 | 2007 | INDEYE | India | Indian | 1101 | WARMGS | ||
| Jonasson et al.25 | 2006 | Reykjavik | Iceland | Icelandic | 48 | 922 | ICSARM | |
| Kawasaki et al.5 | 2008 | Funagata | Japan | Japanese | 43.8 | 70.6 (6.8) | 1625 | WARMGS |
| Kawasaki et al.7 | 2008 | Sing Malay | Singapore | Malay | 48.1 | 58.67 | 3265 | WARMGS |
| Klein et al.26 | 1992 | Beaver Dam | Unites States | European | 44.5 | 4771 | WARMGS | |
| Klein et al.27 | 2003 | CHD | Unites States | African | 363 | WARMGS | ||
| European | 1998 | |||||||
| Klein et al.28 | 2006 | MESA | Unites States | African | 45.1 | 62.4 (9.9) | 1590 | |
| Chinese | 49.4 | 62.4 (10) | 699 | |||||
| Hispanic | 48.1 | 61.6 (10) | 1280 | WARMGS | ||||
| European | 48.8 | 63 (10) | 2315 | |||||
| Klein et al.29 | 1999 | NHANES | Unites States | African | 2129 | |||
| Hispanic | 1925 | WARMGS | ||||||
| European | 4267 | |||||||
| Krishnaiah et al.4 | 2005 | APES | India | Indian | 47 | 54 (10.6) | 3723 | |
| Li et al.30 | 2006 | Beijing | China | Chinese | 56.1 (10.5) | 4376 | WARMGS | |
| Mitchell et al.31 | 1995 | Bluemountain | Australia | European | 43.3 | 3654 | WARMGS | |
| Munoz et al.32 | 2005 | Proyecto VER | Unites States | Hispanic | 68 | 2807 | WARMGS | |
| Oguido et al.33 | 2008 | Brazil | Japanese | 71 | 483 | ICSARM | ||
| Oshima et al.34 | 2001 | Hisayama | Japan | Japanese | 40.1 | 1486 | ICSARM | |
| Schachat et al.6 | 1995 | Barbados | Barbados | African | 3344 | WARMGS | ||
| vanNewkirk et al.35 | 2000 | MVIP | Australia | European | 45 | 60.2 (12.9) | 3271 | ICSARM |
| Varma et al.36 | 2004 | LALES | Unites States | Hispanic | 42 | 54.9 (10.7) | 5875 | WARMGS |
| Vingerling et al.37 | 1995 | Rotterdam | Netherlands | European | 40.3 | 6251 | WARMGS |
BES, Baltimore Eye Study; INDEYE, India Eye Feasibility Study; CHD, cardiovascular Health Study; MESA, Multi-ethnic Study of Atherosclerosis; NHANES, National Health and Nutrition Examination Survey; Proyecto VER, Vision and Eye Research Project; MVIP, Melbourne Visual Impairment Project; LALES, Los Angeles Latino Eye Study; ICS ARM, International Classification System for ARM9; WARMGS, Wisconsin Age-Related Maculopathy Grading System.10
Multicountry study of Norway, Estonia, United Kingdom, France, Italy, Greece, and Spain.
Table 2.
General Characteristics of the Y402H Studies Selected
| Study | Year | Country | Ethnicity | Sample Size* | Mean Age | Disease† and Study |
|---|---|---|---|---|---|---|
| Baird et al.38 | 2006 | Australia | European | 144 | 70 | AMD-MVIP |
| Brantley et al.39 | 2007 | Unites States | European | 189 | 69.5 | AMD-AREDS |
| Chen et al.40 | 2006 | China | Chinese | 244 | 73.5 | AMD |
| Chowers et al.41 | 2008 | Israel | Israeli | 1180 | 70.8 | AMD |
| Conley et al.42 | 2005 | Unites States | European | 210 | AMD | |
| DeJong et al.43 | 2007 | Netherlands | European | 5066 | AMD-Rotterdam | |
| Despriet et al.44 | 2006 | Netherlands | European | 3619 | AMD | |
| 2392 | ||||||
| Droz et al.45 | 2008 | Switzerland | European | 52 | 74.9 | AMD |
| Edwards et al.46 | 2005 | Unites States | European | 134 | AMD | |
| European | 68 | AMD | ||||
| Fisher et al.47 | 2007 | Russia | European | 151 | 71 | AMD |
| Fuse et al.48 | 2006 | Japan | Japanese | 192 | 68.6 | AMD |
| Gotoh et al.49 | 2006 | Japan | Japanese | 105 | 60.2 | AMD |
| Gotting et al.50 | 2008 | German | European | 189 | 0 | AMD |
| Grassi et al.8 | 2006 | Unites States | African | 75 | AMD | |
| European | 148 | |||||
| Hispanic | 81 | |||||
| Japanese | 82 | |||||
| Somali-African | 128 | |||||
| Hageman et al.18 | 2005 | Unites States | European | 131 | 78.4 | AMD |
| European | 275 | 68.84 | ||||
| Haines et al.51 | 2005 | Unites States | European | 24 | 69.8 | AMD |
| Jakobsdottir et al.52 | 2005 | Unites States | European | 117 | AMD | |
