Patients with hypertension are often administered β-adrenergic receptor antagonists for reduction of blood pressure. The authors found that systemic delivery of a β-adrenergic receptor antagonist reduced the b-wave of the electroretinogram and increased IGF-1 receptor signaling and VEGF.
Abstract
Purpose.
To determine whether systemic application of propranolol, a nonselective β-adrenergic receptor antagonist, with an osmotic pump will decrease the b-wave amplitude of the electroretinogram (ERG) and increase insulin-like growth factor (IGF)-1 receptor signaling.
Methods.
Young rats at 8 weeks of age were treated with saline, phentolamine, a nonselective α-adrenergic receptor antagonist, or propranolol, a nonselective β-adrenergic receptor antagonist, delivered by osmotic pumps for 21 days. On the 21st day, all rats underwent electroretinographic analyses followed by collection of the retinas for protein assessment using Western blot analysis for IGF binding protein 3 (IGFBP3), IGF-1 receptor (IGF-1R), Akt, extracellular signal-related kinases 1 and 2 (ERK1/2), and vascular endothelial cell growth factor (VEGF).
Results.
Data indicate that 21 days of propranolol significantly decreased the b-wave amplitude of the ERG. The decrease in the b-wave amplitude occurred concurrently with a decrease in IGFBP3 levels and an increase in tyrosine phosphorylation of IGF-1 receptor on 1135/1136. This phosphorylation of IGF-1 receptor led to increased phosphorylation of Akt and ERK1/2. VEGF protein levels were also increased.
Conclusions.
Overall, β-adrenergic receptor antagonism produced a dysfunctional ERG, which occurred with an increase in IGF-1R phosphorylation and activation of VEGF. Systemic application of β-adrenergic receptor antagonists may have detrimental effects on the retina.
As the overall age of the population increases, so does the need for a better understanding of the aging process in each tissue. We have previously reported that aging is associated with loss of dopamine β-hydroxylase levels in the retina.1 Recently, we have shown that increasing age in rats also produced increased tyrosine phosphorylation of insulin-like growth factor-1 receptor (IGF-1R), a dysfunctional electroretinogram (ERG), and increased apoptosis in retinal lysates.2 Both IGF-1R and vascular endothelial cell growth factor (VEGF) are increased in the aging rat retina, suggesting that normal aging may promote the vascular growth of normally quiescent tissue.3 Occurring concurrently with the increase in VEGF and IGF-1R is an increase in β-1-adrenergic receptor levels, likely because of denervation sensitivity in response to reduced norepinephrine.1 It is unclear whether the changes in IGF-1R and VEGF are related to this loss of norepinephrine in aging.
The role of IGF-1R in the retina is still unclear. IGF-1 receptor (IGF-1R) mutations allow for increased life spans in multiple organisms,4,5 which may occur because these animals are resistant to cancer.6,7 IGF-1R may promote cancer and other age-related diseases through its ability to activate both the mitogen activated kinase pathway and the phosphatidylinositol-3-kinase cascade.8,9 In the retina, IGF-1R signaling has been shown to be involved with neovascularization in retinopathy of prematurity,10 diabetic retinopathy,11,12 normal development,13 and normal aging.2 However, the regulation of IGF-1R in the retina is unknown.
