This report provides the first evidence of a nasal-dominant distribution of blood and lymphatic vessels in the cornea, which not only will shed light on corneal physiology and pathogenesis but also will provide a new concept in designing experimental models using the cornea and developing therapeutic protocols for corneal diseases.
Abstract
Purpose.
Because of its unique characteristics, the cornea has been widely used for blood and lymphatic vessel research. However, whether limbal or corneal vessels are evenly distributed under normal or inflamed conditions has never been studied. The purpose of this study was to investigate this question and to examine whether and how the distribution patterns change during corneal inflammatory lymphangiogenesis (LG) and hemangiogenesis (HG).
Methods.
Corneal inflammatory LG and HG were induced in two most commonly used mouse strains, BALB/c and C57BL/6 (6–8 weeks of age), by a standardized two-suture placement model. Oriented flat-mount corneas together with the limbal tissues were used for immunofluorescence microscope studies. Blood and lymphatic vessels under normal and inflamed conditions were analyzed and quantified to compare their distributions.
Results.
The data demonstrate, for the first time, greater distribution of both blood and lymphatic vessels in the nasal side in normal murine limbal areas. This nasal-dominant pattern was maintained during corneal inflammatory LG, whereas it was lost for HG.
Conclusions.
Blood and lymphatic vessels are not evenly distributed in normal limbal areas. Furthermore, corneal LG and HG respond differently to inflammatory stimuli. These new findings will shed some light on corneal physiology and pathogenesis and on the development of experimental models and therapeutic strategies for corneal diseases.
Both blood and lymph penetrate most tissues in the body, and dysfunction in their circulation is involved in a broad spectrum of diseases. Unlike blood vessels, lymphatic vessels are not easily visible. Although the lymphatic system was discovered at almost the same time as blood circulation, study in this field has made little progress during the past several decades until recently, when several lymphatic endothelium-specific markers, including vascular endothelial growth factor receptor-3 (VEGFR-3) and lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1), were identified.1–6
The cornea is the most well-studied and well-characterized tissue of the eye in blood and lymphatic vessel research.6–8 As the forefront medium in the passage of light to the retina, it by nature maintains transparency and is devoid of any vasculatures. The limbus, however, a transitional zone between the transparent cornea and the opaque sclera, is normally endowed with both vessel types. It is also the site of surgical incisions for certain cataract and glaucoma surgeries.9,10 Although not normally present in the cornea, blood and lymphatic vessels are induced in the cornea after inflammatory, infectious, traumatic, chemical, or toxic insult.6 Once induced, they enhance the high-volume delivery of antigens and antigen-presenting cells through the lymphatic channel and immune effector cells through the blood vessel channel, thus accelerating inflammation and transplant rejection.6–8
The importance of blood and lymphatic vessels in corneal transplantation immunity has also been demonstrated because the risk for corneal graft rejection increases as more quadrants of the cornea are vascularized.11 In addition, it has been found that surgical severing of the lymphatic pathway leads to universal corneal transplant survival without any form of pharmaceutical immune modulation.12 However, this surgical approach is not practical in the clinic. More recent studies have focused on the alternative molecular regulation of the lymphatic pathway, with some promising results on a few factors of the VEGFR and integrin families.13–16
Despite all these recent advances in corneal research, to date no study has been conducted on whether blood and lymphatic vessels are evenly distributed in the limbus and the cornea under normal or inflamed conditions. Answers to these questions are important for our understanding of the pathogenesis of limbal and corneal diseases and the development of more effective therapeutic strategies for them. The purpose of this study was, therefore, to identify and characterize possible organized distribution of blood and lymphatic vessels in the limbus and the cornea. Using a standardized method system, we herein provide the first evidence of a nasal-side polarized distribution of both vessel types under normal condition and their disparate changes during inflammatory responses.
Methods
Animals
Six- to 8-week-old male BALB/c and C57BL/6 mice (Taconic Farms, Germantown, NY) were used for the experiments. All mice were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee of the University of California at Berkeley. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure.
Corneal Suture Placement
The standard suture-induced inflammatory neovascularization model was used as described previously,13,17 with some modifications. Briefly, to appreciate the nasal versus temporal distributions of the vessels, two diametrically opposed 11–0 nylon sutures (AROSurgical, Newport Beach, CA) were placed at the 3 o'clock and 9 o'clock positions of the cornea after a demarcation of a 1.5-mm trephine (Fig. 1). Seven days later, corneas were collected for immunohistochemical studies. The experiments were repeated twice with 10 mice in each group of study.