| Kaur et al.19 | 2006 | India | Indian | 120 | 63.9 | AMD |
| Kim et al.53 | 2008 | Korea | Koreans | 187 | AMD | |
| Lau et al.54 | 2006 | China | Chinese | 232 | AMD | |
| Lee et al.55 | 2008 | Singapore | Chinese | 93 | AMD | |
| LeFur et al.56 | 2008 | France | European | 6348 | Dementia | |
| European | 642 | Alzheimer's | ||||
| Lin et al.57 | 2008 | Taiwan | Chinese | 180 | AMD | |
| Magnusson et al.58 | 2006 | Iceland | Icelandic | 171 | AMD | |
| Icelandic | 891 | AMD | ||||
| Unites States | European | 203 | AMD | |||
| Maller et al.59 | 2006 | Unites States | European | 934 | 74 | AMD-AREDS |
| Mooijaart et al.60 | 2007 | Netherlands | European | 640 | Inflammation, | |
| European | 552 | Cardiovascular-PAMD | ||||
| Mori et al.61 | 2007 | Japan | Japanese | 139 | AMD | |
| Narayanan et al.62 | 2006 | Unites States | European | 58 | 72.5 | AMD |
| Ng et al.63 | 2008 | China | Chinese | 155 | 73.1 | AMD |
| Okamoto et al.64 | 2006 | Japan | Japanese | 89 | AMD | |
| Pai et al.65 | 2007 | Unites States | European | 499 | 65.1 | Coronary |
| European | 473 | 60.3 | Heart Disease | |||
| Pulido et al.66 | 2007 | Unites States | European | 120 | Coronary Heart Disease | |
| Rivera et al.67 | 2005 | Germany | European | 611 | AMD | |
| European | 335 | |||||
| Schaumberg et al.68 | 2007 | Unites States | European | 1071 | 60.2 | AMD-NHS |
| Seddon et al.16 | 2006 | Unites States | European | 280 | AMD | |
| Seitsonen et al.69 | 2006 | Finland | European | 105 | 76.9 | AMD |
| Sepp et al.70 | 2006 | England | European | 262 | 75.8 | AMD |
| Simonelli et al.71 | 2001 | Italy | European | 47 | 0 | AMD |
| Souied et al.72 | 2005 | France | European | 91 | 74.6 | AMD |
| Stark et al.73 | 2007 | Germany | European | 56.9 | Myocardial Infarction-GMI | |
| 973 | ||||||
| Tanimoto et al.74 | 2007 | Japan | Japanese | 99 | 73.5 | AMD |
| Tedeschi-Blok et al.75 | 2007 | Unites States | Hispanic | 570 | AMD-LALES | |
| Uka et al.76 | 2006 | Japan | Japanese | 107 | AMD | |
| Volcick et al.77 | 2008 | Unites States | African | 3010 | Atherosclerosis-ARIC | |
| European | 8217 | |||||
| Wegscheider et al.78 | 2007 | Austria | European | 163 | 77.5 | AMD |
| Xing et al.79 | 2008 | Australia | European | 2381 | AMD-Blue Mountains | |
| Xu et al.80 | 2008 | China | Chinese | 132 | 66.1 | AMD |
| Zareparsi et al.81 | 2005 | Unites States | European | 275 | AMD | |
| Zee et al.82 | 2006 | Unites States | European | 335 | Cardiovascular diseases-PHS | |
| European | 235 | |||||
| European | 34 | |||||
| Zetterberg et al.83 | 2008 | Sweden | European | 1265 | 78.6 | Alzheimer's |
| Ziskind et al.13 | 2008 | South Africa | African | 98 | 76 | AMD |
LALES, Los Angeles Latino Eye Study; MVIP, Melbourne Visual Impairment Project; NHS, Nurses' Health study; BMI, German Myocardial Infarction Study; PHS, Physician Health Study; ARIC, Atherosclerosis Risk in Communities.
Sample sizes of the control group.
Disease studied.
Analysis
Our hypothesis was that the observed differences in the Y402H risk allele frequencies between ethnicities reflects various prevalence rates of late AMD in these ethnicities. We assumed that the estimates given by the samples in the studies are measures of true allele frequencies and prevalence of late AMD in their respective populations and geographic regions. We thus defined clusters of samples within studies based on both ethnicity and geographic region. For example, people of non-Hispanic European origin living in the United States were categorized in the European-American cluster. For each cluster, mean log-transformed prevalence and mean risk allele frequencies were calculated across all samples, weighted by the sample sizes. Spearman's rank correlation coefficient was calculated.
