This study was undertaken to identify the full complement of muscarinic acetylcholine receptors (mAChRs) in the rabbit retina and to assess mAChR distribution and the functional effects of mAChR activation and blockade on retinal response properties. Understanding the distribution and function of mAChRs in the retina has the potential to provide important insights into the visual changes caused by decreased ACh in the retinas of Alzheimer's patients and the potential visual effects of anticholinergic treatments for ocular diseases.
Abstract
Purpose.
The activation and blockade of muscarinic acetylcholine receptors (mAChRs) affects retinal ganglion cell light responses and firing rates. This study was undertaken to identify the full complement of mAChRs expressed in the rabbit retina and to assess mAChR distribution and the functional effects of mAChR activation and blockade on retinal response properties.
Methods.
RT-PCR, Western blot analysis, and immunohistochemistry were used to identify the complement and distribution of mAChRs in the rabbit retina. Extracellular electrophysiology was used to determine the effects of the activation or blockade of mAChRs on ganglion cell response properties.
Results.
RT-PCR of whole neural retina resulted in the amplification of mRNA transcripts for the m1 to m5 mAChR subtypes. Western blot and immunohistochemical analyses confirmed that all five mAChR subtypes were expressed by subpopulations of bipolar, amacrine, and ganglion cells in the rabbit retina, including subsets of cells in cholinergic and glycinergic circuits. Nonspecific muscarinic activation and blockade resulted in the class-specific modulation of maintained ganglion cell firing rates and light responses.
Conclusions.
The expression of mAChR subtypes on subsets of bipolar, amacrine, and ganglion cells provides a substrate for both enhancement and suppression of retinal responses via activation by cholinergic agents. Thus, the muscarinic cholinergic system in the retina may contribute to the modulation of complex stimuli. Understanding the distribution and function of mAChRs in the retina has the potential to provide important insights into the visual changes that are caused by decreased ACh in the retinas of Alzheimer's patients and the potential visual effects of anticholinergic treatments for ocular diseases.
In the retina, tonic and light-stimulated ACh release modulates ganglion cell response properties through the activation of muscarinic and nicotinic acetylcholine receptors (AChRs).1,2 Cholinergic signaling can be mediated, not only by ACh but also by choline, the product of ACh hydrolysis, which can activate both nicotinic (n) and muscarinic (m)AChRs.3 ACh is decreased in the brain and retinas of persons with Alzheimer's disease, and visual disturbances caused by both central and retinal mechanisms have been reported.4,5 In addition to treatments for the symptoms of Alzheimer's disease, anticholinesterases and cholinergic drugs have been used in the treatment of myopia,6 and ocular mAChRs are implicated in tear fluid production, ocular drainage, and lens cell signaling.7 Because functional mAChRs are also expressed in the retina, understanding the distribution and function of cholinergic receptors in the retina has the potential to provide important insights into the understanding of visual changes resulting from decreased ACh in the retinas of Alzheimer's patients and the potential visual effects of cholinergic agents as treatment for ocular diseases.
mAChRs are coupled to heterotrimeric G-proteins. The five muscarinic subtypes are grouped based on the intracellular signaling pathway that is activated by ligand binding. In neurons, m1, m3, and m5 mAChRs are associated with the Gq-α subunit which activates phosphatidyl inositol or phospholipase pathways. Activation increases neuronal excitability through activation of nonspecific cation channels, release of Ca2+ from intracellular stores, or inhibition of Ca2+-activated K+ (SK) channels.8 In contrast, m2 and m4 mAChRs are associated with the Gi-α subunit, activation of which inhibits adenylate cyclase and cyclic adenosine monophosphate (cAMP) production. Activation of m2 and m4 typically decreases neuronal activity, through either the activation of a subset of K+ channels or the inhibition of Ca2+ channels.9 The activation of m2 and m4 AChRs can also inhibit the Ca2+ priming of SK channels.10 Because SK channels are thought to be responsible for the medium and slow after-hyperpolarization (AHP) phase of action potentials,10 the activation of mAChRs can change cell firing rates.
In studies performed in the 1980s, radiolabeled ligands were used to visualize the expression patterns of muscarinic receptors in rabbit11,12 and human retinas.13 These studies demonstrated that muscarinic receptors are present in the inner plexiform layer (IPL) of both species. In the human retina, the strongest muscarinic labeling has been reported in the IPL, with less in the outer plexiform layer (OPL). There were no reported changes in density from the central to the peripheral retina.
More recent immunohistochemical studies have shown that m2 mAChRs are expressed by cells in the inner nuclear layer (INL) and the IPL of rat14 and primate15 retinas. In the rabbit, m2 receptors have been localized in the INL, IPL, and ganglion cell layer (GCL).16,17 Immunoreactive m2 amacrine cells include the cholinoceptive 4′,6′-diamino-2-phenylindole (DAPI)-3 glycinergic amacrine cells.17 These cells also express γ-aminobutyric acid (GABA) receptors and may provide inhibitory feedback to cholinergic amacrine cells.
Populations of horizontal, bipolar, and amacrine cells in the primate retina have been reported to express m3 mAChRs.15 The mAChRs m2, m3, and m4 are expressed in the chick retina,18 and all the mAChRs are reported to be expressed in the retinas of tree shrews. The subtypes are differentially expressed by chick and tree shrew retinal cells, suggesting that the different subtypes subserve different functions.19
The activation of mAChRs affects many retinal ganglion cell (RGC) response properties. For example, application of the muscarinic antagonist scopolamine at specific concentrations has biphasic effects on the rod and cone optic nerve response to light onset.20 Scopolamine at nonspecific concentrations alters the rod and cone b-wave of the electroretinogram (ERG) in the cat retina. The b-wave is a measure of ON bipolar cell activation, and the scopolamine-induced changes suggest that mAChRs are functionally expressed by bipolar cells. Scopolamine has also been reported to decrease the maintained firing rates and center responses of brisk ganglion cells as well as to block ganglion cell responses to exogenously applied ACh.1 Further, carbachol-induced, atropine-sensitive potentiation of light-driven responses in directionally selective ganglion cells21 indicates that mAChRs may be involved in the modulation of specific retinal circuitry, including directional selectivity.
Muscarine application reduces light-evoked ACh release in rabbit retina, an effect that is atropine-sensitive, indicating that muscarinic activation provides negative feedback to cholinergic amacrine cells. Consistent with a role for muscarinic inhibition of ACh release, light-evoked ACh release is increased in the presence of atropine and the cholinesterase inhibitor eserine.22 Muscarinic feedback is also required in early development of the chick retina for the appropriate tiling of starburst amacrine cells.23 In vivo blockade of mAChRs results in changes in the ratio of ON and OFF starburst amacrine cells and disturbs the regular mosaic patterning of the cells.23 The muscarinic receptor antagonists atropine and pirenzipine have been reported to have effects on retinal waves in rabbits, but at this time, the involvement of mAChRs in the generation and propagation of retinal waves is unclear.24,25
Although the specific mAChR subtypes responsible for these effects and the cells that express them are not yet known, there are several lines of evidence that suggest that m2 and m4 receptor activation is involved in visual processing. The activation of m2 and m4 mAChRs decreases dopamine release in the guinea pig retina.26 The activation of m2 in a population of salamander GABAergic amacrine cells results in an increase in cGMP via increased nitric oxide production.27 In the rat retina, m2 mAChRs negatively regulate adenylate cyclase,28 whereas increases in neuronal nitric oxide synthase and cGMP production correlate with the activation of m1 and m3 mAChRs.29 The m1 and m3 mAChRs have also been reported to have direct effects on retinal processing. In the rabbit retina, muscarinic activation results in increased intracellular calcium concentrations in a small percentage of RGCs. These effects are sensitive to the m1 antagonist pirenzepine, but not to the m2 antagonist gallamine.2
The present study was undertaken to identify the full complement of mAChRs in the rabbit retina and to assess how the cell-type–specific expression of these receptors may relate to the described effects of the activation or blockade of mAChRs on retinal response properties. We report the effects of muscarinic activation on RGC firing rates and light responses. RT-PCR and Western blot experiments demonstrated the expression of all five mAChR subtypes in the rabbit retina. We report the expression patterns of mAChR subtypes relative to each other and to retinal cholinergic and glycinergic circuitry and propose how this circuitry may mediate ACh effects on RGC response properties.