Insulin-like growth factor binding proteins (IGFBPs) regulate circulating IGF-I bioavailability. All IGFBPs function at some level to regulate the delivery of IGF molecules to local tissues,14 with the predominating IGFBP species determined by cell type and local conditions. Both IGFBP-3 and IGFBP-5 bind the extracellular matrix,14 which provides a physiological “sink” in which to store these IGFBPs. The extracellular matrix is disrupted in normal aging15,16; hence, protein levels of IGFBP-3 and IGFBP-5 may be decreased because of abnormal extracellular matrix. Work in RPE samples of elderly humans has shown that aging can regulate the expression of IGFBP-2 in vitro.17 In other work, all six of the IGFBPs have been localized in the RPE in culture.18 Retinal Müller cells in culture express IGFBP-2, -3, -4, and -6.19 IGFBP-3 is expressed on the surface and in the cytoplasm of human retinal endothelial cells.20 We have previously shown that both retinal Müller cells and endothelial cells have β-adrenergic receptors and, therefore, may regulate IGFBP levels in the retina.21–23
In addition to the IGFBPs, limited work has demonstrated that β-adrenergic receptors may regulate IGF-1 levels and signaling. Evidence from β-2-adrenergic receptor knockout mice demonstrated that loss of β-2-adrenergic receptors in astrocytes increases IGF1R gene expression.24 Whether these findings can be extended to the retina is not known because most work on IGF-1R signaling has investigated the ability of IGF-1R to regulate β-adrenergic receptor signaling.25
Our goal was to determine whether β-adrenergic receptors can regulate IGF-1R levels in the rat retina. Given that aging is associated with a loss of norepinephrine, we hypothesized that the blockade of β-adrenergic receptors in young rats will induce changes in the retina similar to those noted in aging, specifically increased IGF-1R signaling. To test our hypothesis, we placed osmotic pumps containing saline, phentolamine (nonselective α-adrenergic receptor antagonist), or propranolol (nonselective β-adrenergic receptor antagonist) into 8-week-old rats for 21 days of systemic delivery of the drug. The doses and time course for treatment were based on previous work with this technique, which demonstrated that propranolol at 1 mg/d for 21 days could elicit histologic changes in the rat retina and choroid.26 We evaluated IGF-1R signaling and electroretinographic findings of rats in each treatment group to measure whether the blockade of adrenergic receptor signaling in youth could produce changes similar to those that occur during normal aging of the retina.
Methods
Animal Preparation
Studies were conducted on 15 adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) aged 60 days and weighing 180 to 200 g. Rats were anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (27.5 mg/kg; Sano Winthrop, New York, NY) and xylazine hydrochloride (Rompun, 2.5 mg/kg; Miles, Shawnee Mission, KS). Osmotic mini-pumps (Alzet, model no. 2ML4; Alza Corporation, Palo Alto, CA) were implanted subcutaneously in a dorsal interscapular configuration. These pumps deliver solution at a rate of 2.5 μL/h and provide constant infusion for at least 28 days. Pumps were loaded with saline vehicle alone (n = 5) or saline containing the α-adrenoceptor antagonist phentolamine (Sigma, St. Louis, MO; n = 5) or the β-adrenoceptor antagonist propranolol (Sigma, St. Louis, MO; n = 5). Both drugs were delivered at dosages of 1 mg/d. Table 1 provides the systolic and diastolic blood pressures at kill. Blood pressure was measured under anesthesia using a tail cuff (Kent Scientific, Torrington, CT). Although systolic blood pressure was not significantly reduced, this was likely because of the short duration of the treatment and the low doses used. We have previously shown that these drugs administered at these doses are effective for maintaining adrenoceptor blockade.26–28 At 21 days after pump implantation, rats were killed, and their eyes were removed for analyses. All animal procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center and conformed to National Institutes of Health guidelines.
Table 1.
Treatment Groups and Blood Pressure at 21 Days of Treatment
| Treatment Group | n | Systolic (mmHg) | Diastolic (mmHg) |
|---|---|---|---|
| Saline | 5 | 125 ± 11.55 | 99.40 ± 14.72 |
| Propranolol | 4 | 119 ± 18.27 | 83.75 ± 9.71 |
| Phentolamine | 5 | 121 ± 13.08 | 76.00 ± 9.14* |
Data represent mean ± SD.
P < 0.05 versus saline.
Electroretinogram Analyses
Electroretinographic analyses were performed before kill to evaluate functional changes in vision after β-adrenergic receptor or α-adrenergic receptor inhibition. For the electroretinographic analyses, rats were dark adapted overnight. The following morning, rats were anesthetized with intraperitoneal injection of a ketamine (0.6 mL/kg) and xylazine (0.375 mL/kg) cocktail. The pupil of each eye was fully dilated using a 1% tropicamide solution (Alcon, Fort Worth, TX). To protect the eye and assist in maintaining a good electrical connection, 1 drop of methylcellulose solution was added to each eye (Celluvisc; Allergan, Irvine, CA). Body temperature was maintained at 37°C with a water-based heating pad. Electroretinographic responses were recorded from both eyes simultaneously using platinum wire corneal electrodes, a forehead reference electrode, and a ground electrode in the tail. Electroretinographic stimuli were delivered through the Diagnosys LLC (Lowell, MA) system. All animals were killed after the electroretinographic recording sessions.