Figure 1.
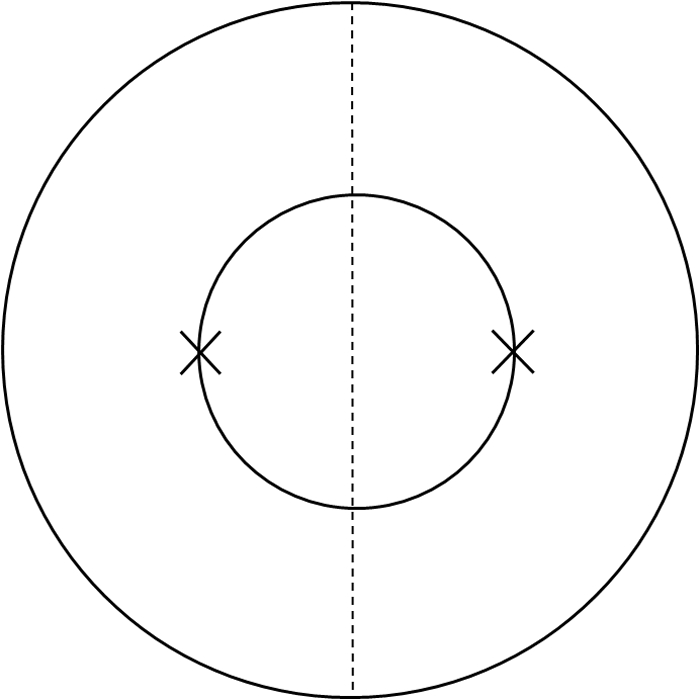
Schematic picture illustrating the suture placement method used to compare the nasal and temporal distributions of vessels. Two sutures were placed at the 3 o'clock and 9 o'clock positions of the cornea, respectively. Outer circle: limbus. Inner circle: demarcation of the trephine. Dashed line: demarcation between the nasal and the temporal sides.
Immunohistochemical Studies
The experiments were performed according to our standard protocol.14,16,17 Briefly, freshly excised corneas, labeled at the 6 o'clock position for orientation, were fixed in acetone for immunofluorescence staining. Nonspecific staining was blocked with anti–Fc CD16/CD32 antibody (BD Biosciences, San Jose, CA). Samples were sequentially stained with FITC-conjugated rat anti–mouse CD31 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and purified rabbit anti–mouse LYVE-1 antibody (Abcam, Cambridge, MA), which was visualized by a rhodamine-conjugated donkey anti–rabbit secondary antibody. Double-stained samples were covered with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA) and examined by an epifluorescence deconvolution microscope (AxioImager M1; Carl Zeiss AG, Göttingen, Germany). Digital images were taken (iVision-Mac software; BioVision Technologies Inc., Exton, PA). Vascular structures stained as CD31+LYVE-1− were identified as blood vessels, whereas those stained as CD31+LYVE-1+ were defined as lymphatic vessels.
Vascular Quantification
Blood and lymphatic vessels were graded and analyzed using the ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), as described previously.16,18,19 Basically, the neovascularization area was normalized to the total corneal area to obtain a percentage coverage score for each sample. Neovascularization area refers to the fraction of the corneal area in which vessels are present. The total corneal area was measured by outlining the innermost vessels of the limbal arcade. The nasal and temporal sides were divided by a midline across the 6 and 12 o'clock positions of the cornea. The degree of disparity in percentage was calculated as follows: (nasal score − temporal score)/temporal score.
Statistical Analysis
Data are expressed as mean ± SEM. The statistical significance of the difference between each group (n = 10) was evaluated using Student's t-test with statistical analysis software (Prism; GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered significant.