Results
The prevalence of late AMD and allele frequency data in our analysis came from independent samples within ethnicities and regions, except for four studies that sampled from the same populations: MVIP (Melbourne Visual Impairment Project), LALES (Los Angeles Latino Eye Study), ProyectoVER (Vision and Eye Research Project), and the Rotterdam Eye Study (Tables 1 and 2). Our selected publications produced 11 clusters on prevalence of AMD and 12 on Y402H studies. Of these, nine clusters had data on both the prevalence and Y402H frequencies. The age groups used in the prevalence studies varied slightly from study to study, and we therefore merged some of these to create three common groups: below 60, between 60 and 69, and above 70 years.
In the studies on prevalence of late AMD, a clear increase was observed with advancing age but there was a marked variation between studies in the prevalence rates. Most notably, lower rates were observed in Chinese, Japanese, and African Americans. At the other extreme were very high prevalence rates in the Icelandic population, whereas the Greenland Inuit had an overall prevalence that was even 2.5 times higher than that of the Icelandics (Table 3, Fig. 1).
Table 3.
Weighted AMD Prevalence by Age-Group and Cluster and Number of Samples Contributing to Each Estimate
| Cluster | Age <60 |
Age 60–70 |
Age 70+ |
Prevalence Total % (SE) | |||
|---|---|---|---|---|---|---|---|
| Samples (n) | % (SE) | Samples (n) | % (SE) | Samples (n) | % (SE) | ||
| African (American and Caribbean) | 4 | 0.28 (0.09) | 4 | 0.29 (0.18) | 5 | 0.47 (0.26) | 0.34 (0.12) |
| Chinese | 2 | 0.14 (0.07) | 3 | 0.32 (0.21) | 3 | 1.67 (0.54) | 0.55 (0.16) |
| European Australia | 0 | — | 2 | 0.24 (0.15) | 2 | 3.4 (0.47) | 1.13 (0.18) |
| European Europe* | 0 | — | 1 | 0.96 (0.24) | 1 | 4.58 (0.0.37) | 2.39 (0.21) |
| European American | 4 | 0.12 (0.08) | 4 | 0.46 (0.1) | 5 | 2.03 (0.35) | 1.18 (0.19) |
| Hispanic American | 4 | 0.07 (0.04) | 4 | 0.38 (0.22) | 4 | 1.15 (0.36) | 0.37 (0.10) |
| Icelandic | 1 | 0.30 (0.29) | 1 | 1.20 (0.59) | 1 | 10.55 (1.9) | 3.47 (0.60) |
| Indian | 2 | 0.97 (0.22) | 2 | 2.63 (0.45) | 2 | 4.06 (1.14) | 1.78 (2.53) |
| Inuit Greenland | 0 | — | 1 | 3.84 (0.94) | 1 | 18.52 (2.4) | 9.24 (1.13) |
| Japanese | 2 | 0.19 (0.14) | 2 | 0.87 (0.39) | 2 | 0.94 (0.39) | 0.67 (0.21) |
| Japanese-Brazilian | 0 | — | 0 | — | 1 | 1.24 (0.50) | 1.24 (0.15) |
| Malay | 1 | 0.11 (0.08) | 1 | 0.38 (0.22) | 1 | 2.49 (0.58) | 0.70 (0.15) |
The European Europe cluster estimates by age group exclude one study (Vingerling et al.37, Rotterdam study) that did not give sample sizes by age group.
Figure 1.
Forest plot of total prevalence across all age groups.
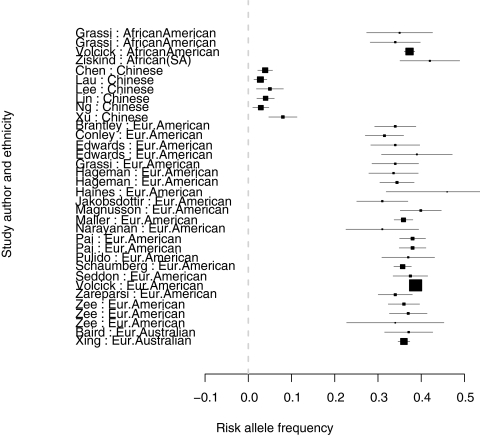
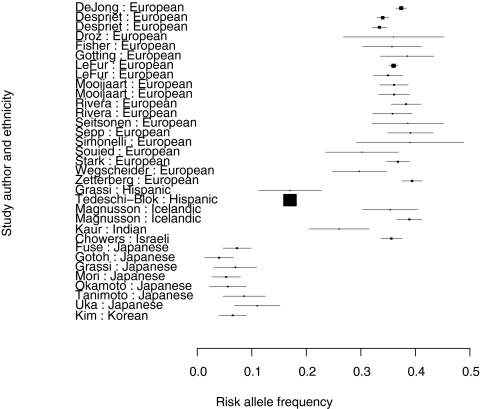
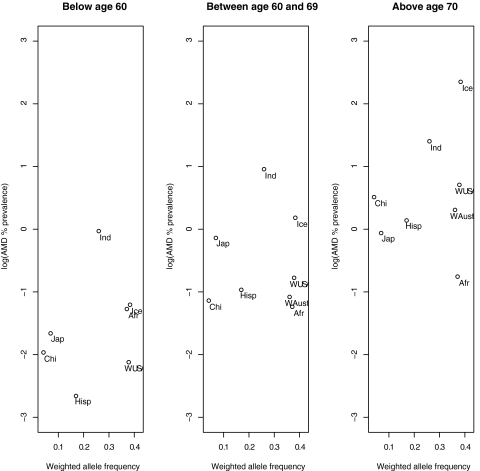
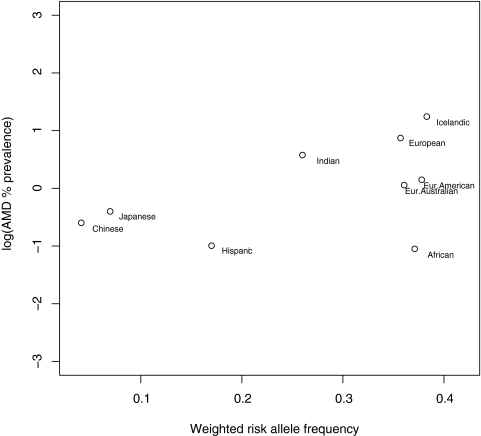
In studies on CFH Y402H allele distribution, low-risk allele frequencies (<1%) were observed in the Japanese, Chinese, and Koreans. In most other populations studied, the allele frequencies were between 36% and 42% (Table 4, Figs. 2, 3). Figure 4 shows a plot of the log transformed, weighted AMD prevalence by age group against the weighted risk allele frequencies. This relationship, across all age groups, is plotted in Figure 5. Only nine clusters are depicted on the graphs because other clusters (Inuit Greenland, South African, Malay, Korean, Israeli, and Japanese-Brazilians) either had prevalence or allele frequency data, but not both, as shown in Tables 3 and 4.