Methods
Animals were maintained in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Protocols and experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. For retinal RNA extraction, protein extraction, and immunohistochemistry, rabbits were overdosed with pentobarbital (Fatal Plus; Vortech, Dearborn, MI) and the eyes enucleated. For electrophysiological experiments, the rabbits were anesthetized with urethane (Sigma Aldrich, St. Louis, MO) followed by pentobarbital (Nembutal; Vedco, St. Joseph, MO). When all reflexive responses were abolished, the eye was removed and placed in ice-cold Ames medium.30 The rabbits were then overdosed with pentobarbital. The eyes were hemisected, and the retinas were isolated and mounted in a recording chamber.
Electrophysiology
Standard extracellular recording techniques were used to record spike frequency, as described elsewhere.31,32 Briefly, action potentials generated by RGCs were extracellularly recorded, digitized by use of a window discriminator, binned, and assessed as peristimulus time (PST) histograms. Changes in spike frequency were used to assess retinal responses to application of AChR agonists and antagonists. Choline was used as a nonspecific muscarinic agonist and atropine as a nonspecific muscarinic antagonist. The sensitivity of choline to atropine was essential in the determination of whether changes in RGC firing are mediated by the activation of mAChRs. Repeated-measures ANOVA followed by Tukey's HSD test was used to identify significant differences in the responses of a single RGC across experimental conditions.
Reverse Transcription–Polymerase Chain Reaction
An RT-PCR kit (OneStep; Qiagen, Inc., Valencia, CA) and primers for mAChR subtypes m1 to m533 were used to identify the presence of mAChR transcripts in total RNA extracted from rabbit neural retina. RNA was isolated by dissection of intact retinas and placement in an RNA stabilizer (RNAlater; Ambion, Austin, TX) immediately after enucleation. RNA was then extracted (Absolutely RNA RT-PCR Miniprep Kit; Stratagene, La Jolla, CA). DNA contamination bound to the fiber matrix was removed by DNase digestion, followed by a series of low- and high-salt buffer rinses.
Reverse transcription occurred at 50°C for 30 minutes, followed by one cycle at 95°C for 15 minutes, to inactivate the reverse transcriptase and activate the Taq polymerase. Denaturing, annealing, and extension consisted of 35 to 40 cycles at 94°C for 1 minute, 52° to 60°C for 1.5 minutes, and 72°C for 2 minutes, respectively. Final extension took place at 72°C for 20 minutes. For second-round PCR amplification of single cell products, 1 to 2 μL of first-round product was used as the template, and the reverse transcription cycle was omitted. Gel electrophoresis was used to separate RT-PCR and PCR products on 2% agarose gels. Ethidium bromide was used to visualize product bands. Products matching the predicted sizes, m1, 573 bp; m2, 469 bp; m3, 434 bp; m4, 592 bp; and m5: 451 bp, were gel purified (QIAquick PCR Purification kit; Qiagen, Inc.) and sequenced (Center for AIDS Research University of Alabama at Birmingham). The sequences from the DNA extraction were aligned with one another with Clustal W (European Bioinformatics Institute, Cambridge, UK; http://www.ebi.ac.uk/clustalw/) and compared to known cDNA sequences through GenBank in an NCBI (National Center for Biotechnology Information) BLAST query (http://www.ncbi.nlm.nih.gov/Genbank; NCBI, Bethesda, MD). Homology with the appropriate human cDNA sequence ranged from 83% to 95% without significant homology to other cDNA sequences.
Western Blot Analysis
Western blot analysis was performed to confirm that mRNA was translated to expressed protein and to confirm the specificity of antibodies against the five AChR subtypes. Retinal protein was extracted from neural retina according to the protocol of Dmitrieva et al.34 Isolated rabbit retinas were homogenized in four volumes of ice-cold lysis buffer (PBS containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail; 1:30, Sigma Aldrich), incubated at 4°C for 30 minutes, and centrifuged (15,000g at 4°C for 30 minutes). The supernatant was retained, mixed with an equal amount of sample buffer, and denatured. For gel electrophoresis, 20 μg of total protein was loaded onto 12% SDS polyacrylamide gels, after which the proteins were transferred to nitrocellulose membranes (Pierce Biotechnology, Inc., Rockford, IL). The membranes were blocked with 7% nonfat milk (Bio-Rad Laboratories, Inc., Hercules, CA) and 1% bovine serum albumin in PBS/0.1% Tween-20 (PBST), followed by incubation with the primary antibodies (Table 1) in PBST/blocking solution. After the membrane was rinsed in PBS (three times for minutes each time), it was incubated for 2 hours in peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) diluted in PBST. Colorimetric detection was used to reveal immunoreactive bands (Opti-4CN; Bio-Rad Laboratories, Inc). In some cases, the secondary antibody labeling was amplified (Amplification module; Bio-Rad Laboratories) before visualization. Control experiments were performed both by omitting the primary antibody and by substituting matched protein concentrations of IgG from the species in which the primary antibody was raised.
Table 1.
Antibodies and Specificity
| Antibody (Antigen) | Species | Catalog No. | Supplier | Specificity |
|---|---|---|---|---|
| ChAT (human placental enzyme; NM_020549.3) | Goat | AB144P | Chemicon, Temecula, CA | Firth et al.35 |
| Glyt-1 (synthetic peptide corresponding to the final 15 amino acids at the carboxyl-terminus of glyt-1) | Guinea pig | N/A | Gift of David Pow, University of Queensland, Australia | Dmitrieva et al.36 Pow and Hendrickson37 |
| m1 mAChR (GST fusion protein and part of the i3 intercellular loop of the human m1 muscarinic acetylcholine receptor; AA227-353; accession P11229) | Rabbit | AB5164 | Chemicon | Dorje et al.38 Hersh et al.39 Levey et al.40 |
| m2 mAChR (KLH-conjugated synthetic peptide corresponding to the third cytoplasmic loop) | Rabbit | NLS1333 | Novus Biologicals Littleton, CO | Immunogen based on NM_000739, 100% homology between mouse, rat, and human |
| m2 mAChR (clone M2-2-B3, i3 loop of m2 receptor [AA225-359], fused to GST) | Rat | mAB367 | Chemicon | Levey et al.41 Zucker and Ehinger16 Yamada et al.15 |
| m3 mAChR (peptide analogue of the carboxyl terminal of muscarinic M3 receptor covalently bonded to carrier protein) | Rabbit | AS-3741S | Research and Diagnostic Antibodies, Benicia, CA | Ndoye et al.42 |
| m4 mAChR (Clone 18C7.2, m4 receptor i3 loop [human], fused to GST, NM_000741.2) | Mouse | mAB1578 | Chemicon | Wang et al.43 |
| m5 mAChR (Synthetic peptide from the 3rd cytoplasmic domain of human mAChR M5, NM_012125.2) | Rabbit | AB9454 | Chemicon | Human immunogen with 44% homology with mouse and rat |
Immunohistochemistry
For immunohistochemistry (IHC), rabbit eye cups were fixed in 2% paraformaldehyde; PFA), 4% PFA, or 1% PFA with 0.34% l-lysine, and 0.05% sodium-m-periodate (1% PLP), and cryoprotected in 30% sucrose in 0.1 M PBS. The retinas were embedded in 50% optimum cutting temperature compound (OCT; VWR Scientific, West Chester, PA)/50% aqueous medium (Aquamount; VWR Scientific) and cryosectioned (12-μm sections). The sections were washed three times for 10 minutes each time with 0.1 M PBS and incubated for 1 hour at room temperature (RT) in 10% donkey normal serum (DKNS) in PBS with 0.3% Triton-X-100. All antibodies were diluted in PBS-Triton. See Table 1 for primary antibody suppliers and specificity. For localization of m1, m3, m5, and ChAT or glyt-1, the sections were incubated overnight at 4°C in primary antibodies. The sections were washed in PBS three times for 10 minutes each time, and incubated in donkey anti-rabbit antibody conjugated to either FITC or rhodamine. For m2 and m4 localization, the sections were blocked with avidin-biotin blocking solution (Vector Laboratories, Burlingame, CA) for 15 minutes each, followed by incubation overnight at 4°C with the primary antibody. The sections were then washed and incubated with biotinylated secondary antibodies raised in donkey, washed, and incubated with fluorophore-conjugated streptavidin. In cases in which three-step IHC was necessary for double-labeling studies, a fluorophore-labeled secondary antibody was used in place of a biotinylated secondary antibody, and an additional fluorophore-labeled tertiary antibody raised in donkey was used. Both three-step methods yielded equivalent results and enabled double labeling between antibodies that required the additional amplification. All double-labeling studies were performed sequentially. Control sections were processed in parallel with experimental sections. To control for the specificity of the primary antibodies, we substituted matched protein concentrations of isotype IgG for the primary antibodies. To control for the specificity of the secondary antibodies, we substituted PBS-Triton for the primary antibodies.