Results
Blood and Lymphatic Vessel Polarization in Normal BALB/c Limbal Area
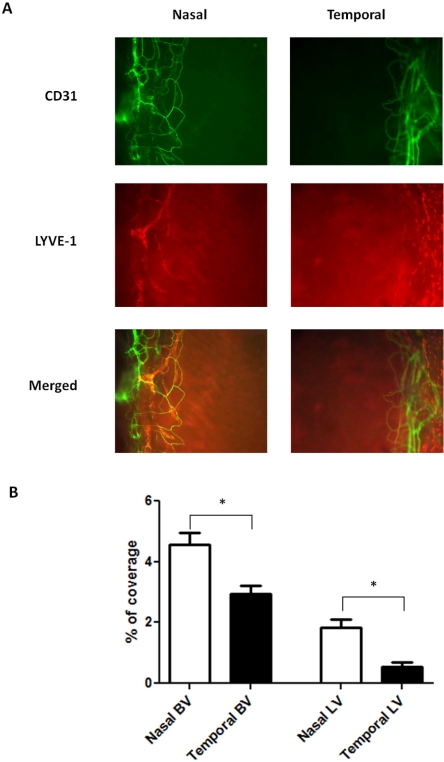
We first set out to investigate the normal distribution of blood and lymphatic vessels in the limbi of BALB/c mice using specific antibodies against CD31 (a pan-endothelial marker) and LYVE-1 (a lymphatic marker). Our results from the immunofluorescence microscope studies confirmed that though the normal cornea was devoid of any vascular structures, the limbal area was endowed with both blood and lymphatic vessels. Surprisingly, it was also found that these vessels were not evenly distributed around the clock. Both blood and lymphatic vessels were present more frequently in the nasal side (Fig. 2A). Although the degree of the disparity between nasal and temporal distributions for the blood vessels was 55% (P = 0.0036), that for the lymphatic vessels was 238% (P = 0.0017), which was 4.3-fold of the previous value. Results from repetitive studies were summarized in Figure 2B.
Figure 2.
Comparison of blood and lymphatic vascular distribution in the nasal versus temporal side of the normal BALB/c limbi. (A) Representative immunofluorescence micrographs demonstrating that both blood and lymphatic vessels were more prominent in the nasal side. Green: CD31. Red: LYVE-1. Yellow: merged. BV, blood vessels; LV, lymphatic vessels. Original magnification, ×100. (B) Summary of results. Significant differences were observed for both blood and lymphatic vessels. *P < 0.05. Error bars represent SEM (n = 10).
Corneal Inflammatory Hemangiogenesis and Lymphangiogenesis in the Nasal versus Temporal Side of BALB/c Mice
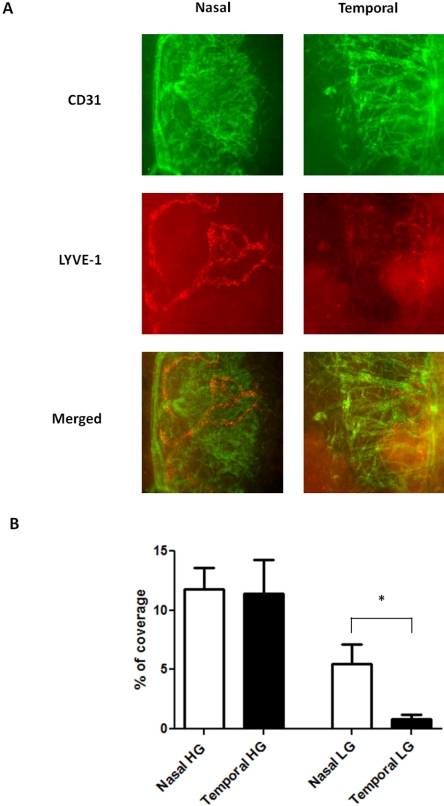
The findings that neither blood nor lymphatic vessels are evenly distributed in the limbal areas are novel. We next investigated, with the use of immunofluorescence microscope assays, whether the nasal dominance pattern observed under normal conditions also applied to corneal inflammatory HG and LG. The standardized suture placement model was used in BALB/c mice as demonstrated in Figure 1. This particular model, with two equally placed sutures, allowed us to perform a precise comparison between nasal and temporal sides of the cornea.
Results from these studies on the newly formed blood vessels demonstrated that robust HG responses were induced from both the nasal and the temporal sides (Fig. 3A). However, there was no significant disparity between these two sides of vascular distribution (P = 0.83). Surprisingly, unlike corneal HG, which lost its original nasal-dominant pattern during inflammation, corneal LG behaved differently and maintained its nasal-dominant arrangement even after inflammatory stimulation (Fig. 3A). More prominent lymphatic vessels were found in the nasal side, and the degree of the disparity between the nasal and the temporal distributions was 581% (P = 0.01), which was 2.4-fold that of the normal condition (Fig. 3B).