Table 4.
Weighted Risk Allele Frequencies by Cluster
| Cluster | Samples (n) | Weighted Risk Allele Frequency (SE) |
|---|---|---|
| African American | 3 | 0.371 (0.008) |
| African South America | 1 | 0.420 (0.035) |
| Chinese | 6 | 0.041 (0.011) |
| European Australia | 2 | 0.361 (0.008) |
| European Europe | 19 | 0.359 (0.078) |
| European American | 22 | 0.378 (0.009) |
| Hispanic American | 2 | 0.170 (0.005) |
| Icelandic | 2 | 0.383 (0.014) |
| Indian | 1 | 0.260 (0.028) |
| Israeli | 1 | 0.356 (0.009) |
| Japanese | 7 | 0.069 (0.016) |
| Korean | 1 | 0.065 (0.013) |
Figure 2.
Forest plot of risk allele frequency for the first 34 studies.
Figure 3.
Forest plot of the risk allele frequency for the last 33 studies.
Figure 4.
Log-transformed AMD prevalence versus risk allele frequencies by age weighted by sample size.
Figure 5.
Log-transformed AMD prevalence across all age groups versus risk allele frequencies weighted by sample size.
Spearman's rank correlation between weighted AMD prevalence and CFH risk allele frequency was 0.40 (P = 0.28) and after the studies undertaken on persons of African ancestry were excluded, it was 0.71 (P = 0.04).
Discussion
Overall, our results suggest evidence of a positive association between the prevalence of late AMD and Y402H risk-allele frequency across ethnicities, except in those of African descent. We observed marked differences in both AMD prevalence and allele frequency in different ethnicities and geographic regions. We therefore used an ecological study design to combine evidence of gene–disease association based on published data. To our knowledge, until now, this approach has not been applied to the study of AMD. It provides a useful way of comparing gene–disease associations between ethnicities and geographic regions when large interpopulation-based studies do not exist. The criteria we used to select studies ensured that we included prevalence estimates that were measured using standard diagnostic techniques in different ethnicities. We did not analyze the results by type of late AMD, because not all studies reported prevalence in that way, and therefore we used estimates of any late AMD.
There are several limitations in our analysis. Only four of the prevalence studies listed in Table 1 had the risk allele frequency derived from the same populations. Most of the studies on the Y402H variant were designed as case–control studies and therefore AMD prevalence was not established within them. Risk allele frequencies were strongly homogeneous within each of the clusters, and therefore it was sensible to cluster study samples in that way. This homogeneity within clusters suggests that, at least for the studies in our analysis, within a given homogenous residential population, a representative sample of genetic information is meaningful for others in the same population. With increased ethnic mixing, populations may be more genetically heterogeneous, and self-reported ethnicity may not reflect this. Ethnic mixing and migration may apply to populations of African origin, but the results of the three African American studies and one South African study show similar allele frequencies.