Imaging
Fluorescent images were collected with a laser scanning confocal microscope (model TCS 4D; Leica Microsystems, Mannheim, Germany) equipped with argon, krypton, helium-neon, and UV lasers. Images were typically collected with a 40× oil-immersion objective with a numerical aperture of 1.25. Each channel was scanned separately with 0.5-μm intervals between optical sections. Five optical sections were used to assess the distribution of a single receptor subtype. This method allowed for a standard number of sections to be compared in each experiment, as well as enough depth to assess morphology and stratification. The final determination of colocalization was performed only on single optical sections. Because there were no systematic differences between colocalization as determined from the single optical sections and projections of five optical sections and because additional morphologic information was contained in the projection images, the figures were made with the projection images. The images were then processed for contrast and brightness, and the figures were created with image-management software (Photoshop; Adobe Systems, Mountain View, CA).
Results
Physiological Responses
We have shown in other work44,45 that subsets of brisk sustained, brisk transient, and directionally selective RGCs respond to choline, and this response can be blocked by nanomolar concentrations of methyllycaconitine (MLA), indicating that the choline responses are mediated by α7 nAChRs. However, choline is also a nonspecific agonist of mAChRs. The previously reported responses of RGCs with high maintained firing rates to choline application were not MLA-sensitive, which suggests that these responses may have been mediated by mAChRs.44 To test this hypothesis, we sought to determine whether choline responses can be blocked by the bath application of atropine. Consistent with data from the previous study, the application of choline resulted in excitatory and suppressive effects on both the light responses and maintained firing rates of nine RGCs with high maintained firing rates (Table 2). Sustained OFF, transient OFF, transient ON, and ON-OFF RGCs displayed atropine-sensitive responses to choline application.
Table 2.
Summary of the Effects of the Application of Cholinergic Agents on Ganglion Cell Light Responses and Maintained Firing Rates
| Light Response | P | Choline Response | P | Baseline Firing Rate | P |
|---|---|---|---|---|---|
| Choline, 1 mM (n = 7) | Atropine, 3 μM (n = 8) | Choline, 1 mM (n = 8) | |||
| Suppressed (2) | 0.001 | Suppressed (5) | 0.001 | Suppressed (2) | 0.01 |
| Enhanced (4) | 0.001 | Enhanced (1) | 0.001 | Enhanced (4) | 0.05 |
| No effect (1) | NS | No effect (2) | NS | Both (1) | 0.001 |
| No effect (1) | NS | ||||
| Atropine, 3 μM (n = 6) | Atropine, 3 μM (n = 8) | ||||
| Suppressed (3) | 0.001 | Suppressed (3) | 0.001 | ||
| Enhanced (0) | NA | Enhanced (1) | 0.001 | ||
| No effect (3) | NS | No effect (4) | NS |
Data in parentheses are the number of cells affected.
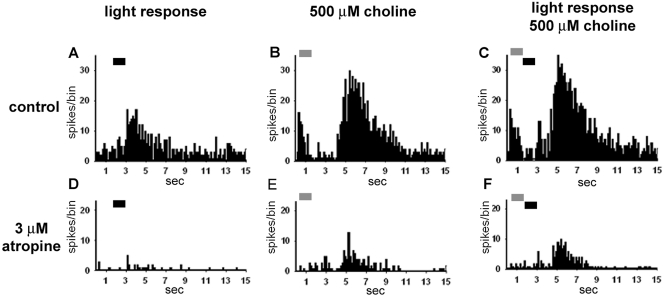
Choline application suppressed the light responses of two OFF RGCs (P < 0.001); enhanced the light responses of four, including transient ON and ON-OFF RGCs (P < 0.001); and had no effect on one. Choline application also had differential effects on the maintained firing rates of the RGCs, suppressing the rate in two cases (P < 0.01) and enhancing it in four (P < 0.05). Choline application had no effect on the firing of one ON-OFF cell. Bath application of atropine was used to examine the presumptive mAChR-mediated component of RGC responses to choline. Atropine significantly (P < 0.001) decreased the effects of choline in the majority of the RGCs tested (five/eight choline-positive cells). Of interest, whereas the light responses of the OFF RGCs were inhibited by choline and the responses of ON and ON-OFF RGCs were enhanced, light responses were either reduced or unaffected by atropine application without choline stimulation. In general, atropine was more effective at relieving choline-induced suppression than at blocking choline-induced enhancement, as evidenced by the incomplete blockade of choline-induced enhancement. Atropine almost completely blocked choline-induced suppression, indicating the preferential involvement of mAChRs in choline-mediated suppression. For example, 1-second puffs of 500 μM choline (Fig. 1A) suppressed the maintained firing rate, with a slow recovery to control levels (Fig. 1B). Bath application of 3 μM atropine decreased the maintained firing rate (Fig. 1D) and shortened the time course of the suppression (Fig. 1C). These data suggest that the firing rates of some RGCs are maintained, in part, by resting levels of ACh or choline and are suppressed by higher concentrations. This interpretation is supported by the atropine-induced suppression of the light responses and the maintained firing rate in three of eight cells and the enhancement of maintained firing by atropine in one additional RGC.
Figure 1.
PST histograms (nine trials) of the responses of an RGC to puff application of 500 μM choline. Choline application (A, horizontal bar) suppressed the firing of the cell (P < 0.001) compared with the normal maintained firing rate (B). The time course of choline-induced suppression was reduced by bath application of 3 μM atropine (C, P < 0.01), and the maintained firing rate was decreased (D).
As mentioned, atropine decreased but did not fully block choline-induced increases in firing rates. Figure 2B demonstrates a multiphasic response to the application of 500 μM choline. The initial transient excitatory response was followed immediately by suppression and then by a large sustained excitatory response. Bath application of 3 μM atropine significantly decreased the light responses (P < 0.001; Fig. 2D) and the suppressive responses to choline application (P < 0.001; Fig. 2F) but did not block the larger sustained choline excitatory response. Thus, the choline-induced responses resulted largely but not completely from mAChR activation. The atropine-insensitive choline excitatory responses most likely were mediated by nAChRs44,45 and indicate the potential for interaction between nicotinic and muscarinic cholinergic systems.
Figure 2.
PST histograms (15 trials) showing the responses of an OFF RGC to light flashes (A), light flashes paired with choline (C), and choline alone (B). In regular Ames medium, the cell responded to 1-second light stimuli with sustained OFF response (black bars). The response to a 1-second application of 1 mM choline (gray bars) was multiphasic and included both enhancement and suppression of firing. Bath application of 3 μM atropine (D–F) resulted in decreases across all conditions, but did not completely eliminate the choline-induced enhancement of the firing rate.