Electroretinographic responses were recorded in response to brief (4 ms) white LED and then from the xenon arc lamp delivered at 2.1-second intervals for dim stimuli and 35-second frames for brighter stimuli. The range of stimulus intensities extended from −4.0 to 3.0 log (cd · s/m2) for analysis of the b-wave amplitudes. Electroretinographic waveforms were recorded with bandwidths of 0.3 to 500 Hz and were sampled at 2 kHz by a digital acquisition system (Diagnosys). Data were analyzed (Espion System; Diagnosys). Plots of intensity-response functions for the b-waves were fit to a hyperbolic (Naka-Rushton) function of the form R(I)/Rmax = Ik/Ik + Kn, where R is the response amplitude at flash intensity I, Rmax is the asymptotic amplitude of the b-wave (in microvolts), K is the intensity at which b-wave amplitude is half its asymptotic value (in cd · s/m2), and n determines the slope of the function at I = K.
For assessment of oscillatory potential, stimuli were administered at 3 log (cd · s/m2). Data analysis for the oscillatory potentials was analyzed using the Fast Fourier Transform Algorithm for a time-based signal (MatLab software; The MathWorks, Natick, MA) with a digital band-pass filter set for 60 to 300 Hz. Peak wavelets of the four wavelets were measured from trough to peak.29,30
Protein Measurements
Western blot analyses for IGF-1R and its downstream signaling intermediaries were performed on retinal lysates, as previously published.31 Primary antibodies used were phosphorylated IGF-1R (Tyr 1135/1136, 1:500; Cell Signaling Technologies, Beverly, MA), total IGF-1R (1:500; Cell Signaling Technologies), total Akt (1:500; Cell Signaling Technologies), phosphorylated Akt (Ser473, 1:500; Cell Signaling Technologies), phosphorylated ERK1/2 (1:500; Cell Signaling Technologies), total ERK1/2 (1:500; Cell Signaling Technologies), VEGF (1:250; Millipore, Billerica, MA), and IGFBP3 (1:500; Millipore). For analyses of the data, mean densitometry values were obtained using commercial software (Kodak 5.0; Eastman Kodak, Carestream Health, Woodbridge, CT) and presented in arbitrary units (AU). For phosphorylated proteins, the ratio of phosphorylated protein was compared to levels of total protein as an assessment of protein activity. Total levels of each protein are presented as blots and as their phosphorylated form, as appropriate.
Statistical Analysis
Statistical analysis was conducted with Kruskal-Wallis nonparametric analysis and then by a Mann-Whitney U test to compare treatments against those with saline using statistical analysis software (Prism; GraphPad, San Diego, CA). P < 0.05 was taken as statistically significant.
Results
Treatment with Propranolol for 21 Days Significantly Reduced b-Wave Amplitudes
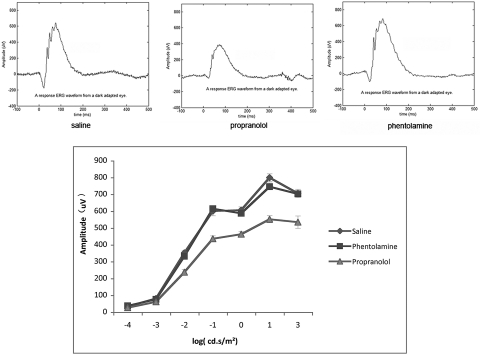
Eight-week-old rats were treated with saline, phentolamine, or propranolol for 21 days using osmotic pumps for systemic delivery. At the end of the 21 days, electroretinographic analyses were performed. Systemic delivery of propranolol to a young rat produced a significant loss in b-wave amplitude (Fig. 1). The b-wave amplitude of the propranolol-treated rat was similar to that noted in a 32-month-old rat with no treatment.2
Figure 1.
Top: electroretinographic recordings from a rat treated with saline (left), propranolol (middle), and phentolamine (right) for 21 days. Bottom: line graph of the mean of five animals from each treatment group for 21 days. Error bars represent mean ± SD for the b-wave amplitude of each treatment group at the 21-day point.