Figure 3.
Comparison of HG and LG in the nasal versus temporal sides of the inflamed BALB/c corneas. (A) Representative immunofluorescence micrographs demonstrating more lymphatic vessels in the nasal side. Green: CD31. Red: LYVE-1. Yellow: merged. HG, hemangiogenesis; LG, lymphangiogenesis. Original magnification, ×100. (B) Summary of the results. Although no significant difference was observed for corneal inflammatory HG (P = 0.83), LG was more prominent in the nasal side. *P < 0.05. Error bars represent SEM (n = 10).
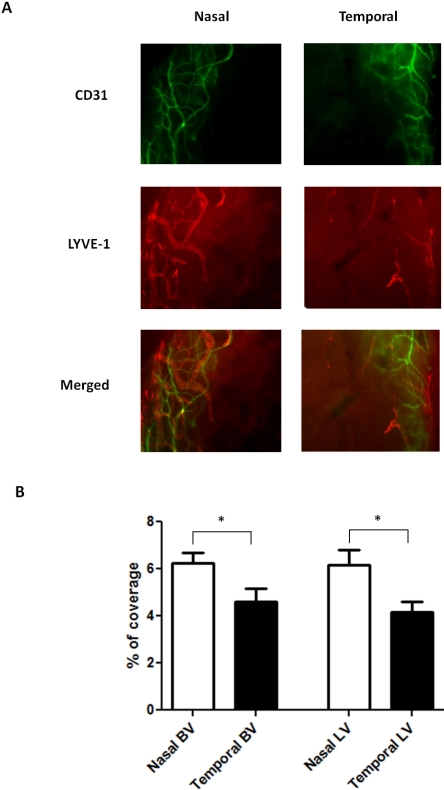
Blood and Lymphatic Vessel Polarization in Normal C57BL/6 Limbal Area
To further investigate whether the novel findings on the blood and lymphatic vessel distributions are specific to BALB/c strain, we also examined another commonly used murine strain, C57BL/6. We first set out to study the normal condition using similar immunofluorescence microscope assays. Results from these studies clearly demonstrated that a nasal-dominant distribution pattern also occurs in the normal C57BL/6 mice (Fig. 4A). The degree of the disparity between the nasal and temporal blood vessel distributions was 36% (P = 0.004), and that of the lymphatic vessel distributions was 48% (P = 0.0026). Moreover, it was found that, compared with BALB/c mice, C57BL/6 mice have higher baseline levels for both vessel types. Results from repetitive studies are summarized in Figure 4B.
Figure 4.
Comparison of blood and lymphatic vascular distribution in the nasal versus temporal sides of the normal C57BL/6 limbi. (A) Representative immunofluorescence micrographs demonstrating that both blood and lymphatic vessels were more prominent in the nasal side. Green: CD31. Red: LYVE-1. Yellow: merged. BV, blood vessels; LV, lymphatic vessels. Original magnification, ×100. (B) Summary of the results. Significant differences were observed for both blood and lymphatic vessels. *P < 0.05. Error bars represent SEM (n = 10).
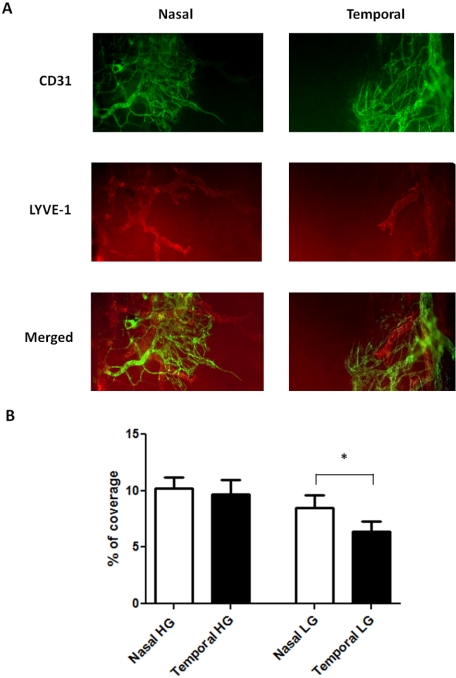
Corneal Inflammatory Hemangiogenesis and Lymphangiogenesis in the Nasal versus the Temporal Side of C57BL/6 Mice
Similarly, as for BALB/c mice, we also examined possible inflammatory changes to the nasal-dominant patterns observed in normal C57BL/6 mice using the standardized suture placement model. Results from these parallel studies revealed that corneal blood vessels in this strain also lost their nasal polarity (P = 0.62) and became evenly distributed on both sides during inflammation (Fig. 5A). However, the lymphatic vessels still obeyed the nasal dominant principle after inflammatory stimulation (Fig. 5A). The degree of the disparity between the nasal and the temporal distributions for corneal LG was 33% (P = 0.04). Results from repetitive studies are summarized in Figure 5B.