RNA and Protein Expression
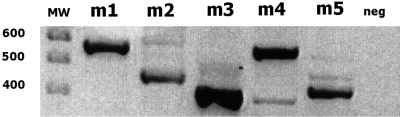
The physiological data in this study extend findings from previous studies indicating that mAChR activation affects retinal processing. However, the expression patterns of all the mAChR subtypes have not yet been described in rabbit retina. Accordingly, mRNA extracted from whole neural retina was screened for the presence of mAChR mRNA transcripts. Figure 3 shows the products that were amplified by using primers for the m1 to m5 mAChRs, separated by electrophoresis on 2% agarose gels, extracted, and sequenced. The resulting sequences were 83% to 95% homologous to human mAChRs and had no significant homology to other cDNA sequences. No products were amplified in the no-template control experiments.
Figure 3.
RT-PCR of mRNA extracted from rabbit retina demonstrated the presence of m1 to m5: m1, 573 bp; m2, 469 bp; m3, 434 bp; m4, 592 bp; and m5, 451 bp. Product identity was confirmed by sequencing. Sequence homology to human ranged from 83% to 95%.
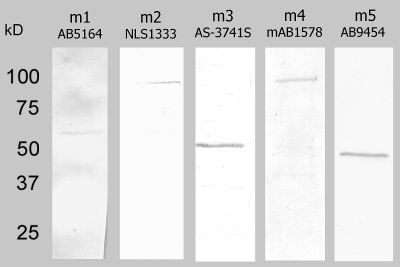
To confirm that the mRNA transcripts reflected the expression of the corresponding proteins and to confirm the specificity of the antibodies used for immunohistochemical studies, we performed Western blot analyses with protein extracted from whole neural retina and antibodies against each of the mAChR subtypes (Table 1).
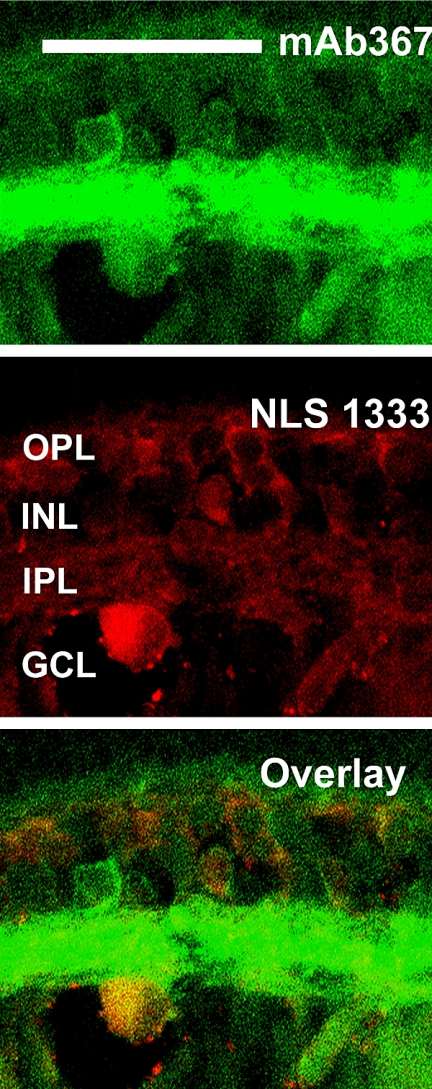
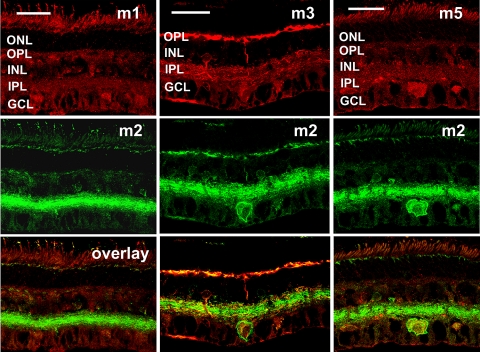
Figure 4 shows that the antibodies against each of the mAChR subtypes bound to single proteins at or very near the molecular weight for that subtype, as predicted by human mAChR protein sequences, suggesting that the proteins are expressed in the retina and that the antibodies are specific. No bands were labeled when the primary antibodies were omitted, or when isotype IgG was substituted for the primary antibodies (data not shown). Although the molecular weights of both the m2 and m4 bands were larger than predicted from human mAChR protein sequences (∼54 kDa), neither the protein sequences nor the posttranslational modifications have been described for rabbit, but the size differences are probably attributable to posttranslational modifications and/or species differences. The m2 antibody mAb367 has been reported to be a specific immunohistochemical label for m2 mAChRs in rabbit and primate retinas,15,16,41 and labeling was abolished in m2-knockout mice,46 but this mAb is unsuitable for Western analysis. To confirm the specificity of the monoclonal m2 antibody in rabbit retina, we used a second polyclonal anti-m2 antibody (NLS1333) for Western blot analysis, and the results yielded a single band. Further, sequential double-label immunohistochemistry with both anti-m2 antibodies demonstrated that the antibodies yielded equivalent labeling patterns (Fig. 5), which were consistent with previous reports of m2 expression in mammalian retina.15,16,41 The dim labeling and lack of labeling in the IPL by the NLS antibody were two of the reasons why we chose to continue the double-labeling studies with mAb367. In addition, the NLS1333 antibody was also raised in rabbit, which precluded colocalization studies with m1, m3, or m5 (antibodies all raised in rabbit; see Table 1). All further m2 immunohistochemistry was performed with mAb367.
Figure 5.
Comparison of labeling patterns of two antibodies against m2: mAb367 (green) and NLS 1333 (red). There was expected correspondence in the labeling of cell bodies (overlay). Confocal images of five optical sections taken at 0.5-μm intervals. Scale bar, 50 μm.
Figure 4.
Western blot analysis with antibodies to the five muscarinic mAChR subtypes resulted in single bands, indicating the presence of mAChR protein and showed that each antibody was specific for a single protein in retinal neural extracts.
Expression Patterns
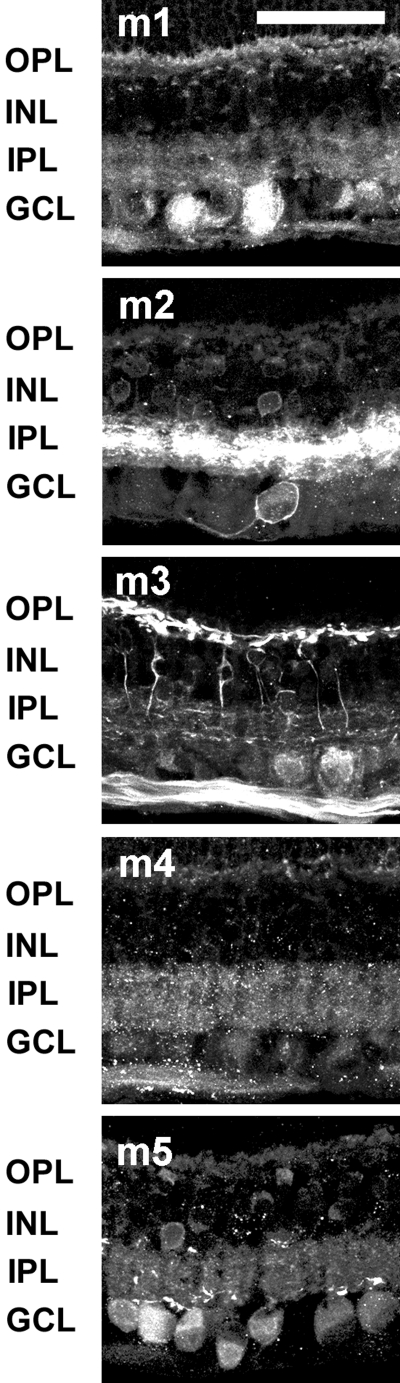
Indirect immunohistochemistry was used to assess the distribution of mAChRs (Fig. 6). Antibodies against muscarinic subtypes m1 to m5 yielded labeling in the inner retina, including subsets of bipolar, amacrine, and RGCs. Fluorescence in photoreceptors and the ONL was nonspecific, since it persisted in some isotype IgG control sections. Processes in the OPL and presumptive horizontal and bipolar cell bodies in the INL were prominently labeled with antibodies against m3 mAChRs. Bipolar cell dendrites labeled with m3 stratified narrowly in both the ON and OFF sublamina of the IPL, and other labeled dendrites were more broadly but sparsely distributed throughout the entire IPL. Antibodies against m2 and m5 mAChRs also labeled bipolar cells, but immunoreactivity was less intense and less consistent than that observed with antibodies against m3 mAChRs.