It is interesting that the prevalence of late AMD among African descendants does not match their allele frequencies. This mismatch is unlikely to be due to bias in the corresponding prevalence studies, as they have been carefully designed and rigorously implemented. It may be that an association of the Y402H risk allele with late AMD does not exist or is less pronounced in these populations. At present, there are not sufficient data from association studies on those of African ancestry. There is only one study in Africa in which the association between Y204H and AMD (early form) was investigated.13 The investigators found a nonsignificant odds ratio of 1.56 (95% confidence interval [CI] = 0.75–3.33) for the risk allele, but it was based on a very small sample size. A neighboring deletion allele delCFHR1 confers a protective effect in Europeans.14 Based on high frequencies of Y402H and delCHFR1 alleles in African Americans, the hypothesis in one study15 of the complement factor H and related genes was that the effect of these explains the low prevalence of late AMD in African Americans. This notion is in line with what our ecological analysis showed. Further studies are needed in African populations to substantiate this conclusion. It is also plausible that differences between Africans and Europeans in risk factors such as smoking and sunlight may partly explain the lower prevalence of AMD in people of African descent, but at present, there is insufficient evidence to address this point.
There is a notably high prevalence of late AMD among the Icelandics and the Greenland Inuit. Only one prevalence study was available from each of these two fairly environmentally similar but racially different regions, with sample sizes of 922 and 660 for Iceland and Greenland, respectively. The prevalence estimates in these studies may be imprecise due to small sample sizes, but the response rates for both studies were high (76% and 75%), and so the estimates are unlikely to be biased. The allele frequency for the Icelandic study was 0.38, which is comparable to those of European populations. There may be environmental and lifestyle factors that contribute to such a high prevalence of late AMD in these two populations.
Differences in observed prevalences within a given cluster may reflect the paucity of people in the very elderly age group in some studies, perhaps due to response bias. (Older people may be less likely to participate for reasons associated with AMD.) Differences between clusters may also have arisen because, in some ethnicities, the proportion of the older age group is lower in the population. A low number of people in the oldest age groups, where AMD rates are the highest, can lead to imprecision in the estimation of the prevalence (i.e., sampling error). More detailed data, such as the mean ages within the age categories, would have enabled us to investigate potential age bias between ethnicities.
We could not include environmental risk factors that are known to be associated with AMD, such as tobacco smoking,16,17 because most papers reporting the association of these factors with AMD do not mention the prevalence of the risk factors for the population controls. It is unlikely, however, that even a strong risk factor such as smoking can explain much of the variation in prevalence of AMD. For example, most studies document a lower prevalence of smoking in women, but there is little, if any, variation in prevalence between men and women. In addition, there is no reason to suspect that allele frequency would be related to such environmental factors. We note that the Y402H SNP has been found to be in linkage disequilibrium with other loci on the CFH gene,18,19 suggesting that there is a haplotype effect. We limited our study to this particular SNP because it is the most widely reported genetic variant in relation to AMD and appears to have very strong effects. We note that there are other genes that increase the risk of development of AMD and that such genes may differ across ethnic groups.
In conclusion, we found evidence at the population level to support a positive association with the Y204H risk allele and the prevalence of AMD. We highlight the anomalous result of the high-risk allele frequency and the low prevalence of late AMD in African Americans. There are none or very few articles on populations from the African continent or indeed from other regions, such as the Middle East, parts of Asia, and South America. In some of these areas, only the proportion of blindness due to AMD in a population has been reported, rather than prevalence of late AMD among the elderly. This deficiency highlights the need for better reporting of findings from these populations obtained with standard diagnostic techniques to quantify the effects of the Y402H SNP (and other risk genetic variants) on AMD prevalence across ethnicities and regions.
Footnotes
Supported by British Heart Foundation (Schillingford) Clinical Training Fellowship FS/07/011 (RS) and by a Wellcome Trust Senior Clinical Fellowship (LS).