Figure 5.
Comparison of HG and LG in the nasal versus temporal sides of the inflamed C57BL/6 corneas. (A) Representative immunofluorescence micrographs demonstrating more lymphatic vessels in the nasal side. Green: CD31. Red: LYVE-1. Yellow: merged. HG, hemangiogenesis; LG, lymphangiogenesis. Original magnification, ×100. (B) Summary of the results. Although no significant difference was observed for corneal inflammatory HG, corneal LG was more prominent in the nasal side. *P < 0.05. Error bars represent SEM (n = 10).
Discussion
In this study, we provide the first analysis on the physiological and pathologic distributions of blood and lymphatic vessels in the limbus and the cornea, which is important for our understanding and management of corneal diseases. At least two important conclusions can be drawn from our data: blood and lymphatic vessels are not evenly distributed in normal limbus, and corneal LG and HG respond differently to inflammatory stimuli. Although blood vessels lose their original nasal polarity during inflammation, lymphatic vessels maintain it.
Our data on blood vessel polarity in the normal limbus is new but consistent with previous findings on other ocular tissues. Similar asymmetric distribution of blood vessels has been reported in the developing retina.20,21 Indeed, the blood vessel supply of human retinas is completed at 8 months of embryonic life in the nasal periphery but not until after birth in the temporal periphery.22 Another study shows a predominant orientation of retinal veins where posterior crossing occurs more frequently in the superotemporal quadrant than in the inferotemporal quadrant.22 This polarization is considered an important predisposing factor of the superotemporal occurrence of the branch retinal vein occlusion.22 In the ocular surface, similar nasal preference patterns have been observed in antigen-induced conjunctiva-associated lymphoid tissue, which is located in the nictitating membrane at the nasal side.23,24 It has also been long observed that certain diseases, such as pterygium, predominantly affect the nasal side of the cornea, though the mechanisms remain largely unknown. Our findings that the nasal side is naturally more vascularized might explain in part why it is more prone to pathologic irritations. Further in-depth studies are required to define possible new mechanisms of such disorders of nasal preference.
There are no reports on disparate lymphatic distribution in the limbus or the cornea. Our findings that lymphatic vessels are nasally dominated in these areas under both normal and inflamed conditions are novel and have at least three implications. First, they provide strong evidence that lymphatic vessels behave independently of or differently from blood vessels under certain circumstances. Unlike blood vessels, which have been studied extensively in the past, the lymphatics are a field of new discovery. Many questions remain unanswered, including whether and how lymphatic vessels depend on blood vessels. Our results provide a new conceptual framework for further investigations on the relationship between these two vessel types. These data demonstrating that corneal LG and HG behave differently during inflammation also correlate with other recent findings that corneal LG can occur in the absence of HG,25 and its regression follows a different time course.26 Second, these data reveal novel insights into the experimental design and data analysis using corneal models. It should be taken into account that the vasculogenic responses of the cornea are not equivalent across areas. For example, when conducting the micropocket assay,27 one of the most widely applicable models for vascular research both inside and outside the eye, the choice of the pellet site is crucial in data generation and interpretation. The same pellet may yield significantly different results when it is implanted on the nasal or temporal side. Researchers using this or similar corneal models should, therefore, consider the side orientation issue in their experimental protocols and in data analysis. Third, from a clinical point of view, these data will also have an impact on the pharmaceutical administrations and surgical procedures. Corneal LG is an emerging focus of therapy to treat inflammation and transplant rejection. The fact that lymphatic vessels are richer at baseline and more prone to develop in the nasal side implies that a nasal-side preferential procedure may yield better outcomes for certain treatments, such as subconjunctival injection of anti–lymphangiogenic reagents. Similar propositions may be made regarding surgical procedures, such as limbal and corneal transplantation. The side orientation between the donor and recipient tissues may produce dissimilar outcomes. Interestingly, it has been reported that limbal epithelial crypts were most likely to be encountered in the nasal side.28 It is anticipated that further in-depth investigation on these polarization phenomena will reveal novel molecular and cellular mechanisms of ocular surface physiology and pathogenesis, holding the great promise of new therapeutic protocols.