Figure 6.
Antibodies against muscarinic AChRs labeled specific subpopulations of horizontal, bipolar, amacrine, and ganglion cells, and immunoreactive processes were broadly distributed throughout the IPL. Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 50 μm.
Subsets of amacrine cells and RGCs were labeled with antibodies against all the mAChRs, and immunoreactivity was broadly distributed throughout the IPL.
The specific labeling patterns differed by subtype. For example, processes that were m2 and m4 immunoreactive appeared to be dense, whereas m3-immunoreactive dendrites were the most sparsely distributed. The m3 and m4 receptor antibodies strongly labeled the nerve fiber layer (NFL), whereas antibodies against other muscarinic subtypes did not. Antibodies against m5 mAChRs yielded the broadest distribution of all the subtypes. Labeling was apparent in processes throughout the IPL and in many amacrine and RGC bodies, as well as in a subset of bipolar cells. Subsets of amacrine cells were immunoreactive to antibodies against m1 and m4 mAChRs, and a larger proportion of cells in the GCL were labeled. The processes of m1 muscarinic receptors were distributed in two broad bands in the ON and OFF sublamina of the IPL, indicating that both ON and OFF RGCs most likely express m1 mAChRs. Although the cellular labeling patterns for m1 and m4 were similar, m4 processes were more evenly distributed throughout the IPL than were the m1 processes. In contrast to m1 and m4, many amacrine cells and a smaller proportion of RGCs were labeled with antibodies against m2 mAChRs, and the labeling appeared to be the densest in a broad band in the center of the IPL.
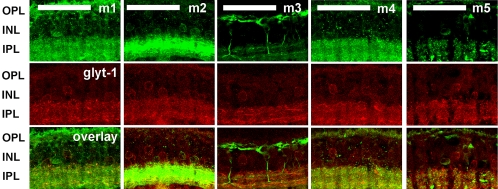
To determine whether cholinergic and glycinergic amacrine cells express mAChRs, retinas were double labeled with antibodies against ChAT and against the glycine transporter glyt-1.
Figure 7 shows the labeling of the m1 to m5 mAChRs relative to ChAT. The broad distribution of the m1, m2, m4, and m5 receptors relative to the narrow stratification of the ChAT bands is reminiscent of the distributions of nAChRs throughout the IPL.47 Processes exhibiting labeled m3 were observed between and below the ChAT bands; thus, the dendrites of both ON and OFF bipolar cells passed though one or both of the cholinergic plexuses. Of interest, a small proportion of starburst amacrine cells expressed the mAChR subtypes, as seen by colocalization with ChAT immunoreactivity (Fig. 7, bottom row). However, the starburst cells comprised a relatively small proportion of the cells labeled by antibodies against mAChRs.
Figure 7.
The distribution of muscarinic AChR subtypes relative to ChAT. Maximum projection images of five optical confocal sections showed immunoreactivity to the mAChR subtypes (top row) and ChAT (middle row). Immunoreactivity for all subtypes predominated in the inner retina, with a broad distribution through the IPL, although m3 immunoreactivity was closely apposed to ChAT immunoreactivity in the IPL. Subsets of ChAT-immunoreactive cells express each of the mAChR subtypes. Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 40 μm.
Colocalization of mAChRs with the glyt-1 was very limited (Fig. 8), suggesting that most of the mAChR-expressing amacrine cells are GABAergic rather than glycinergic. A small proportion of m1-, m2-, and m4-immunoreactive amacrine cells were also labeled by antibodies against glyt-1, whereas the m3 and m5 subtypes showed no colocalization.
Figure 8.
The distribution of muscarinic subtypes relative to glyt-1. Maximum projection images of five optical confocal sections showed immunoreactivity to the mAChR subtypes (top row) and glyt-1 (middle row). Colocalization was limited. A small proportion of amacrine cells that expressed m1, m2, and m4 also expressed glyt-1. Cells expressing m3 and m5 did not express glyt-1. Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 50 μm.
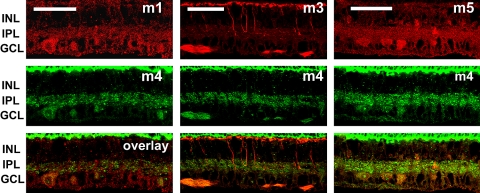
To assess the distribution of mAChRs relative to one another, we made pair-wise comparisons between m1, m3, and m5 relative to m2 (Fig. 9); m1, m3, m5, relative to m4 (Fig. 10); and m2 relative to m4 (Fig. 11). Because the m1, m3, and m5 polyclonal antibodies were all raised in rabbit, it was not possible to make direct comparisons of the distributions of these mAChR subtypes by double-labeling immunohistochemistry.
Figure 9.
Pair-wise comparisons of m1, m3, and m5 immunoreactivity relative to that of m2. Maximum projection images of five optical confocal sections shows immunoreactivity to m1, m3, and m5 (top row) relative to m2 immunoreactivity (middle row). There was very limited colocalization between m1 and m2 (left). Subsets of bipolar, amacrine, and ganglion cells expressed both m3 and m2 (middle), whereas only cells in the GCL expressed both m5 and m2 (right). Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 32 μm.
Figure 10.
Pair-wise comparisons of m1, m3, and m5 immunoreactivity relative to m4. Maximum projection images of five optical confocal sections showed immunoreactivity to m1, m3, and m5 (top row) relative to m4 immunoreactivity (middle row). The labeling patterns of m1 and m4 (left) and m5 and m4 (right) were distinctly similar but limited to the IPL and GCL. There was no apparent colocalization of m3 and m4 in neural retina, but there was apparent colocalization in the NFL (middle). Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 50 μm.
Figure 11.
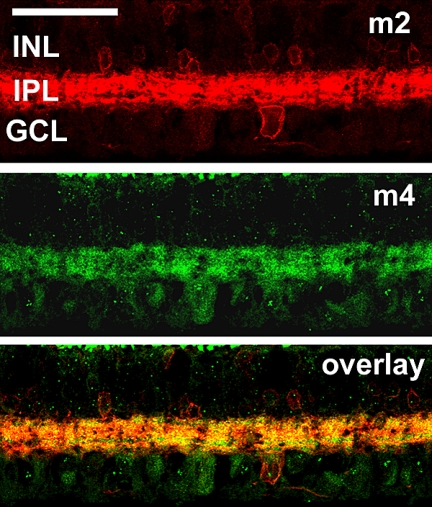
Comparison of m2 mAChR expression relative to m4 mAChR expression. Maximum projection images of five optical confocal sections showed immunoreactivity to antibodies against m2 (top) and antibodies against m4 (middle). Most of the cells in the INL expressed m2 but not m4. Immunoreactivity for both subtypes was broadly distributed throughout the IPL. More detailed examination showed two narrow bands in the IPL that were immunoreactive for m4 but not m2. One band was located in the outer portion of the OFF sublamina, and the other band was located in the outer portion of the ON sublamina. Most of the cells in the GCL expressed m4 but not m2. A small subset of RGCs was immunoreactive to antibodies against both m2 and m4. Confocal images of five optical sections were obtained in 0.5-μm intervals. Scale bar, 50 μm.