Disclosure: B.A.S. Nonyane, None; D. Nitsch, None; J.C. Whittaker, None; R. Sofat, None; L. Smeeth, None; U. Chakravarthy, None; A.E. Fletcher, None
References
- 1.Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol 2007;14(4):184–187 [DOI] [PubMed] [Google Scholar]
- 2.Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol 2006;51(4):316–363 [DOI] [PubMed] [Google Scholar]
- 3.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308(5720):385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnaiah S, Das T, Nirmalan PK, et al. Risk factors for age-related macular degeneration: findings from the Andhra Pradesh eye disease study in South India. Invest Ophthalmol Vis Sci 2005;46(12):4442–4449 [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki R, Wang JJ, Ji GJ, et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology 2008;115(8): 1376–1381, 1381, e1–2 [DOI] [PubMed] [Google Scholar]
- 6.Schachat AP, Hyman L, Leske MC, Connell AM, Wu SY. Features of age-related macular degeneration in a black population. The Barbados Eye Study Group. Arch Ophthalmol 1995;113(6):728–735 [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki R, Wang JJ, Aung T, et al. Prevalence of age-related macular degeneration in a Malay population: The Singapore Malay Eye Study. Ophthalmology 2008;115(10):1735–1741 [DOI] [PubMed] [Google Scholar]
- 8.Grassi MA, Fingert JH, Scheetz TE, et al. Ethnic variation in AMD-associated complement factor H polymorphism p.Tyr402His. Hum Mutat 2006;27(9):921–925 [DOI] [PubMed] [Google Scholar]
- 9.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39(5):367–374 [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991;98(7):1128–1134 [DOI] [PubMed] [Google Scholar]
- 11.Beckmann JS, Estivill X, Antonarakis SE. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat Rev Genet 2007;8(8):639–646 [DOI] [PubMed] [Google Scholar]
- 12.Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet 2006;15(18):2784–2790 [DOI] [PubMed] [Google Scholar]
- 13.Ziskind A, Bardien S, van der Merwe L, Webster AR. The frequency of the H402 allele of CFH and its involvement with age-related maculopathy in an aged Black African Xhosa population. Ophthalmic Genet 2008;29(3):117–119 [DOI] [PubMed] [Google Scholar]
- 14.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 2006;38(10):1173–1177 [DOI] [PubMed] [Google Scholar]
- 15.Hageman GS, Hancox LS, Taiber AJ, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med 2006;38(8):592–604 [PMC free article] [PubMed] [Google Scholar]
- 16.Seddon JM, George S, Rosner B, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered 2006;61(3):157–165 [DOI] [PubMed] [Google Scholar]
- 17.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001;108(4):697–704 [DOI] [PubMed] [Google Scholar]
- 18.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 2005;102(20):7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur I, Hussain A, Hussain N, et al. Analysis of CFH, TLR4, and APOE polymorphism in India suggests the Tyr402His variant of CFH to be a global marker for age-related macular degeneration. Invest Ophthalmol Vis Sci 2006;47(9):3729–3735 [DOI] [PubMed] [Google Scholar]
- 20.Andersen MV, Rosenberg T, la Cour M, et al. Prevalence of age-related maculopathy and age-related macular degeneration among the inuit in Greenland. The Greenland Inuit Eye Study. Ophthalmology 2008;115(4): 700–707 e1 [DOI] [PubMed] [Google Scholar]
- 21.Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol 2006;124(4):529–535 [DOI] [PubMed] [Google Scholar]
- 22.Chen SJ, Cheng CY, Peng KL, et al. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2008;49(7):3126–3133 [DOI] [PubMed] [Google Scholar]
- 23.Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999;106(6):1049–1055 [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Murthy GV, Morrison N, et al. Prevalence of early and late age-related macular degeneration in a rural population in northern India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci 2007;48(3):1007–1011 [DOI] [PubMed] [Google Scholar]
- 25.Jonasson F, Arnarsson A, Sasaki H, Peto T, Sasaki K, Bird AC. The prevalence of age-related maculopathy in iceland: Reykjavik eye study. Arch Ophthalmol 2003;121(3):379–385 [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99(6):933–943 [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE, Marino EK, et al. Early age-related maculopathy in the cardiovascular health study. Ophthalmology 2003;110(1):25–33 [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006;113(3):373–380 [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology 1999;106(6):1056–1065 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Xu L, Jonas JB, Yang H, Ma Y, Li J. Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol 2006;142(5):788–793 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995;102(10):1450–1460 [DOI] [PubMed] [Google Scholar]
- 32.Munoz B, Klein R, Rodriguez J, Snyder R, West SK. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch Ophthalmol 2005;123(11):1575–1580 [DOI] [PubMed] [Google Scholar]