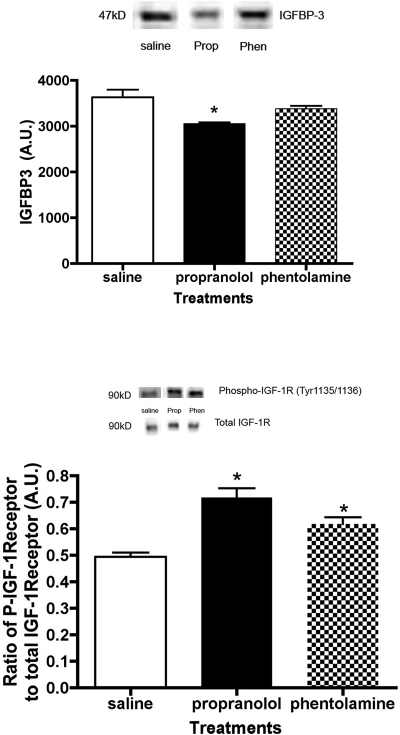
Propranolol Treatment Significantly Decreases Levels of IGFBP3 in the Rat Retina, Producing Increased Tyrosine Phosphorylation of the IGF-1R
Occurring concurrently with the decreased b-wave amplitude, a significant decrease in IGFBP3 was noted in the retina of propranolol-treated rats (Fig. 2, top). IGFBPs regulate the bioavailability of IGF-1 to bind its receptor, IGF-1R. Increased expression of IGF-1R has been associated with ocular disease. As expected, the decrease in IGFBP3 was associated with a significant increase in IGF-1R tyrosine phosphorylation in the propranolol-treated rat retina compared with saline treatment (Fig. 2, bottom). Phentolamine-treatment also increased IGF-1R phosphorylation levels when compared with saline. No significant changes in total IGF-1R protein levels were noted between the treatment groups (data not shown).
Figure 2.
Top: representative blot and bar graph of IGFBP3 protein levels in the retina of saline-, propranolol-, and phentolamine-treated rats at 21 days of treatment. *P < 0.05 versus saline (n = 4). Bottom: representative blots and bar graph of the ratio of phosphorylated IGF-1R to total IGF-1R on tyrosine 1135/1136 in the retinas of saline-, propranolol-, and phentolamine-treated rats after 21 days of treatment. *P < 0.05 versus saline (n = 4).
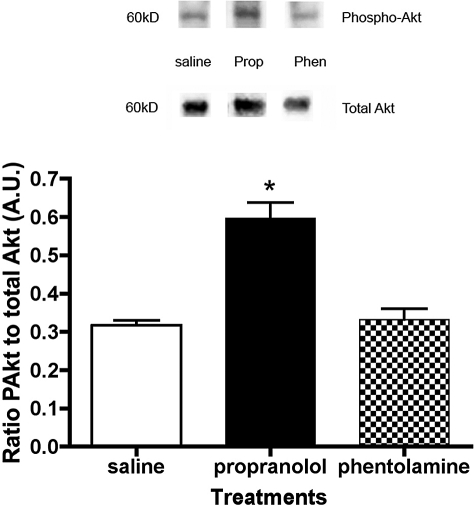
Akt Activation Increased in the Retinas of Propranolol-Treated Rats
IGF-1R phosphorylation often produces an increase in serine phosphorylation of Akt (Ser473).32 As occurs in other tissues, we found that Akt phosphorylation was significantly increased in the retinas of propranolol-treated rats (Fig. 3). The stimulus of increased phosphorylation of IGF-1R in phentolamine-treated rats was not transduced to Akt in this model.
Figure 3.
Representative blot and bar graph of phosphorylated Akt (Ser473) to total Akt in the retinas of treated rats. *P < 0.05 versus saline (n = 4).
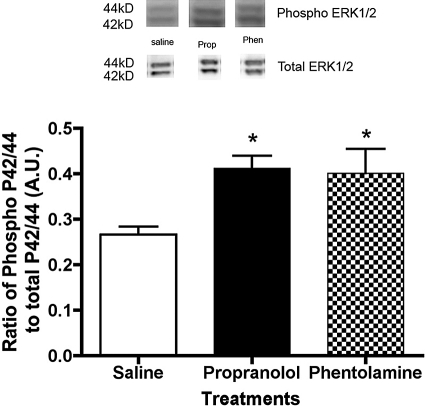
ERK1/2 Phosphorylation Is Increased in the Retinas of Propranolol- and Phentolamine-Treated Rats
In addition to Akt, IGF-1R is reported to increase tyrosine phosphorylation of ERK1/2, a key member of the mitogen-activated protein kinase pathway. We did find that both propranolol and phentolamine treatment of rats led to a significant increase in ERK1/2 phosphorylation in the retina (Fig. 4).
Figure 4.
Blots and bar graph of phosphorylated ERK1/2 to total ERK1/2 in the retinas of saline-, propranolol-, and phentolamine-treated rats. ERK1/2 was increased after both α-adrenergic receptor and β-adrenergic receptor blockade. *P < 0.05 versus saline (n = 4).