Figure 9 shows the distribution of m1, m3, and m5 immunoreactivity (top row) relative to m2 immunoreactivity (middle row) and the merged immunoreactivity images (bottom row). Both m1 and m2 mAChRs were expressed by subsets of amacrine and RGCs (left), but there was little overlap between the populations of m1- and m2-immunoreactive cells. Subsets of bipolar, amacrine, and RGCs expressed both m3 and m2 mAChRs, and there were areas of distinct colocalization in both the OPL and the IPL (middle). The overlap in the IPL appeared to be due largely to coexpression of the subtypes by bipolar cells and RGCs. Only a small subpopulation of amacrine cells expressed both m3 and m2 (middle column), whereas the labeled bipolar cell populations appeared to overlap. Of interest, colocalization of m5 and m2 mAChRs (right column) appeared to be limited to the somata in the GCL.
A second set of double-labeling experiments was undertaken to determine the distribution of m1, m3, and m5 mAChRs (Fig. 10, row 1) relative to m4 (Fig. 10, row 2). Colocalization between m1 and m4 mAChRs (left) and m1 and m5 mAChRs (right) was limited to processes in the IPL and cells in the GCL, including RGCs and either small RGCs and/or displaced amacrine cells. There was no apparent colocalization between m3 and m4 mAChRs (middle column). Specifically, the overlap between m3 and m4 mAChR immunoreactivity appeared to be limited to labeling in the NFL. This is an interesting contrast with the almost complete colocalization between m3 and m2 mAChRs. These findings indirectly suggest that m2 and m4 mAChR activation may have different roles in visual processing. The m4 labeling in this set of experiments was limited to the IPL and the GCL; however, this was not the case in all retinal sections, and it may be related to differences in retinal locations such as eccentricity.
The final set of double-labeling experiments was performed to assess the distribution of m2 and m4 mAChRs relative to one another (Fig. 11). Although immunoreactivity for both subtypes was broadly distributed throughout the IPL, two narrow bands in the IPL were immunoreactive for m4 but not m2. One band was located in the outer portion of the OFF sublamina, and the other band was located in the outer portion of the ON sublamina. Most of the cells in the INL expressed m2 but not m4 mAChRs, and most cells in the GCL expressed m4 but not m2 mAChRs. A small subset of presumptive RGCs was immunoreactive to antibodies against both m2 and m4 mAChRs. Both m2 and m4 mAChRs have inhibitory effects on resting membrane potential, and the differential distribution of these two subtypes suggests that each receptor modulates a different aspect of visual processing.
Discussion
In this study, we used choline and atropine to assess the functional roles of mAChRs in the retina. Choline is constitutively present in many parts of the nervous system and brain.48 When choline is taken up by the high-affinity choline transporter on cholinergic neurons, it is used as a precursor for ACh synthesis and/or for phospholipid and membrane production.49,50 Levels of choline in the plasma are reportedly stable at 10 μM,48 and extracellular choline concentrations in the brain can be sufficient to activate AChRs.51 In the retina, choline is taken up by photoreceptors, predominantly for the synthesis of phospholipids and photoreceptor membrane production52,53; however, the expression of mAChRs in the OPL may indicate that choline also serves to modulate retinal processing in the OPL. In the inner retina, choline is also present at synaptic sites as a product of the hydrolysis of ACh and thus may provide a mechanism for secondary or extended activation of mAChRs. Thus, at physiological concentrations, choline can act as a specific endogenous ligand for AChRs.
To assess the global effects of choline-mediated mAChR activation, the responses of nine RGCs to puff application of choline were tested. Because choline can also activate some nicotinic receptor subtypes, mAChR activation was confirmed by application of the nonspecific muscarinic antagonist atropine. Choline application resulted in increased firing by ON and ON-OFF RGCs and suppressed the firing of OFF RGCs. Atropine significantly decreased both choline-induced excitation and suppression in most of the RGCs tested. Atropine application without choline also affected the light responses and maintained firing rates of the RGCs. The responses of individual RGCs to cholinergic agonists and antagonists were quite robust, statistically significant, and consistent with those in previous studies. Although the sample size was small, these data suggest that the tonic release of ACh and the availability of its breakdown product choline contribute to overall retinal excitability.
Atropine was more effective at relieving choline-induced suppression than choline-induced excitation. Thus, choline-induced muscarinic modulation of RGC activity appeared to be preferentially weighted toward suppression of the firing rate, with a lesser contribution to the enhancement of RGC firing rates. Preferential suppression may be due to indirect as well as direct effects. One study has shown that ACh release in the retina is inhibited by muscarine due to an inhibitory feedback loop that includes glycinergic amacrine cells.22 The reported expression of m2 mAChRs by a subset of glycinergic amacrine cells17 and the preferential suppression of RGC firing by choline in the present study are consistent with that model. However, choline application inhibits ACh release via scopolamine-sensitive presynaptic mAChRs in the porcine enteric system.54 Since our immunohistochemical data indicate that some cholinergic amacrine cells express mAChRs we cannot rule out presynaptic effects or direct muscarinic feedback to starburst amacrine cells. Only a small subset of starburst cells in the adult retina express heteromeric nAChRs,47 or α7 nAChRs, though α7 processes closely stratify with cholinergic processes.34 It seems likely that nicotinic feedback to starburst amacrine cells is mediated by feedback from glycinergic or GABAergic amacrine cells, to avoid a positive feedback loop. However, muscarinic receptors may provide a substrate for direct feedback to cholinergic amacrine cells. Retinal bipolar cells express several different potassium channels,55–57 and cyclic nucleotide-gated channels,58 which may be affected by mAChR activation, providing for indirect effects on RGC response properties. Alternatively, direct suppression of RGC firing may be mediated by m2 and m4 mAChR activation of K+ channels,9 as inwardly rectifying K+ channels have been described in rat retinal RGCs.59
To begin to assess the effects of mAChR activation, it was necessary to first determine which muscarinic subtypes are expressed in rabbit retina. RT-PCR data indicated that mRNA transcripts for all subunits were present in the rabbit retina. Western blot data confirmed mAChR protein expression and demonstrated the specificity of each antibody against the targeted mAChR. Specificity was further confirmed by control experiments that substituted matched concentrations of isotype IgG. The tree shrew is the only other species in which the full complement of mAChRs has been systematically identified, due to the reported involvement of muscarinic activation on the development of myopia.19 Although the expression patterns of mAChRs in tree shrew inner retina are similar to those in the rabbit, these authors also reported muscarinic immunoreactivity in photoreceptor outer segments and Müller cells, whereas in the rabbit, labeling in the photoreceptors and ONL appeared to be nonspecific. These differences may be attributable to differences in methodology or species differences.
Recent reports46,60 call into question the specificity of many commercially available antibodies against mAChRs. Many antibodies result in labeling in knockout mouse or label multiple bands in Western blots experiments, indicating that multiple proteins are labeled. The antibodies against m1 and m2 used in this study were also tested in the knockout mouse.46 In this report, m2 labeling was abolished in the m2-knockout mouse, whereas m1 labeling was retained. In another recent report, antibodies against m3 mAChRs resulted in multibanded Western blots of extracts from cell lines stably expressing m3 mAChRs.60 Because fixation and blocking can affect the specificity of antibody labeling,61,62 it is necessary to test antibody specificity for each species and each application. The single band in the Western blots for each antibody tested confirmed the specificity of the antibodies for rabbit retinal proteins. The specificity of the m2 antibody mAb367 has been confirmed in the knockout mouse. However, we wanted to be certain that we could obtain a single band on Western blot, to assure that m2 labeling in the rabbit retina was specific. mAb367, although well documented for immunohistochemistry, does not work for Western blot analysis. This problem was reported by the manufacturer of the antibody (Chemicon) and was evident in the original report of the characterizations of the antibody,41 in which the protein identified by the antibody did not migrate during electrophoreses. Because this antibody is unsuitable for Western blot analysis, we compared the labeling pattern with that of a second m2 antibody that did yield a specific single band. The labeling patterns were consistent with each other and with those in previous reports.