- 33.Oguido AP, Casella AM, Matsuo T, Ramos Filho EH, Berbel R, Silva RM. Prevalence of age-related macular degeneration in Japanese immigrants and their descendants living in Londrina (PR)-Brazil (in Protuguese). Arq Bras Oftalmol 2008;71(3):375–380 [DOI] [PubMed] [Google Scholar]
- 34.Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T. Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol 2001;85(10):1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanNewkirk MR, Nanjan MB, Wang JJ, Mitchell P, Taylor HR, McCarty CA. The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology 2000;107(8):1593–1600 [DOI] [PubMed] [Google Scholar]
- 36.Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology 2004;111(7):1288–1297 [DOI] [PubMed] [Google Scholar]
- 37.Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 1995;102(2):205–210 [DOI] [PubMed] [Google Scholar]
- 38.Baird PN, Islam FM, Richardson AJ, Cain M, Hunt N, Guymer R. Analysis of the Y402H variant of the complement factor H gene in age-related macular degeneration. Invest Ophthalmol Vis Sci 2006;47(10):4194–4198 [DOI] [PubMed] [Google Scholar]
- 39.Brantley MA, Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology 2007;114(12):2168–2173 [DOI] [PubMed] [Google Scholar]
- 40.Chen LJ, Liu DT, Tam PO, et al. Association of complement factor H polymorphisms with exudative age-related macular degeneration. Mol Vis 2006;12:1536–1542 [PubMed] [Google Scholar]
- 41.Chowers I, Cohen Y, Goldenberg-Cohen N, et al. Association of complement factor H Y402H polymorphism with phenotype of neovascular age related macular degeneration in Israel. Mol Vis 2008;14:1829–1834 [PMC free article] [PubMed] [Google Scholar]
- 42.Conley YP, Thalamuthu A, Jakobsdottir J, et al. Candidate gene analysis suggests a role for fatty acid biosynthesis and regulation of the complement system in the etiology of age-related maculopathy. Hum Mol Genet 2005;14(14):1991–2002 [DOI] [PubMed] [Google Scholar]
- 43.de Jong FJ, Ikram MK, Despriet DD, et al. Complement factor h polymorphism, inflammatory mediators, and retinal vessel diameters: The Rotterdam Study. Invest Ophthalmol Vis Sci 2007;48(7):3014–3018 [DOI] [PubMed] [Google Scholar]
- 44.Despriet DD, Klaver CC, Witteman JC, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006;296(3):301–309 [DOI] [PubMed] [Google Scholar]
- 45.Droz I, Mantel I, Ambresin A, Faouzi M, Schorderet DF, Munier FL. Genotype-phenotype correlation of age-related macular degeneration: influence of complement factor H polymorphism. Br J Ophthalmol 2008;92(4):513–517 [DOI] [PubMed] [Google Scholar]
- 46.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308(5720):421–424 [DOI] [PubMed] [Google Scholar]
- 47.Fisher SA, Rivera A, Fritsche LG, Babadjanova G, Petrov S, Weber BH. Assessment of the contribution of CFH and chromosome 10q26 AMD susceptibility loci in a Russian population isolate. Br J Ophthalmol 2007;91(5):576–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuse N, Miyazawa A, Mengkegale M, et al. Polymorphisms in Complement Factor H and Hemicentin-1 genes in a Japanese population with dry-type age-related macular degeneration. Am J Ophthalmol 2006;142(6):1074–1076 [DOI] [PubMed] [Google Scholar]
- 49.Gotoh N, Yamada R, Hiratani H, et al. No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet 2006;120(1):139–143 [DOI] [PubMed] [Google Scholar]
- 50.Gotting C, Hendig D, Zarbock R, Szliska C, Kleesiek K. Complement factor H variant p.Y402H in pseudoxanthoma elasticum patients. Genet Test 2008;12(3):431–436 [DOI] [PubMed] [Google Scholar]
- 51.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308(5720):419–421 [DOI] [PubMed] [Google Scholar]
- 52.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet 2005;77(3):389–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim NR, Kang JH, Kwon OW, Lee SJ, Oh JH, Chin HS. Association between complement factor H gene polymorphisms and neovascular age-related macular degeneration in Koreans. Invest Ophthalmol Vis Sci 2008;49(5):2071–2076 [DOI] [PubMed] [Google Scholar]
- 54.Lau LI, Chen SJ, Cheng CY, et al. Association of the Y402H polymorphism in complement factor H gene and neovascular age-related macular degeneration in Chinese patients. Invest Ophthalmol Vis Sci 2006;47(8):3242–3246 [DOI] [PubMed] [Google Scholar]
- 55.Lee KY, Vithana EN, Mathur R, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2008;49(6):2613–2619 [DOI] [PubMed] [Google Scholar]
- 56.Le Fur I, Laumet G, Richard F, et al. Association study of the CFH Y402H polymorphism with Alzheimer's disease. Neurobiol Aging 2009;31(1):165–166 [DOI] [PubMed] [Google Scholar]
- 57.Lin JM, Wan L, Tsai YY, et al. HTRA1 polymorphism in dry and wet age-related macular degeneration. Retina 2008;28(2):309–313 [DOI] [PubMed] [Google Scholar]
- 58.Magnusson KP, Duan S, Sigurdsson H, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med 2006;3(1):e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet 2006;38(9):1055–1059 [DOI] [PubMed] [Google Scholar]
- 60.Mooijaart SP, Koeijvoets KM, Sijbrands EJ, Daha MR, Westendorp RG. Complement Factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Exp Gerontol 2007;42(11):1116–1122 [DOI] [PubMed] [Google Scholar]