Acknowledgments
The authors thank Jeffrey LeDue (University of California Berkeley) for excellent technical assistance with the immunofluorescence microscopy studies.
Footnotes
Supported in part by research grants from the National Institutes of Health, the Department of Defense, and the University of California at Berkeley (LC).
Disclosure: T. Ecoiffier, None; D. Yuen, None; L. Chen, None
References
- 1.Brown P. Lymphatic system: unlocking the drains. Nature 2005;436:456–458 [DOI] [PubMed] [Google Scholar]
- 2.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS 2004;112:526–538 [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Kaipainen A, Folkman J. Lymphangiogenesis new mechanisms. Ann N Y Acad Sci 2002;979:111–119 [DOI] [PubMed] [Google Scholar]
- 4.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev 2002;16:773–783 [DOI] [PubMed] [Google Scholar]
- 5.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005;438:946–953 [DOI] [PubMed] [Google Scholar]
- 6.Chen L. Ocular lymphatics: state-of-the-art review. Lymphology 2009;42:66–76 [PMC free article] [PubMed] [Google Scholar]
- 7.Chong EM, Dana MR. Graft failure, IV: immunologic mechanisms of corneal transplant rejection. Int Ophthalmol 2008;28:209–222 [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003;22:273–281 [DOI] [PubMed] [Google Scholar]
- 9.Van Buskirk EM. The anatomy of the limbus. Eye 1989;3:101–108 [DOI] [PubMed] [Google Scholar]
- 10.Meyer PA. The circulation of the human limbus. Eye 1989;3:121–127 [DOI] [PubMed] [Google Scholar]
- 11.Hill JC. The relative importance of risk factors used to define high-risk keratoplasty. Ger J Ophthalmol 1996;5:36–41 [PubMed] [Google Scholar]
- 12.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci 2001;42:1293–1298 [PubMed] [Google Scholar]
- 13.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med 2004;10:813–815 [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol 2007;125:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci 2004;45:2666–2673 [DOI] [PubMed] [Google Scholar]
- 16.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004;113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci 2005;46:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bock F, Onderka J, Hos D, Horn F, Martus P, Cursiefen C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res 2008;87:462–470 [DOI] [PubMed] [Google Scholar]
- 19.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol 2009;127:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cogan DG. Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc U K 1963;83:465–489 [PubMed] [Google Scholar]
- 21.Patz A. The role of oxygen in retrolental fibroplasia. Sinai Hosp J (Balt) 1957;6:3–22 [PubMed] [Google Scholar]
- 22.Weinberg DV, Egan KM, Seddon JM. Asymmetric distribution of arteriovenous crossings in the normal retina. Ophthalmology 1993;100:31–36 [DOI] [PubMed] [Google Scholar]
- 23.Sakimoto T, Shoji J, Inada N, Saito K, Iwasaki Y, Sawa M. Histological study of conjunctiva-associated lymphoid tissue in mouse. Jpn J Ophthalmol 2002;46:364–369 [DOI] [PubMed] [Google Scholar]
- 24.Steven P, Rupp J, Huttmann G, et al. Experimental induction and three-dimensional two-photon imaging of conjunctiva-associated lymphoid tissue. Invest Ophthalmol Vis Sci 2008;49:1512–1517 [DOI] [PubMed] [Google Scholar]
- 25.Chang LK, Garcia-Cardena G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A 2004;101:11658–11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 2006;25:443–447 [DOI] [PubMed] [Google Scholar]
- 27.Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc 2007;2:2545–2550 [DOI] [PubMed] [Google Scholar]
- 28.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol 2005;89:529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]