Each mAChR subtype was expressed throughout the IPL, although the density of immunoreactivity varied by subtype. Subtypes m2 and m4 had the densest IPL labeling, whereas m3 immunoreactivity was more sparsely distributed in the INL. Subsets of amacrine and RGC somata were also immunoreactive, indicating that muscarinic activation has the potential for direct and indirect effects on RGC response properties.
The m3 subtype, and to a lesser extent, the m2 and m5 subtypes were also expressed by bipolar cells. Because m1, m3, and m5 mAChR activation tends to have excitatory effects, and m2 activation tends to have inhibitory effects, these labeling patterns suggest that the excitatory effects of activation through Gq-coupled subtypes might be counterbalanced by activation of the Gi-coupled subtypes. The expression pattern of m3 mAChRs was consistent with that in primates.15 Bipolar cells labeled by m3 antibodies were similar in morphology and stratification to rabbit CBmb4 and CBmb5 ON cone bipolar cells,63 and the rat CB-6 on cone bipolar cells.64 Subpopulations of OFF bipolar cells also expressed m3 mAChRs and were similar in stratification and morphology to CBmb3 OFF cone bipolar cells. The expression patterns of m2 mAChRs by ganglion and amacrine cells are consistent with previous reports of m2 mAChR distribution in the rabbit retina.14,17
We performed a series of double-label immunohistochemical comparisons, including the assessment of mAChR distribution relative to ChAT immunoreactivity and the glycine transporter and to the distribution of mAChRs relative to one another.
Cholinergic amacrine cells expressed all mAChR subtypes, suggesting several possible feedback pathways. However, previous reports of m2 immunoreactivity in the rabbit retina16,17 did not describe bipolar cell labeling or colocalization of m2 immunoreactivity with ChAT immunoreactivity. Although the same antibodies were used against m2 mAChRs in the current and previous studies, different fixation protocols were used. The 1% PLP fixation used in the present study may retain antigenicity and result in decreased background fluorescence due to the decreased amount of paraformaldehyde.
Colocalization of mAChRs with the glycine transporter was very limited, suggesting that the most mAChR-expressing amacrine cells are GABAergic rather than glycinergic. A small proportion of m1, m2, and m4 immunoreactive amacrine cells were also labeled by antibodies against the glycine transporter, whereas the m3 and m5 subtypes showed no colocalization.
This is the first study in which the distribution of mAChR subtypes has been investigated relative to one another in the retina. Gq-coupled (m1, m3, and m5) muscarinic receptors tended to be colocalized with Gi -coupled (m2 and m4) muscarinic receptors, but not with other Gq-coupled receptors. There were differential distributions within these groups, as m1 was preferentially colocalized with m4, m3 was preferentially colocalized with m2, and m5 was colocalized with both m2 and m4. The m2 and m4 subtypes were not colocalized with one another in amacrine cells but were colocalized in some RGCs. Although we were not able to do pair-wise assessments of the distribution of m1, m3, and m5 mAChRs relative to one another, the preferential colocalization with m2 and m4 suggests that m1 and m3 mAChRs are expressed by different subsets of cells in the inner retina.
The expression of mAChR subtypes with excitatory and inhibitory effects by individual neurons provides a substrate for excitation and inhibition via activation by ACh and/or choline. Thus, initial ACh release may activate one subtype of receptor, but may be counterbalanced by activation of another type via residual choline, with the opposite effect. These receptors may be expressed by the same cell or by cells at different points in the retinal circuitry. This expression pattern provides a mechanism by which very small increments and decrements in light could be rapidly modulated. Additional complexity is provided by cholinergic modulation of upstream inputs to these same cells via feedback to starburst amacrine cells or to a small proportion of retinal glycinergic circuitry. Thus, the muscarinic cholinergic system in the retina may be well positioned to contribute to the modulation of complex stimuli.
Our understanding of the role of mAChRs in retinal processing is complicated by the widespread expression of nicotinic AChRs, often by the same cells that express mAChRs.44 An exploration of the effects of muscarinic and nicotinic activation in individual neurons will be helpful for the identification of synergistic or antagonistic interactions of AChRs in the retina. This understanding has the potential to provide important insights into complex retinal information processing as well as a baseline to assess potential visual effects of anticholinergic treatments for ocular diseases and brain diseases.
Acknowledgments
The authors thank John Tootle for computer programming and Virginia Wotring for many helpful thoughts and comments.
Footnotes
Supported by National Eye Institute Grants R01 EY07845 and P30 EY03039.
Disclosure: C.E. Strang, None; J.M. Renna, None; F.R. Amthor, None; K.T. Keyser, None
References
- 1.Schmidt M, Humphrey MF, Wassle H. Action and localization of acetylcholine in the cat retina. J Neurophysiol 1987;58:997–1015 [DOI] [PubMed] [Google Scholar]
- 2.Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci 1996;16:5060–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa LG, Murphy SD. Interaction of choline with nicotinic and muscarinic cholinergic receptors in the rat brain in vitro. Clin Exp Pharmacol Physiol 1984;11:649–654 [DOI] [PubMed] [Google Scholar]
- 4.Strenn K, Dal-Bianco P, Weghaupt H, Koch G, Vass C, Gottlob I. Pattern electroretinogram and luminance electroretinogram in Alzheimer's disease. J Neural Transm Suppl 1991;33:73–80 [DOI] [PubMed] [Google Scholar]
- 5.Daniels R, Harding GF, Anderson SJ. Effect of dopamine and acetylcholine on the visual evoked potential. Int J Psychophysiol 1994;16:251–261 [DOI] [PubMed] [Google Scholar]
- 6.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006;113:2285–2291 [DOI] [PubMed] [Google Scholar]
- 7.Duncan G, Collison DJ. Role of the non-neuronal cholinergic system in the eye: a review. Life Sci 2003;72:2013–2019 [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Abogadie FC, Allen TG, et al. Muscarinic mechanisms in nerve cells. Life Sci 1997;60:1137–1144 [DOI] [PubMed] [Google Scholar]
- 9.Wess J, Liu J, Blin N, Yun J, Lerche C, Kostenis E. Structural basis of receptor/G protein coupling selectivity studied with muscarinic receptors as model systems. Life Sci 1997;60:1007–1014 [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, Selyanko AA. Membrane currents underlying the cholinergic slow excitatory post-synaptic potential in the rat sympathetic ganglion. J Physiol 1985;365:365–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal M. Transmitter interactions in the rabbit retina. Biochemical Society Transactions 1983;11:684–686 [DOI] [PubMed] [Google Scholar]
- 12.Neal MJ, Dawson C. Muscarinic cholinergic receptors in rabbit retina. J Pharm Pharmacol 1985;37:60–61 [DOI] [PubMed] [Google Scholar]
- 13.Hutchins JB, Hollyfield JG. Acetylcholine receptors in the human retina. Invest Ophthalmol Vis Sci 1985;26:1550–1557 [PubMed] [Google Scholar]
- 14.Wasselius J, Johansson K, Bruun A, Zucker C, Ehinger B. Correlations between cholinergic neurons and muscarinic m2 receptors in the rat retina (published correction appears in Neuroreport 1998;9(13):2436). Neuroreport 1998;9:1799–1802 [DOI] [PubMed] [Google Scholar]
- 15.Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol 2003:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucker CL, Ehinger B. Complexities of retinal circuitry revealed by neurotransmitter receptor localization. Prog Brain Res 2001;131:71–81 [DOI] [PubMed] [Google Scholar]