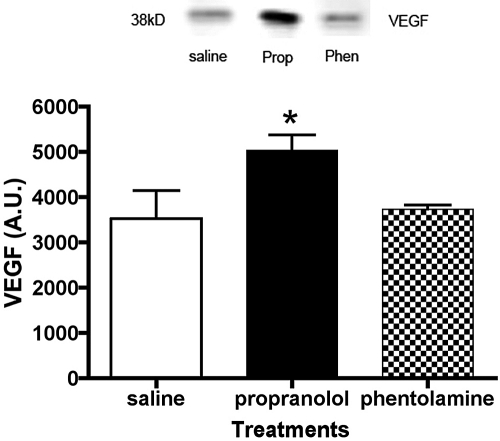
Propranolol Treatment Increases VEGF Protein Levels in the Retina
A key protein that can be activated by IGF-1R signaling, Akt, and ERK1/2 phosphorylation is VEGF. Increased VEGF levels are associated with retinal neovascularization and macular edema.33–35 We found that systemic administration of propranolol for 21 days significantly increased VEGF protein levels (Fig. 5; P < 0.05 vs. saline). In addition to the ability of ERK1/2 to activate VEGF, increased VEGF has also been reported to increase ERK1/2 phosphorylation.36
Figure 5.
Protein levels of VEGF in saline-, propranolol-, and phentolamine-treated rat retinas after 21 days of treatment. *P < 0.05 versus saline (n = 4).
Discussion
Aging of the eye is often associated with age-related macular degeneration. Although this disease can result in debilitating blindness, increasing our understanding of the normal aging process of the eye may aid in treatment development. The newest form of treatment for age-related macular degeneration is based on the knowledge that retinal neovascularization occurs because of the activation of VEGF, and blockade of VEGF can prevent this blood vessel growth from the choroid into the macula of the retina.37,38 Although these treatments can slow neovascularization, optimal conditions would prevent the activation of VEGF. One factor that can increase VEGF levels is activation of the IGF-1R signaling cascade.10,39 Therefore, the goal of this study was to determine whether adrenergic receptors regulate IGF-1R signaling to reduce VEGF levels.
IGF-1R signaling is regulated by the bioavailability of IGF-1 in the tissue of interest, which is controlled by the expression of IGFBPs. The IGFBPs are a family of six secreted proteins and a related protein (IGFBP-rP1).40 IGFBP gene expression, synthesis, and posttranslational modifications are regulated by IGF-1R signaling in some cells.41 IGFBP-3 and -5 are the major IGFBPs that regulate circulating IGF-1 levels, but all IGFBPs function at some level to regulate the delivery of IGF-1 molecules to local tissues.14 In our data, we found that IGFBP3 levels were significantly decreased in propranolol-treated rats, which occurred concurrently with a significant increase in IGF-1R phosphorylation. Our data agree with those of other studies showing that IGF-1 and IGFBPs are expressed in retinal cells and tissues in both physiological and pathologic states.2,10,11
The regulation of IGFBP expression and IGF-1R signaling by β-adrenergic receptors has not been investigated in ocular tissues. Previous work42 has shown that isoproterenol, a nonselective β-adrenergic receptor agonist, can increase IGF-1R activity in cardiac myocytes.42 Using IGF-1 receptor–deficient mice, Lembo et al.43 found that cross-talk between β-adrenergic receptors and IGF-1R signaling regulates ventricular contractility.43 Others44 have shown that the regulation of IGF-1 by β-adrenergic receptors may occur through the modulation of IGFBPs. Work using β2-adrenergic receptor knockout mice has shown that loss of β2-adrenergic receptors increased astrocyte proliferation through the activation of IGF-1R.24 Our data are in contrast to those reported in cardiac tissue, but they match well the data from astrocytes. Because the retina is derived from central nervous system tissue, it is likely that astrocytes and retinal neurons will have similar features. The differences in β-adrenergic receptor regulation of IGF-1R are likely related to the tissue-specific levels of IGFBPs in the heart versus the retina and brain. We have previously shown that retinal Müller cells have β-adrenergic receptors21; hence, it is possible that β-adrenergic receptors may regulate IGF-1R in these cells. In this manner, activation or inhibition of β-adrenergic receptors can control cell proliferation versus apoptosis through tissue-specific levels of IGFBPs.