- 61.Mori K, Gehlbach PL, Kabasawa S, et al. Coding and noncoding variants in the CFH gene and cigarette smoking influence the risk of age-related macular degeneration in a Japanese population. Invest Ophthalmol Vis Sci 2007;48(11):5315–5319 [DOI] [PubMed] [Google Scholar]
- 62.Narayanan R, Butani V, Boyer DS, et al. Complement factor H polymorphism in age-related macular degeneration. Ophthalmology 2007;114(7):1327–1331 [DOI] [PubMed] [Google Scholar]
- 63.Ng TK, Chen LJ, Liu DT, et al. Multiple gene polymorphisms in the complement factor h gene are associated with exudative age-related macular degeneration in chinese. Invest Ophthalmol Vis Sci 2008;49(8):3312–3317 [DOI] [PubMed] [Google Scholar]
- 64.Okamoto H, Umeda S, Obazawa M, et al. Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol Vis 2006;12:156–158 [PubMed] [Google Scholar]
- 65.Pai JK, Manson JE, Rexrode KM, Albert CM, Hunter DJ, Rimm EB. Complement factor H (Y402H) polymorphism and risk of coronary heart disease in US men and women. Eur Heart J 2007;28(11):1297–1303 [DOI] [PubMed] [Google Scholar]
- 66.Pulido JS, McConnell JP, Lennon RJ, et al. Relationship between age-related macular degeneration-associated variants of complement factor H and LOC387715 with coronary artery disease. Mayo Clin Proc 2007;82(3):301–307 [DOI] [PubMed] [Google Scholar]
- 67.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 2005;14(21):3227–3236 [DOI] [PubMed] [Google Scholar]
- 68.Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch Ophthalmol 2007;125(1):55–62 [DOI] [PubMed] [Google Scholar]
- 69.Seitsonen S, Lemmela S, Holopainen J, et al. Analysis of variants in the complement factor H, the elongation of very long chain fatty acids-like 4 and the hemicentin 1 genes of age-related macular degeneration in the Finnish population. Mol Vis 2006;12:796–801 [PubMed] [Google Scholar]
- 70.Sepp T, Khan JC, Thurlby DA, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci 2006;47(2):536–540 [DOI] [PubMed] [Google Scholar]
- 71.Simonelli F, Frisso G, Testa F, et al. Polymorphism p. 402Y>H in the complement factor H protein is a risk factor for age related macular degeneration in an Italian population. Br J Ophthalmol 2006;90(9):1142–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Souied EH, Leveziel N, Richard F, et al. Y402H complement factor H polymorphism associated with exudative age-related macular degeneration in the French population. Mol Vis 2005;11:1135–1140 [PubMed] [Google Scholar]
- 73.Stark K, Neureuther K, Sedlacek K, et al. The common Y402H variant in complement factor H gene is not associated with susceptibility to myocardial infarction and its related risk factors. Clin Sci (Lond) 2007;113(4):213–218 [DOI] [PubMed] [Google Scholar]
- 74.Tanimoto S, Tamura H, Ue T, et al. A polymorphism of LOC387715 gene is associated with age-related macular degeneration in the Japanese population. Neurosci Lett 2007;414(1):71–74 [DOI] [PubMed] [Google Scholar]
- 75.Tedeschi-Blok N, Buckley J, Varma R, Triche TJ, Hinton DR. Population-based study of early age-related macular degeneration: role of the complement factor H Y402H polymorphism in bilateral but not unilateral disease. Ophthalmology 2007;114(1):99–103 [DOI] [PubMed] [Google Scholar]
- 76.Uka J, Tamura H, Kobayashi T, et al. No association of complement factor H gene polymorphism and age-related macular degeneration in the Japanese population. Retina 2006;26(9):985–987 [DOI] [PubMed] [Google Scholar]
- 77.Volcik KA, Ballantyne CM, Braun MC, Coresh J, Mosley TH, Boerwinkle E. Association of the complement factor H Y402H polymorphism with cardiovascular disease is dependent upon hypertension status: The ARIC study. Am J Hypertens 2008;21(5):533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wegscheider BJ, Weger M, Renner W, et al. Association of complement factor H Y402H gene polymorphism with different subtypes of exudative age-related macular degeneration. Ophthalmology 2007;114(4):738–742 [DOI] [PubMed] [Google Scholar]
- 79.Xing C, Sivakumaran TA, Wang JJ, et al. Complement factor H polymorphisms, renal phenotypes and age-related macular degeneration: the Blue Mountains Eye Study. Genes Immun 2008;9(3):231–239 [DOI] [PubMed] [Google Scholar]
- 80.Xu Y, Guan N, Xu J, et al. Association of CFH, LOC387715, and HTRA1 polymorphisms with exudative age-related macular degeneration in a northern Chinese population. Mol Vis 2008;14:1373–1381 [PMC free article] [PubMed] [Google Scholar]
- 81.Zareparsi S, Branham KE, Li M, et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet 2005;77(1):149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zee RY, Diehl KA, Ridker PM. Complement factor H Y402H gene polymorphism, C-reactive protein, and risk of incident myocardial infarction, ischaemic stroke, and venous thromboembolism: a nested case-control study. Atherosclerosis 2006;187(2):332–335 [DOI] [PubMed] [Google Scholar]
- 83.Zetterberg M, Landgren S, Andersson ME, et al. Association of complement factor H Y402H gene polymorphism with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet 2008;147B(6):720–726 [DOI] [PubMed] [Google Scholar]