- 17.Zucker CL, Nilson JE, Ehinger B, Grzywacz NM. Compartmental localization of gamma-aminobutyric acid type B receptors in the cholinergic circuitry of the rabbit retina. J Comp Neurol 2005;493:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol 1998;392:273–284 [PubMed] [Google Scholar]
- 19.McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol Vis 2009;15:464–475 [PMC free article] [PubMed] [Google Scholar]
- 20.Jurklies B, Kaelin-Lang A, Niemeyer G. Cholinergic effects on cat retina In vitro: changes in rod- and cone-driven b-wave and optic nerve response. Virus Res 1996;36:797–816 [DOI] [PubMed] [Google Scholar]
- 21.Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol 1997;77:675–689 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham JR, Dawson C, Neal MJ. Evidence for a cholinergic inhibitory feed-back mechanism in the rabbit retina. J Physiol (Lond) 1983;340:455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanke JJ, Lehman B, Fischer AJ. Muscarinic signaling influences the patterning and phenotype of cholinergic amacrine cells in the developing chick retina. BMC Dev Biol 2008;8:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. J Neurosci 2000;20:6570–6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol 2004;560:533–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber B, Schlicker E. Modulation of dopamine release in the guinea-pig retina by G(i)- but not by G(s)- or G(q)-protein-coupled receptors. Fundam Clin Pharmacol 2001;15:393–400 [DOI] [PubMed] [Google Scholar]
- 27.Cimini BA, Strang CE, Wotring VE, Keyser KT, Eldred WD. Role of acetylcholine in nitric oxide production in the salamander retina. J Comp Neurol 2008;507:1952–1963 [DOI] [PubMed] [Google Scholar]
- 28.Qu ZX, Fertel R, Neff NH, Hadjiconstantinou M. Pharmacological characterization of muscarinic receptors mediating inhibition of adenylate cyclase activity in the rat retina. J Pharmacol Exp Therapeut 1988;246:839–842 [PubMed] [Google Scholar]
- 29.Borda E, Berra A, Saravia M, Ganzinelli S, Sterin-Borda L. Correlations between neuronal nitric oxide synthase and muscarinic M3/M1 receptors in the rat retina. Exp Eye Res 2005;80:391–399 [DOI] [PubMed] [Google Scholar]
- 30.Ames A, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem 1981;37:867–877 [DOI] [PubMed] [Google Scholar]
- 31.Renna JM, Strang CE, Amthor FR, Keyser KT. Strychnine, but not PMBA, inhibits neuronal nicotinic acetylcholine receptors expressed by rabbit retinal ganglion cells. Vis Neurosci 2007;24:503–511 [DOI] [PubMed] [Google Scholar]
- 32.Strang CE, Amthor FR, Keyser KT. Rabbit retinal ganglion cell responses to nicotine can be mediated by β2-containing nicotinic acetylcholine receptors. Vis Neurosci 2003;20:651–662 [DOI] [PubMed] [Google Scholar]
- 33.Hellstrom-Lindahl E, Nordberg A. Muscarinic receptor subtypes in subpopulations of human blood mononuclear cells as analyzed by RT-PCR technique. Journal of Neuroimmunology 1996;68:139–144 [DOI] [PubMed] [Google Scholar]
- 34.Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem 2007;55:461–476 [DOI] [PubMed] [Google Scholar]
- 35.Firth SI, Li W, Massey SC, Marshak DW. AMPA receptors mediate acetylcholine release from starburst amacrine cells in the rabbit retina. J Comp Neurol 2003;466:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dmitrieva NA, Pow DV, Lindstrom JM, Keyser KT. Identification of cholinoceptive glycinergic neurons in the mammalian retina. J Comp Neurol 2003;456:167–175 [DOI] [PubMed] [Google Scholar]
- 37.Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci 1999;16:231–239 [DOI] [PubMed] [Google Scholar]
- 38.Dorje F, Levey AI, Brann MR. Immunological detection of muscarinic receptor subtype proteins (m1–m5) in rabbit peripheral tissues. Mol Pharmacol 1991;40:459–462 [PubMed] [Google Scholar]
- 39.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 1994;14:3351–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AI, Stormann TM, Brann MR. Bacterial expression of human muscarinic receptor fusion proteins and generation of subtype-specific antisera. FEBS Lett 1990;275:65–69 [DOI] [PubMed] [Google Scholar]
- 41.Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol 1995;351:339–356 [DOI] [PubMed] [Google Scholar]
- 42.Ndoye A, Buchli R, Greenberg B, et al. Identification and mapping of keratinocyte muscarinic acetylcholine receptor subtypes in human epidermis. J Invest Dermatol 1998;111:410–416 [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Han H, Zhang L, et al. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol Pharmacol 2001;59:1029–1036 [DOI] [PubMed] [Google Scholar]
- 44.Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit retinal ganglion cells express functional α7 nAChRs. Am J Physiol Cell Physiol 2005;289:C644–C655 [DOI] [PubMed] [Google Scholar]
- 45.Strang CE, Renna JM, Amthor FR, Keyser KT. Nicotinic acetylcholine receptor expression by directionally selective ganglion cells. Vis Neurosci 2007;24:523–533 [DOI] [PubMed] [Google Scholar]
- 46.Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 2009;379:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Vis Neurosci 2000;1 55 :743–752 [DOI] [PubMed] [Google Scholar]
- 48.Lockman PR, Allen DD. The transport of choline: review. Drug Dev Ind Pharm 2002;28:749–771 [DOI] [PubMed] [Google Scholar]
- 49.Guo JZ, Chiappinelli VA. A novel choline-sensitive nicotinic receptor subtype that mediates enhanced GABA release in the chick ventral lateral geniculate nucleus. Neuroscience 2002;110:505–513 [DOI] [PubMed] [Google Scholar]
- 50.Apparsundaram S, Ferguson SM, Blakely RD. Molecular cloning and characterization of a murine hemicholinium-3-sensitive choline transporter. Biochem Soc Trans 2001;29:711–716 [DOI] [PubMed] [Google Scholar]
- 51.Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 1999;19:2693–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masland RH, Mills JW. Choline accumulation by photoreceptor cells of the rabbit retina. Proc Natl Acad Sci U S A 1980;77:1671–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masland RH. Choline metabolism and the maintenance of photoreceptor cell structure. Retina 1982;2:282–287 [PubMed] [Google Scholar]
- 54.Kilbinger H, Kruel R. Choline inhibits acetylcholine release via presynaptic muscarine receptors. Naunyn Schmiedebergs Arch 1981;316:131–134 [DOI] [PubMed] [Google Scholar]
- 55.Ma YP, Cui J, Pan ZH. Heterogeneous expression of voltage-dependent Na+ and K+ channels in mammalian retinal bipolar cells. Vis Neurosci 2005;22:119–133 [DOI] [PubMed] [Google Scholar]
- 56.Ma YP, Cui J, Hu HJ, Pan ZH. Mammalian retinal bipolar cells express inwardly rectifying K+ currents (IKir) with a different distribution than that of Ih. J Neurophysiol 2003;90:3479–3489 [DOI] [PubMed] [Google Scholar]
- 57.Pan ZH. Voltage-activated Ca2+ channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Vis Neurosci 2001;18:279–288 [DOI] [PubMed] [Google Scholar]
- 58.Muller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci 2003;17:2084–2096 [DOI] [PubMed] [Google Scholar]
- 59.Lee SC, Ishida AT. Ih without Kir in adult rat retinal ganglion cells. J Neurophysiol 2007;97:3790–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 2009;379:397–402 [DOI] [PubMed] [Google Scholar]
- 61.Lorincz A, Nusser Z. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci 2008;28:9083–9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 1998;10:478–487 [DOI] [PubMed] [Google Scholar]
- 63.McGillem GS, Dacheux RF. Rabbit cone bipolar cells: correlation of their morphologies with whole-cell recordings. Vis Neurosci 2001;18:675–685 [DOI] [PubMed] [Google Scholar]
- 64.Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 1998;79:1384–1395 [DOI] [PubMed] [Google Scholar]