Once IGF-1R is activated, it will promote the phosphorylation of phosphatidylinositol-3-kinase and the activation of Akt, a known antiapoptotic factor. IGF-1R can also activate ERK1/2.32 We found that β-adrenergic receptor antagonism activated IGF-1R signaling in the retina, leading to the phosphorylation of Akt and ERK1/2. Others45 have reported that Akt and ERK1/2 can work cooperatively to activate VEGF. In retinal neovascularization, IGF-1 has been shown to activate VEGF.46 Although the mechanisms for this have only more recently become clear, it is likely that IGF-1R signaling promotes VEGF activation through both Akt and ERK1/2 phosphorylation.
Work on β-adrenergic receptor agents in the retina has previously focused on the antiglaucoma aspects because many antiglaucoma agents are β-adrenergic receptor antagonists. One of the mechanisms of action for betaxolol, one of the most potent β-adrenergic receptor antagonists for glaucoma, is its ability to alter calcium levels.47,48 In a recent review, Osborne49 reported that the beneficial effects of β-adrenergic receptor blockers in glaucoma therapy do not appear to involve their actions on β-adrenergic receptors. These findings suggest that the role of β-adrenergic receptor antagonists as neuroprotective drugs for glaucoma therapy may be related to non–β-adrenergic receptor actions of these agents. In diabetic retinopathy, two studies have shown that β-adrenergic receptor antagonists do not reduce diabetic retinopathy, in spite of reduced systemic blood pressure.50,51 Therefore, previous work on β-adrenergic receptor antagonists in the retina suggests that they do not prevent diabetic retinopathy in rodents. They likely prevent glaucoma damage through the regulation of calcium or sodium channel responses.49,51 Nonetheless, the downstream effects of β-adrenergic receptor blockade mediated by receptor signaling in the retina are unknown. No work has previously shown the role of β-adrenergic receptor antagonists in the regulation of IGF-1R in the retina. We find that blockade of β-adrenergic receptor signaling in the normal retina is detrimental, as evidenced by the reduced b-wave amplitude of the ERG. This agrees with previous work in our laboratory that has shown positive effects on the retina after application of β-adrenergic receptor agonists in reducing inflammatory marker levels.21,22,52
The key finding of this study is that β-adrenergic receptors can regulate IGFBP3 levels to control the phosphorylation of IGF-1R and downstream signaling. These occur concurrently with a significant loss of b-wave amplitude of the ERG, suggesting a loss of retinal electrical activity. The increase in IGF-1R leads to the increased activity of Akt and ERK1/2. Ultimately, VEGF is activated, which can promote neovascularization. In this manner, systemic administration of a β-adrenergic receptor antagonist may have detrimental effects on the retina through increased IGF-1R signaling.
Footnotes
Supported by Juvenile Diabetes Research Foundation Career Development Award (JJS), a Translational Award from the Juvenile Diabetes Research Foundation (JJS), the William and Mary Greve Special Scholar Award from Research to Prevent Blindness, an unrestricted grant from Research to Prevent Blindness, and National Eye Institute Vision Core Grant PHS 3P30 EY013080.
Disclosure: Y. Jiang; None; J.J. Steinle, None
References
- 1.Smith CP, Sharma S, Steinle JJ. Age-related changes in sympathetic neurotransmission in rat retina and choroid. Exp Eye Res 2007;84:75–81 [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Walker RJ, Steinle JJ. Age-associated increase in cleaved caspase 3 despite phosphorylation of IGF-1 receptor in the rat retina. J Gerontol A Biol Sci Med Sci 2009;64:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith CP, Steinle JJ. Changes in growth factor expression in normal aging of the rat retina. Exp Eye Res 2007;85:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holzenberger M. The GH/IGF-I axis and longevity. Eur J Endocrinol 2004;151(suppl 1):S23–S27 [DOI] [PubMed] [Google Scholar]
- 5.Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell 2008;7:285–290 [DOI] [PubMed] [Google Scholar]
- 6.Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology 2002;143:4139–4142 [DOI] [PubMed] [Google Scholar]
- 7.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell 2005;120:449–460 [DOI] [PubMed] [Google Scholar]
- 8.Allen TR, Krueger KD, Hunter WJ, 3rd, Agrawal DK. Evidence that insulin-like growth factor-1 requires protein kinase C-epsilon, PI3-kinase and mitogen-activated protein kinase pathways to protect human vascular smooth muscle cells from apoptosis. Immunol Cell Biol 2005;83:651–667 [DOI] [PubMed] [Google Scholar]
- 9.O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 2008;252:91–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate 2005;88:237–244 [DOI] [PubMed] [Google Scholar]
- 11.Seigel GM, Lupien SB, Campbell LM, Ishii DN. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J Diabetes Complications 2006;20:196–204 [DOI] [PubMed] [Google Scholar]
- 12.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2006;2:71–98 [DOI] [PubMed] [Google Scholar]
- 13.Koriyama Y, Homma K, Sugitani K, et al. Upregulation of IGF-I in the goldfish retinal ganglion cells during the early stage of optic nerve regeneration. Neurochem Int 2007;50:749–756 [DOI] [PubMed] [Google Scholar]
- 14.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 1999;20:761–787 [DOI] [PubMed] [Google Scholar]
- 15.Ugarte M, Hussain AA, Marshall J. An experimental study of the elastic properties of the human Bruch's membrane-choroid complex: relevance to ageing. Br J Ophthalmol 2006;90:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verzar F. The ageing of connective tissue. Gerontologia 1957;1:363–378 [PubMed] [Google Scholar]
- 17.Miyamura N, Mishima K, Honda S, et al. Age and topographic variation of insulin-like growth factor-binding protein 2 in the human RPE. Invest Ophthalmol Vis Sci 2001;42:1626–1630 [PubMed] [Google Scholar]
- 18.Yang H, Chaum E. A reassessment of insulin-like growth factor binding protein gene expression in the human retinal pigment epithelium. J Cell Biochem 2003;89:933–943 [DOI] [PubMed] [Google Scholar]
- 19.King JL, Guidry C. Muller cell production of insulin-like growth factor-binding proteins in vitro: modulation with phenotype and growth factor stimulation. Invest Ophthalmol Vis Sci 2004;45:4535–4542 [DOI] [PubMed] [Google Scholar]
- 20.Spoerri PE, Ellis EA, Tarnuzzer RW, Grant MB. Insulin-like growth factor: receptor and binding proteins in human retinal endothelial cell cultures of diabetic and nondiabetic origin. Growth Horm IGF Res 1998;8:125–132 [DOI] [PubMed] [Google Scholar]
- 21.Walker R, Steinle J. Role of beta-adrenergic receptors in inflammatory marker expression in Muller cells. Invest Ophthalmol Vis Sci 2007;48:5276–5281 [DOI] [PubMed] [Google Scholar]
- 22.Steinle JJ, Chin VC, Williams KP, Panjala SR. Beta-adrenergic receptor stimulation modulates iNOS protein levels through p38 and ERK1/2 signaling in human retinal endothelial cells. Exp Eye Res 2008;87:30–34 [DOI] [PubMed] [Google Scholar]
- 23.Steinle JJ, Booz GW, Meininger CJ, Day JN, Granger HJ. Beta 3-adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem 2003;278:20681–20686 [DOI] [PubMed] [Google Scholar]
- 24.Chesik D, Glazenburg L, De Keyser J, Wilczak N. Enhanced proliferation of astrocytes from beta(2)-adrenergic receptor knockout mice is influenced by the IGF system. J Neurochem 2007;100:1555–1564 [DOI] [PubMed] [Google Scholar]
- 25.Baltensperger K, Karoor V, Paul H, Ruoho A, Czech MP, Malbon CC. The beta-adrenergic receptor is a substrate for the insulin receptor tyrosine kinase. J Biol Chem 1996;271:1061–1064 [DOI] [PubMed] [Google Scholar]
- 26.Steinle JJ, Smith PG. Role of adrenergic receptors in vascular remodelling of the rat choroid. Br J Pharmacol 2002;136:730–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg S, Wilborn WM. Effect of chronic administration of clonidine and propranolol on the myocardium of spontaneously hypertensive rats. Arch Int Pharmacodyn Ther 1982;255:141–161 [PubMed] [Google Scholar]
- 28.Holmang A, Jennische E, Bjorntorp P. The effects of long-term hyperinsulinaemia on insulin sensitivity in rats. Acta Physiol Scand 1995;153:67–73 [DOI] [PubMed] [Google Scholar]
- 29.Forte JD, Bui BV, Vingrys AJ. Wavelet analysis reveals dynamics of rat oscillatory potentials. J Neurosci Methods 2007;169:191–200 [DOI] [PubMed] [Google Scholar]
- 30.Weymouth AE, Vingrys AJ. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog Retin Eye Res 2008;27:1–44 [DOI] [PubMed] [Google Scholar]
- 31.Steinle JJ. Sympathetic neurotransmission modulates expression of inflammatory markers in the rat retina. Exp Eye Res 2007;84:118–125 [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld RG, Charles T, Roberts J. The IGF System: Molecular Biology, Physiology, and Clinical Application Totowa, NJ: Humana Press; 1999. [Google Scholar]
- 33.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 2002;29:10–14 [DOI] [PubMed] [Google Scholar]
- 34.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001;42:2408–2413 [PubMed] [Google Scholar]
- 35.Murata T, Ishibashi T, Khalil A, Hata Y, Yoshikawa H, Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res 1995;27:48–52 [DOI] [PubMed] [Google Scholar]
- 36.Murata M, Kador PF, Sato S. Vascular endothelial growth factor (VEGF) enhances the expression of receptors and activates mitogen-activated protein (MAP) kinase of dog retinal capillary endothelial cells. J Ocul Pharmacol Ther 2000;16:383–391 [DOI] [PubMed] [Google Scholar]
- 37.Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol 2006;141:149–156 [DOI] [PubMed] [Google Scholar]
- 38.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997;81:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruberte J, Ayuso E, Navarro M, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest 2004;113:1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartke A, Chandrashekar V, Dominici F, et al. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology 2003;4:1–8 [DOI] [PubMed] [Google Scholar]
- 41.Feldman EL, Randolph AE. Regulation of insulin-like growth factor binding protein synthesis and secretion in human retinal pigment epithelial cells. J Cell Physiol 1994;158:198–204 [DOI] [PubMed] [Google Scholar]
- 42.Suzuki J, Ohno I, Nawata J, Miura S, Ikeda J, Shirato K. Overexpression of insulin-like growth factor-I in hearts of rats with isoproterenol-induced cardiac hypertrophy. J Cardiovasc Pharmacol 1999;34:635–644 [DOI] [PubMed] [Google Scholar]
- 43.Lembo G, Rockman HA, Hunter JJ, et al. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clin Invest 1996;98:2648–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miura S, Ohno I, Suzuki J, et al. Inhibition of matrix metalloproteinases prevents cardiac hypertrophy induced by beta-adrenergic stimulation in rats. J Cardiovasc Pharmacol 2003;42:174–181 [DOI] [PubMed] [Google Scholar]
- 45.Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem Biophys Res Commun 2001;287:292–300 [DOI] [PubMed] [Google Scholar]
- 46.Punglia RS, Lu M, Hsu J, et al. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I. Diabetes 1997;46:1619–1626 [DOI] [PubMed] [Google Scholar]
- 47.Hirooka K, Kelly ME, Baldridge WH, Barnes S. Suppressive actions of betaxolol on ionic currents in retinal ganglion cells may explain its neuroprotective effects. Exp Eye Res 2000;70:611–621 [DOI] [PubMed] [Google Scholar]
- 48.Wood JP, Schmidt KG, Melena J, Chidlow G, Allmeier H, Osborne NN. The beta-adrenoceptor antagonists metipranolol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp Eye Res 2003;76:505–516 [DOI] [PubMed] [Google Scholar]
- 49.Osborne NN, Wood JP, Chidlow G. Invited review: neuroprotective properties of certain beta-adrenoceptor antagonists used for the treatment of glaucoma. J Ocul Pharmacol Ther 2005;21:175–181 [DOI] [PubMed] [Google Scholar]
- 50.Phipps JA, WilkinsonBerka JL, Fletcher EL. Retinal dysfunction in diabetic ren-2 rats is ameliorated by treatment with valsartan but not atenolol. Invest Ophthalmol Vis Sci 2007;48:927–934 [DOI] [PubMed] [Google Scholar]
- 51.WilkinsonBerka JL, Tan G, Jaworski K, Ninkovic S. Valsartan but not atenolol improves vascular pathology in diabetic Ren-2 rat retina. Am J Hypertens 2007;20:423–430 [DOI] [PubMed] [Google Scholar]
- 52.Williams KP, Steinle JJ. Maintenance of beta-adrenergic receptor signaling can reduce Fas signaling in human retinal endothelial cells. Exp Eye Res 2009;89:448–455 [DOI] [PubMed] [Google Scholar]